Vine Foliar Treatments at Veraison and Post-Veraison with Methyl Jasmonate Enhanced Aromatic, Phenolic and Nitrogen Composition of Tempranillo Blanco Grapes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Vineyard, Treatments and Grape Samples
2.2. Determination of General Parameters in Musts
2.3. Analysis of Must Volatile Composition by HS-SPME-GC-MS
2.4. Determination of Grape Phenolic Composition by HPLC-DAD
2.4.1. Extraction of Grape Phenolic Compounds
2.4.2. Analysis of Grape Phenolic Compounds by HPLC-DAD
2.5. Analysis of Must Nitrogen Composition by HPLC-DAD-FLD
2.6. Statistical Analyses
3. Results and Discussion
3.1. General Parameters in the Musts
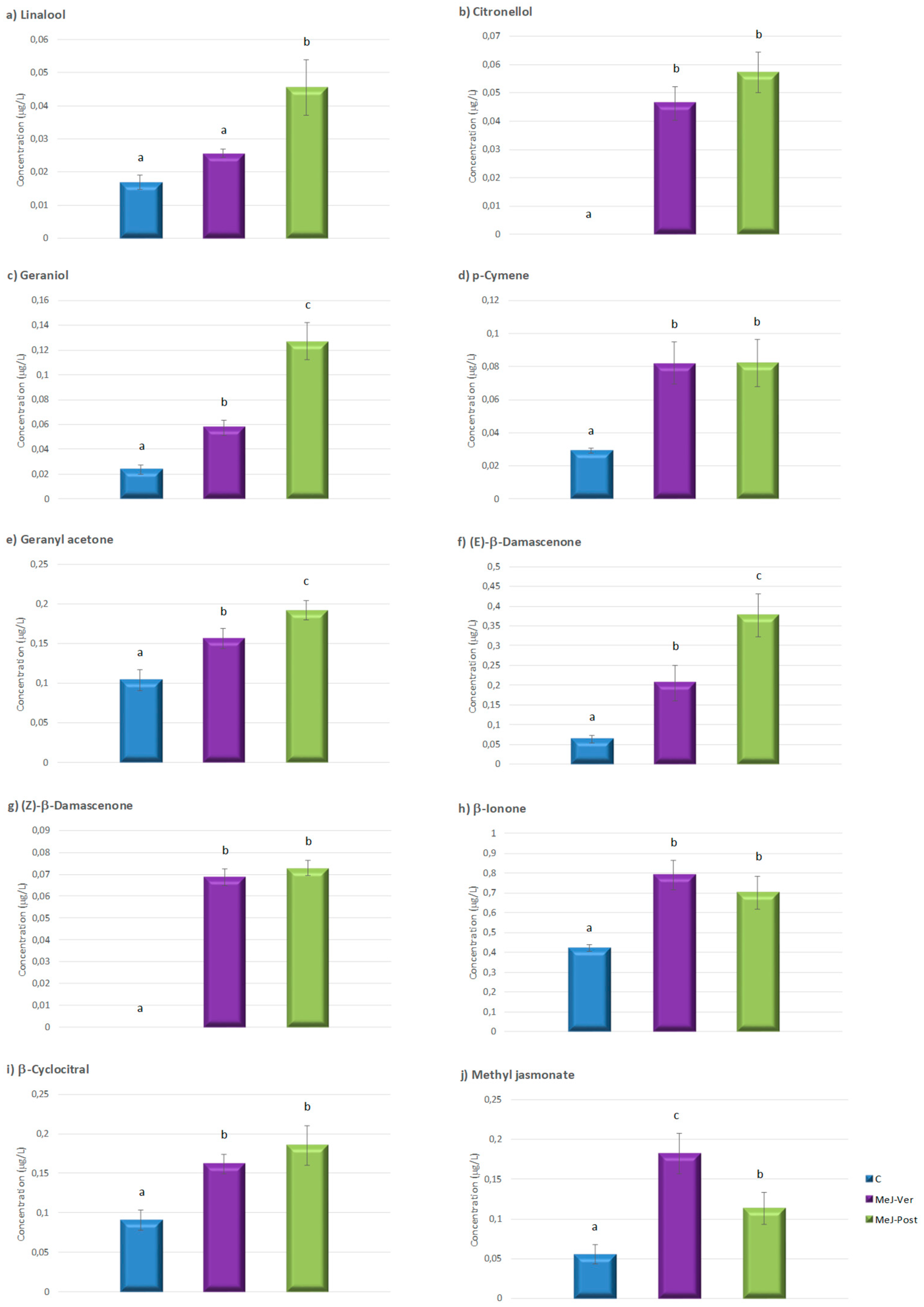
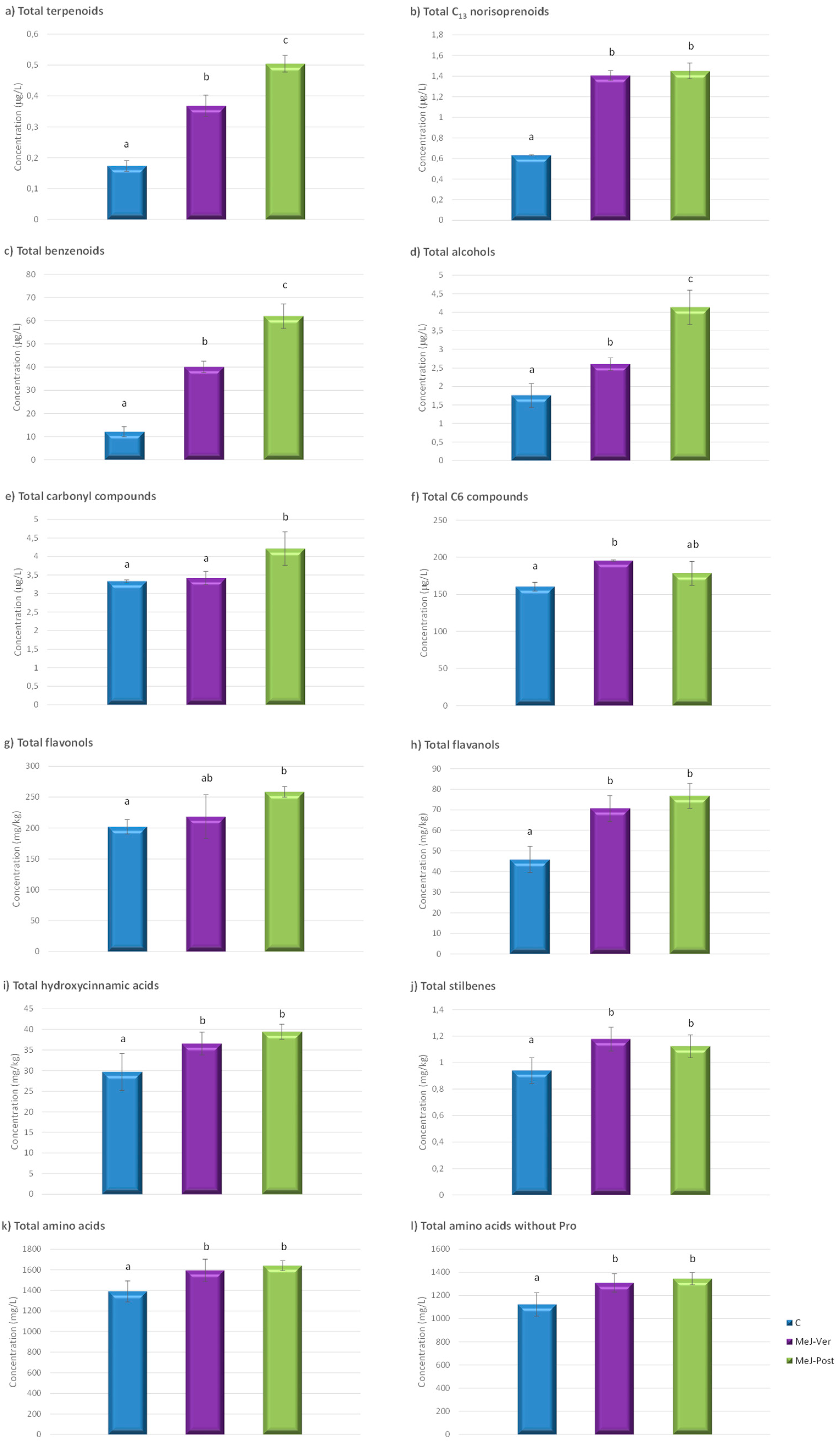
3.2. Influence of the Foliar MeJ Treatments on Must Volatile Compounds
3.3. Effect of the Foliar MeJ Treatments on Grape Phenolic Compounds
3.4. Influence of the Foliar MeJ Treatments on Must Nitrogen Compounds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garde-Cerdán, T.; da Costa, N.L.; Rubio-Bretón, P.; Barbosa, R.; Baroja, E.; Martínez-Vidaurre, J.M.; Román, S.M.-S.; de Urturi, I.S.; Pérez-Álvarez, E.P. The Most Important Parameters to Differentiate Tempranillo and Tempranillo Blanco Grapes and Wines through Machine Learning. Food Anal. Methods 2021, 14, 2221–2236. [Google Scholar] [CrossRef]
- Carbonell-Bejerano, P.; Ibáñez, J.; Royo, C.; Baroja, E.; Martínez, J.; García-Escudero, E.; Martínez-Zapater, J.M. Tempranillo Blanco: Origen, presente y futuro. Cuad. Campo 2018, 61, 36–39. [Google Scholar]
- Martínez, J.; García-Escudero, E. Tempranillo blanco: Una variedad fruto de la variación genética de la vid (Vitis vinifera L). ACE Rev. De Enol. 2017, 3, 159. [Google Scholar]
- de Orduña, R.M. Climate change associated effects on grape and wine quality and production. Food Res. Int. 2010, 43, 1844–1855. [Google Scholar] [CrossRef]
- Rienth, M.; Vigneron, N.; Darriet, P.; Sweetman, C.; Burbidge, C.; Bonghi, C.; Walker, R.P.; Famiani, F.; Castellarin, S.D. Grape Berry Secondary Metabolites and Their Modulation by Abiotic Factors in a Climate Change Scenario–A Review. Front. Plant Sci. 2021, 12, 643258. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-García, Y.; Gómez-Plaza, E. Elicitors: A Tool for Improving Fruit Phenolic Content. Agriculture 2013, 3, 33–52. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-García, Y.; Romero-Cascales, I.; Gil-Muñoz, R.; Fernández-Fernández, J.I.; López-Roca, J.M.; Gómez-Plaza, E. Improving Grape Phenolic Content and Wine Chromatic Characteristics through the Use of Two Different Elicitors: Methyl Jasmonate versus Benzothiadiazole. J. Agric. Food Chem. 2012, 60, 1283–1290. [Google Scholar] [CrossRef]
- Portu, J.; Santamaría, P.; López-Alfaro, I.; López, R.; Garde-Cerdán, T. Methyl Jasmonate Foliar Application to Tempranillo Vineyard Improved Grape and Wine Phenolic Content. J. Agric. Food Chem. 2015, 63, 2328–2337. [Google Scholar] [CrossRef]
- Portu, J.; López, R.; Baroja, E.; Santamaría, P.; Garde-Cerdán, T. Improvement of grape and wine phenolic content by foliar application to grapevine of three different elicitors: Methyl jasmonate, chitosan, and yeast extract. Food Chem. 2016, 201, 213–221. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Portu, J.; López, R.; Santamaría, P. Effect of methyl jasmonate application to grapevine leaves on grape amino acid content. Food Chem. 2016, 203, 536–539. [Google Scholar] [CrossRef]
- Gil-Muñoz, R.; Giménez-Bañón, M.J.; Moreno-Olivares, J.D.; Paladines-Quezada, D.F.; Bleda-Sánchez, J.A.; Fernández-Fernández, J.I.; Parra-Torrejón, B.; Ramírez-Rodríguez, G.B.; Delgado-López, J.M. Effect of Methyl Jasmonate Doped Nanoparticles on Nitrogen Composition of Monastrell Grapes and Wines. Biomolecules 2021, 11, 1631. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Álvarez, E.; Rubio-Bretón, P.; Intrigliolo, D.; Parra-Torrejón, B.; Ramírez-Rodríguez, G.; Delgado-López, J.; Garde-Cerdán, T. Year, watering regime and foliar methyl jasmonate doped nanoparticles treatments: Effects on must nitrogen compounds in Monastrell grapes. Sci. Hortic. 2022, 297, 110944. [Google Scholar] [CrossRef]
- Gómez-Plaza, E.; Mestre-Ortuño, L.; Ruiz-García, Y.; Fernández-Fernández, J.I.; López-Roca, J.M. Effect of Benzothiadiazole and Methyl Jasmonate on the Volatile Compound Composition of Vitis vinifera L. Monastrell Grapes and Wines. Am. J. Enol. Vitic. 2012, 63, 394–401. [Google Scholar] [CrossRef]
- D’Onofrio, C.; Matarese, F.; Cuzzola, A. Effect of methyl jasmonate on the aroma of Sangiovese grapes and wines. Food Chem. 2018, 242, 352–361. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Gamboa, G.G.; Baroja, E.; Rubio-Bretón, P.; Pérez-Álvarez, E.P. Influence of methyl jasmonate foliar application to vineyard on grape volatile composition over three consecutive vintages. Food Res. Int. 2018, 112, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; VanderWeide, J.; Yan, Y.; Tindjau, R.; Pico, J.; Deluc, L.; Zandberg, W.F.; Castellarin, S.D. Impact of hormone applications on ripening-related metabolites in Gewürztraminer grapes (Vitis vinifera L.): The key role of jasmonates in terpene modulation. Food Chem. 2022, 388, 132948. [Google Scholar] [CrossRef]
- Ranjbaran, E.; Gholami, M.; Jensen, M. Near-harvest application of methyl jasmonate affected phenolic content and antioxidant properties in “Thompson Seedless” grape. Food Sci. Nutr. 2022, 10, 477–486. [Google Scholar] [CrossRef]
- Gil-Muñoz, R.; Fernández-Fernández, J.I.; Crespo-Villegas, O.; Garde-Cerdán, T. Elicitors used as a tool to increase stilbenes in grapes and wines. Food Res. Int. 2017, 98, 34–39. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Lorenzo, C.; Lara, J.F.; Pardo, F.; Ancín-Azpilicueta, C.; Salinas, M.R. Study of the Evolution of Nitrogen Compounds during Grape Ripening. Application to Differentiate Grape Varieties and Cultivated Systems. J. Agric. Food Chem. 2009, 57, 2410–2419. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Ancín-Azpilicueta, C. Effect of the addition of different quantities of amino acids to nitrogen-deficient must on the formation of esters, alcohols, and acids during wine alcoholic fermentation. LWT 2008, 41, 501–510. [Google Scholar] [CrossRef]
- Arias-Gil, M.; Garde-Cerdán, T.; Ancín-Azpilicueta, C. Influence of addition of ammonium and different amino acid concentrations on nitrogen metabolism in spontaneous must fermentation. Food Chem. 2007, 103, 1312–1318. [Google Scholar] [CrossRef]
- Pérez-Álvarez, E.P.; Martínez-Vidaurre, J.M.; García-Escudero, E.; Garde-Cerdán, T. Amino acids content in ‘Tempranillo’ must from three soil types over four vintages. Vitis 2019, 58, 3–12. [Google Scholar] [CrossRef]
- Oliva, J.; Garde-Cerdán, T.; Martínez-Gil, A.M.; Salinas, M.R.; Barba, A. Fungicide effects on ammonium and amino acids of Monastrell grapes. Food Chem. 2011, 129, 1676–1680. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; López, R.; Portu, J.; González-Arenzana, L.; López-Alfaro, I.; Santamaría, P. Study of the effects of proline, phenylalanine, and urea foliar application to Tempranillo vineyards on grape amino acid content. Comparison with commercial nitrogen fertilisers. Food Chem. 2014, 163, 136–141. [Google Scholar] [CrossRef]
- Román, S.M.-S.; Rubio-Bretón, P.; Pérez-Álvarez, E.P.; Garde-Cerdán, T. Advancement in analytical techniques for the extraction of grape and wine volatile compounds. Food Res. Int. 2020, 137, 109712. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Rubio-Bretón, P.; Román, S.M.-S.; Baroja, E.; de Urturi, I.S.; Pérez-Álvarez, E.P. Study of Wine Volatile Composition of Tempranillo versus Tempranillo Blanco, a New White Grape Variety. Beverages 2021, 7, 72. [Google Scholar] [CrossRef]
- Román, S.M.-S.; Garde-Cerdán, T.; Baroja, E.; Rubio-Bretón, P.; Pérez-Álvarez, E.P. Foliar application of phenylalanine plus methyl jasmonate as a tool to improve Grenache grape aromatic composition. Sci. Hortic. 2020, 272, 109515. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; de Urturi, I.S.; Rubio-Bretón, P.; Román, S.M.-S.; Baroja, E.; Ramírez-Rodríguez, G.; Delgado-López, J.; Pérez-Álvarez, E. Foliar application of methyl jasmonate and methyl jasmonate supported on nanoparticles: Incidence on grape phenolic composition over two seasons. Food Chem. 2022, 402, 134244. [Google Scholar] [CrossRef]
- OIV. Compendium of International Methods of Wine and Must Analysis; OIV: Paris, France, 2003. [Google Scholar]
- Garde-Cerdán, T.; Rubio-Bretón, P.; Román, S.M.-S.; de Urturi, I.S.; Pérez-Álvarez, E.P. Pre-fermentative maceration with SO2 enhanced the must aromatic composition. Food Chem. 2020, 345, 128870. [Google Scholar] [CrossRef]
- Castillo-Muñoz, N.; Gómez-Alonso, S.; García-Romero, E.; Hermosín-Gutiérrez, I. Flavonol Profiles of Vitis vinifera Red Grapes and Their Single-Cultivar Wines. J. Agric. Food Chem. 2007, 55, 992–1002. [Google Scholar] [CrossRef]
- Yue, X.; Shi, P.; Tang, Y.; Zhang, H.; Ma, X.; Ju, Y.; Zhang, Z. Effects of methyl jasmonate on the monoterpenes of Muscat Hamburg grapes and wine. J. Sci. Food Agric. 2020, 101, 3665–3675. [Google Scholar] [CrossRef]
- Mele, M.A.; Kang, H.-M.; Lee, Y.-T.; Islam, M.Z. Grape terpenoids: Flavor importance, genetic regulation, and future potential. Crit. Rev. Food Sci. Nutr. 2020, 61, 1429–1447. [Google Scholar] [CrossRef] [PubMed]
- Dubery, I.A.; Teodorczuk, L.G.; Louw, A.E. Early Responses in Methyl Jasmonate-Preconditioned Cells toward Pathogen-Derived Elicitors. Mol. Cell Biol. Res. Commun. 2000, 3, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Tomasino, E.; Bolman, S. The Potential Effect of β-Ionone and β-Damascenone on Sensory Perception of Pinot Noir Wine Aroma. Molecules 2021, 26, 1288. [Google Scholar] [CrossRef] [PubMed]
- Cordente, A.G.; Solomon, M.; Schulkin, A.; Francis, I.L.; Barker, A.; Borneman, A.R.; Curtin, C.D. Novel wine yeast with ARO4 and TYR1 mutations that overproduce ‘floral’ aroma compounds 2-phenylethanol and 2-phenylethyl acetate. Appl. Microbiol. Biotechnol. 2018, 102, 5977–5988. [Google Scholar] [CrossRef]
- Marín-San Román, S.; Pérez-Álvarez, E.P.; Baroja, E.; Sáenz de Urturi, I.; Rubio-Bretón, P.; Garde-Cerdán, T. Changes on grape volatile composition after elicitors and nitrogen compounds foliar applications to ‘Garnacha’, ‘Tempranillo’ and ‘Graciano’ vines. Vitis 2022, 61, 133–146. [Google Scholar] [CrossRef]
- Zalacain, A.; Marín, J.; Alonso, G.L.; Salinas, M. Analysis of wine primary aroma compounds by stir bar sorptive extraction. Talanta 2007, 71, 1610–1615. [Google Scholar] [CrossRef]
- Castillo-Muñoz, N.; Gómez-Alonso, S.; García-Romero, E.; Hermosín-Gutiérrez, I. Flavonol profiles of Vitis vinifera white grape cultivars. J. Food Compos. Anal. 2010, 23, 699–705. [Google Scholar] [CrossRef]
- Montealegre, R.R.; Peces, R.R.; Vozmediano, J.C.; Gascueña, J.M.; Romero, E.G. Phenolic compounds in skins and seeds of ten grape Vitis vinifera varieties grown in a warm climate. J. Food Compos. Anal. 2006, 19, 687–693. [Google Scholar] [CrossRef]
- Flamini, R.; Mattivi, F.; De Rosso, M.; Arapitsas, P.; Bavaresco, L. Advanced Knowledge of Three Important Classes of Grape Phenolics: Anthocyanins, Stilbenes and Flavonols. Int. J. Mol. Sci. 2013, 14, 19651–19669. [Google Scholar] [CrossRef]
- Tomada, S.; Agati, G.; Serni, E.; Michelini, S.; Lazazzara, V.; Pedri, U.; Sanoll, C.; Matteazzi, A.; Robatscher, P.; Haas, F. Non-destructive fluorescence sensing for assessing microclimate, site and defoliation effects on flavonol dynamics and sugar prediction in Pinot blanc grapes. PLoS ONE 2022, 17, e0273166. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-García, Y.; Romero-Cascales, I.; Bautista-Ortín, A.B.; Gil-Muñoz, R.; Martínez-Cutillas, A.; Gómez-Plaza, E. Increasing Bioactive Phenolic Compounds in Grapes: Response of Six Monastrell Grape Clones to Benzothiadiazole and Methyl Jasmonate Treatments. Am. J. Enol. Vitic. 2013, 64, 459–465. [Google Scholar] [CrossRef]
- Pérez-Navarro, J.; Cazals, G.; Enjalbal, C.; Izquierdo-Cañas, P.M.; Gómez-Alonso, S.; Saucier, C. Flavanol Glycoside Content of Grape Seeds and Skins of Vitis vinifera Varieties Grown in Castilla-La Mancha, Spain. Molecules 2019, 24, 4001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yilmaz, Y.; Toledo, R.T. Major Flavonoids in Grape Seeds and Skins: Antioxidant Capacity of Catechin, Epicatechin, and Gallic Acid. J. Agric. Food Chem. 2003, 52, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Moro, L.; da Mota, R.V.; Purgatto, E.; Mattivi, F.; Arapitsas, P. Investigation of Brazilian grape juice metabolomic profile changes caused by methyl jasmonate pre-harvest treatment. Int. J. Food Sci. Technol. 2023, in press. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Ancín-Azpilicueta, C. Review of quality factors on wine ageing in oak barrels. Trends Food Sci. Technol. 2006, 17, 438–447. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Portu, J.; López, R.; Santamaría, P. Effect of Foliar Applications of Proline, Phenylalanine, Urea, and Commercial Nitrogen Fertilizers on Stilbene Concentrations in Tempranillo Musts and Wines. Am. J. Enol. Vitic. 2015, 66, 542–547. [Google Scholar] [CrossRef]
- Benbouguerra, N.; Hornedo-Ortega, R.; Garcia, F.; El Khawand, T.; Saucier, C.; Richard, T. Stilbenes in grape berries and wine and their potential role as anti-obesity agents: A review. Trends Food Sci. Technol. 2021, 112, 362–381. [Google Scholar] [CrossRef]
- Gutiérrez-Gamboa, G.; Garde-Cerdán, T.; Rubio-Bretón, P.; Pérez-Álvarez, E. Study of must and wine amino acids composition after seaweed applications to Tempranillo blanco grapevines. Food Chem. 2019, 308, 125605. [Google Scholar] [CrossRef]
- Bell, S.-J.; Henschke, P.A. Implications of nitrogen nutrition for grapes, fermentation and wine. Aust. J. Grape Wine Res. 2005, 11, 242–295. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Gutiérrez-Gamboa, G.; Portu, J.; Fernández-Fernández, J.I.; Gil-Muñoz, R. Impact of phenylalanine and urea applications to Tempranillo and Monastrell vineyards on grape amino acid content during two consecutive vintages. Food Res. Int. 2017, 102, 451–457. [Google Scholar] [CrossRef] [PubMed]
- González-Lázaro, M.; de Urturi, I.S.; Murillo-Peña, R.; Román, S.M.-S.; Pérez-Álvarez, E.P.; Rubio-Bretón, P.; Garde-Cerdán, T. Effect of Methyl Jasmonate and Methyl Jasmonate Plus Urea Foliar Applications on Wine Phenolic, Aromatic and Nitrogen Composition. Beverages 2022, 8, 52. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Pérez-Álvarez, E.P.; Fernández-Fernández, J.I.; Gil-Muñoz, R. Influence of elicitors application to Monastrell and Tempranillo vineyards on grape nitrogen composition over two vintages. OENO One 2021, 56, 295–303. [Google Scholar] [CrossRef]
| Control | MeJ-Ver | MeJ-Post | |
|---|---|---|---|
| Weight of 100 berries (g) | 181.13 ± 15.04 ab | 187.73 ± 9.85 b | 161.50 ± 19.27 a |
| ºBrix | 23.53 ± 1.31 a | 22.33 ± 0.50 a | 21.47 ± 2.06 a |
| Probable alcohol (% v/v) | 13.82 ± 0.90 a | 12.99 ± 0.34 a | 12.41 ± 1.40 a |
| Glucose + Fructose (g/L) | 241.61 ± 14.35 a | 225.76 ± 0.93 a | 214.58 ± 25.75 a |
| Glucose (g/L) | 118.34 ± 7.14 b | 111.18 ± 2.22 ab | 103.31 ± 13.63 a |
| Fructose (g/L) | 123.27 ± 7.38 a | 122.92 ± 12.62 a | 103.26 ± 25.26 a |
| pH | 3.77 ± 0.09 a | 3.77 ± 0.07 a | 3.80 ± 0.06 a |
| Total acidity (g/L) * | 4.89 ± 0.40 a | 5.25 ± 0.34 a | 5.01 ± 0.76 a |
| Tartaric acid (g/L) | 6.83 ± 0.19 a | 7.35 ± 0.93 a | 6.44 ± 0.26 a |
| Malic acid (g/L) | 2.44 ± 0.22 a | 2.76 ± 0.41 a | 2.77 ± 0.54 a |
| Total phenols (mg/L) | 554.67 ± 50.15 a | 546.97 ± 37.60 a | 589.13 ± 26.34 a |
| Ammonium nitrogen (mg N/L) | 93.34 ± 5.48 a | 127.92 ± 31.29 b | 106.08 ± 17.25 ab |
| Amino nitrogen (mg N/L) | 164.85 ± 20.64 a | 228.21 ± 39.60 b | 220.00 ± 25.93 b |
| YAN (mg N/L) | 258.19 ± 22.96 a | 356.13 ± 70.89 b | 326.08 ± 39.27 ab |
| Control | MeJ-Ver | MeJ-Post | |
|---|---|---|---|
| Benzenoid compounds | |||
| 2-Phenylethanol | 1.44 ± 0.23 a | 8.48 ± 1.62 b | 21.88 ± 2.12 c |
| 2-Phenylethanal | 10.48 ± 1.91 a | 31.00 ± 2.29 b | 39.28 ± 4.94 c |
| Benzyl alcohol | 0.20 ± 0.03 a | 0.55 ± 0.06 b | 0.83 ± 0.08 c |
| Alcohols | |||
| 1-Heptanol | 0.029 ± 0.004 a | 0.084 ± 0.006 b | 0.133 ± 0.014 c |
| 1-Octanol | 0.428 ± 0.090 a | 0.647 ± 0.033 a | 0.931 ± 0.196 b |
| 1-Nonanol | 0.104 ± 0.019 a | 0.263 ± 0.026 b | 0.355 ± 0.072 c |
| 1-Octen-3-ol | 0.579 ± 0.100 a | 1.130 ± 0.039 b | 1.863 ± 0.218 c |
| 2-Ethyl-1-hexanol | 0.619 ± 0.123 ab | 0.479 ± 0.146 a | 0.853 ± 0.145 b |
| Carbonyl compounds | |||
| Heptanal | 0.057 ± 0.010 a | 0.057 ± 0.005 a | 0.072 ± 0.012 a |
| (E)-2-Heptenal | 0.135 ± 0.017 a | 0.172 ± 0.016 b | 0.272 ± 0.016 c |
| Octanal | 0.032 ± 0.002 a | 0.050 ± 0.006 b | 0.054 ± 0.010 b |
| (E)-2-Octenal | 0.190 ± 0.023 a | 0.135 ± 0.020 a | 0.802 ± 0.168 b |
| Nonanal | 0.919 ± 0.168 a | 0.792 ± 0.109 a | 1.050 ± 0.171 a |
| (E)-2-Nonenal | 0.112 ± 0.001 a | 0.144 ± 0.020 b | 0.123 ± 0.010 ab |
| Decanal | 0.053 ± 0.008 a | 0.089 ± 0.007 b | 0.093 ± 0.017 b |
| (E,E)-2,4-Hexadienal | 1.715 ± 0.193 a | 1.818 ± 0.247 a | 1.590 ± 0.197 a |
| (E,E)-2,4-Nonadienal | 0.078 ± 0.014 a | 0.090 ± 0.019 a | 0.068 ± 0.014 a |
| γ-Decalactone | 0.041 ± 0.013 a | 0.067 ± 0.004 b | 0.088 ± 0.018 b |
| C6 compounds | |||
| Hexanal | 87.73 ± 15.33 a | 79.34 ± 2.46 a | 77.77 ± 9.04 a |
| n-Hexanol | 35.37 ± 6.24 a | 55.71 ± 1.54 b | 44.62 ± 5.26 a |
| (E)-2-Hexenal | 30.24 ± 4.46 a | 43.48 ± 1.70 b | 43.29 ± 3.94 b |
| (E)-2-Hexen-1-ol | 5.73 ± 1.03 a | 16.07 ± 2.21 c | 12.08 ± 1.72 b |
| (Z)-3-Hexen-1-ol | 1.28 ± 0.20 c | 0.85 ± 0.08 b | 0.50 ± 0.10 a |
| Control | MeJ-Ver | MeJ-Post | |
|---|---|---|---|
| Flavonols | |||
| Quercetin-3-glcU | 71.79 ± 2.23 a | 76.98 ± 15.88 a | 94.42 ± 0.11 a |
| Quercetin-3-glc | 98.58 ± 9.03 a | 102.13 ± 18.80 a | 122.94 ± 10.39 a |
| Kaempferol-3-gal | 4.84 ± 0.24 a | 6.05 ± 0.20 b | 5.95 ± 0.21 b |
| Kaempferol-3-glc | 17.07 ± 3.89 a | 18.37 ± 0.11 a | 18.04 ± 0.22 a |
| Isorhamnetin-3-glc | 9.67 ± 1.28 a | 14.85 ± 1.89 b | 16.98 ± 2.47 b |
| Flavanols | |||
| Catechin | 23.45 ± 4.29 a | 37.01 ± 3.23 b | 36.82 ± 1.59 b |
| Epicatechin | 22.40 ± 2.89 a | 33.62 ± 3.24 b | 39.85 ± 6.41 b |
| Hydroxybenzoic acids | |||
| Gallic acid | 11.04 ± 0.95 a | 15.66 ± 1.32 b | 17.12 ± 1.36 b |
| Hydroxycinnamic acids | |||
| trans-Caftaric acid | 17.17 ± 2.55 a | 20.96 ± 2.16 ab | 22.39 ± 1.44 b |
| trans+cis-Coutaric acids | 11.28 ± 1.92 a | 14.11 ± 0.56 ab | 15.52 ± 1.07 b |
| Caffeic acid | 0.11 ± 0.02 a | 0.10 ± 0.02 a | 0.09 ± 0.01 a |
| p-Coumaric acid | 0.21 ± 0.04 a | 0.34 ± 0.02 b | 0.32 ± 0.03 b |
| trans-Fertaric acid | 0.93 ± 0.01 a | 1.03 ± 0.03 b | 1.14 ± 0.03 c |
| Stilbenes | |||
| trans-Piceid | 0.37 ± 0.07 a | 0.61 ± 0.13 a | 0.55 ± 0.09 a |
| trans-Resveratrol | 0.56 ± 0.14 a | 0.57 ± 0.01 a | 0.57 ± 0.03 a |
| Control | MeJ-Ver | MeJ-Post | |
|---|---|---|---|
| Aspartic acid (Asp) | 8.69 ± 1.83 a | 15.01 ± 2.96 b | 18.36 ± 1.57 b |
| Glutamic acid (Glu) | 19.77 ± 1.34 a | 29.03 ± 5.03 b | 28.75 ± 3.00 b |
| Asparagine (Asn) | 3.29 ± 0.02 a | 7.62 ± 0.40 c | 4.84 ± 0.72 b |
| Serine (Ser) | 45.04 ± 4.12 a | 51.47 ± 5.77 a | 49.78 ± 2.69 a |
| Histidine (His) | 7.63 ± 0.72 a | 11.74 ± 1.45 b | 17.47 ± 1.15 c |
| Glycine (Gly) | 13.18 ± 2.91 a | 12.88 ± 1.24 a | 11.39 ± 1.08 a |
| Threonine (Thr) | 49.11 ± 6.37 a | 57.45 ± 4.48 a | 58.26 ± 0.66 a |
| Citrulline (Cit) | 10.46 ± 0.43 a | 14.95 ± 2.86 a | 13.99 ± 1.07 a |
| Arginine (Arg) | 674.83 ± 67.56 a | 751.42 ± 51.14 a | 793.42 ± 63.75 a |
| Alanine (Ala) | 53.13 ± 6.91 a | 70.39 ± 7.61 b | 61.81 ± 6.17 ab |
| γ-Aminobutyric acid (Gaba) | 123.14 ± 11.37 a | 121.39 ± 10.13 a | 117.60 ± 13.98 a |
| Tyrosine (Tyr) | 1.25 ± 0.26 a | 1.57 ± 0.07 a | 1.59 ± 0.31 a |
| Cysteine (Cys) | 4.34 ± 0.73 a | 5.36 ± 0.63 a | 4.55 ± 0.41 a |
| Valine (Val) | 26.46 ± 4.03 a | 35.07 ± 3.23 b | 39.62 ± 2.44 b |
| Methionine (Met) | 6.08 ± 1.29 a | 9.06 ± 0.06 a | 8.81 ± 1.42 a |
| Tryptophan (Trp) | 29.31 ± 4.01 a | 35.59 ± 1.56 b | 37.73 ± 0.66 b |
| Phenylalanine (Phe) | 20.94 ± 2.01 a | 39.00 ± 1.61 b | 39.64 ± 3.49 b |
| Isoleucine (Ile) | 12.14 ± 2.33 a | 17.63 ± 1.39 b | 19.35 ± 1.33 b |
| Ornithine (Orn) | 5.65 ± 0.92 a | 7.14 ± 1.77 a | 5.81 ± 1.12 a |
| Leucine (Leu) | 15.61 ± 2.06 a | 28.22 ± 2.23 b | 27.85 ± 2.68 b |
| Lysine (Lys) | 1.63 ± 0.27 a | 1.69 ± 0.12 a | 1.94 ± 0.32 a |
| Proline (Pro) | 257.22 ± 11.49 a | 269.96 ± 26.55 a | 276.81 ± 8.45 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sáenz de Urturi, I.; Ribeiro-Gomes, F.M.; Marín-San Román, S.; Murillo-Peña, R.; Torres-Díaz, L.; González-Lázaro, M.; Pérez-Álvarez, E.P.; Garde-Cerdán, T. Vine Foliar Treatments at Veraison and Post-Veraison with Methyl Jasmonate Enhanced Aromatic, Phenolic and Nitrogen Composition of Tempranillo Blanco Grapes. Foods 2023, 12, 1142. https://doi.org/10.3390/foods12061142
Sáenz de Urturi I, Ribeiro-Gomes FM, Marín-San Román S, Murillo-Peña R, Torres-Díaz L, González-Lázaro M, Pérez-Álvarez EP, Garde-Cerdán T. Vine Foliar Treatments at Veraison and Post-Veraison with Methyl Jasmonate Enhanced Aromatic, Phenolic and Nitrogen Composition of Tempranillo Blanco Grapes. Foods. 2023; 12(6):1142. https://doi.org/10.3390/foods12061142
Chicago/Turabian StyleSáenz de Urturi, Itziar, Freud M. Ribeiro-Gomes, Sandra Marín-San Román, Rebeca Murillo-Peña, Lesly Torres-Díaz, Miriam González-Lázaro, Eva P. Pérez-Álvarez, and Teresa Garde-Cerdán. 2023. "Vine Foliar Treatments at Veraison and Post-Veraison with Methyl Jasmonate Enhanced Aromatic, Phenolic and Nitrogen Composition of Tempranillo Blanco Grapes" Foods 12, no. 6: 1142. https://doi.org/10.3390/foods12061142







