Differentiation of Geographic Origin of South African Wines from Austrian Wines by IRMS and SNIF-NMR
Abstract
:1. Introduction
2. Materials and Methods
2.1. IRMS
2.2. SNIF-NMR-Analysis
3. Results
4. Discussion
4.1. Irrigation in Austria (Central Europe) and Cape Province (South Africa)
4.2. Small-Scale Vinification Wine Samples (Stellenbosch Area)
4.3. Outlook
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barisan, L.; Galletto, L. How do sparkling wine producers adopt a sub-appellation? Evidence from an exploratory study on heroic Prosecco Superiore Rive. Wine Econ. Policy 2021, 10, 45–59. [Google Scholar] [CrossRef]
- Camanzi, L.; Grazia, C.; Giraud-Héraud, E.; Malorgio, G. Quality differentiation in the Italian wine industry: Terroir-based vs. brand-based strategies. Int. J. Glob. Small Bus. 2017, 9, 86–104. [Google Scholar] [CrossRef]
- Giraud-Heraud, E.E.; Soler, L.G.; Steinmetz, S.; Tanguy, H. La régulation interprofessionnelle dans le secteur vitivinicole est-elle fondée économiquement? Bull. L’oiv 1998, 813–814, 1060–1084. [Google Scholar]
- Roßmann, A.; Schmidt, H.L.; Reniero, F.; Versini, G.; Moussa, I.; Merle, M.H. Stable carbon isotope content in ethanol of EC data bank wines from Italy, France and Germany. Z. Lebensm. Unters. Forsch. 1996, 203, 293–230. [Google Scholar] [CrossRef]
- Versini, G.; Monetti, A.; Reniero, F. Monitoring Authenticity and Regional Origin of Wines by Natural Stable Isotope Ratios Analysis; ACS Publications: Washington, DC, USA, 1997. [Google Scholar]
- Christoph, N.; Rossmann, A.; Voerkelius, S. Possibilities and limitations of wine authentication using stable isotope and meteorological data, data banks and statistical tests—Part 1: Wines from Franconia and Lake Constance 1992 to 2001. Mitt. Klosterneubg. 2003, 53, 23–40. [Google Scholar]
- Christoph, N.; Baratossy, G.; Kubanovic, V.; Kozina, B.; Roßmann, A.; Schlicht, C. Possibilities and limitations of wine authentication using stable isotope ratio analysis and traceability—Part 2: Wines from Hungary, Croatia and other European countries. Mitt. Klosterneubg. 2004, 54, 155–169. [Google Scholar]
- Christoph, N.; Hermann, A.; Wachter, H. 25 Years authentication of wine with stable isotope analysis in the European Union–Review and outlook. BIO Web Conf. 2015, 5, 02020. [Google Scholar] [CrossRef] [Green Version]
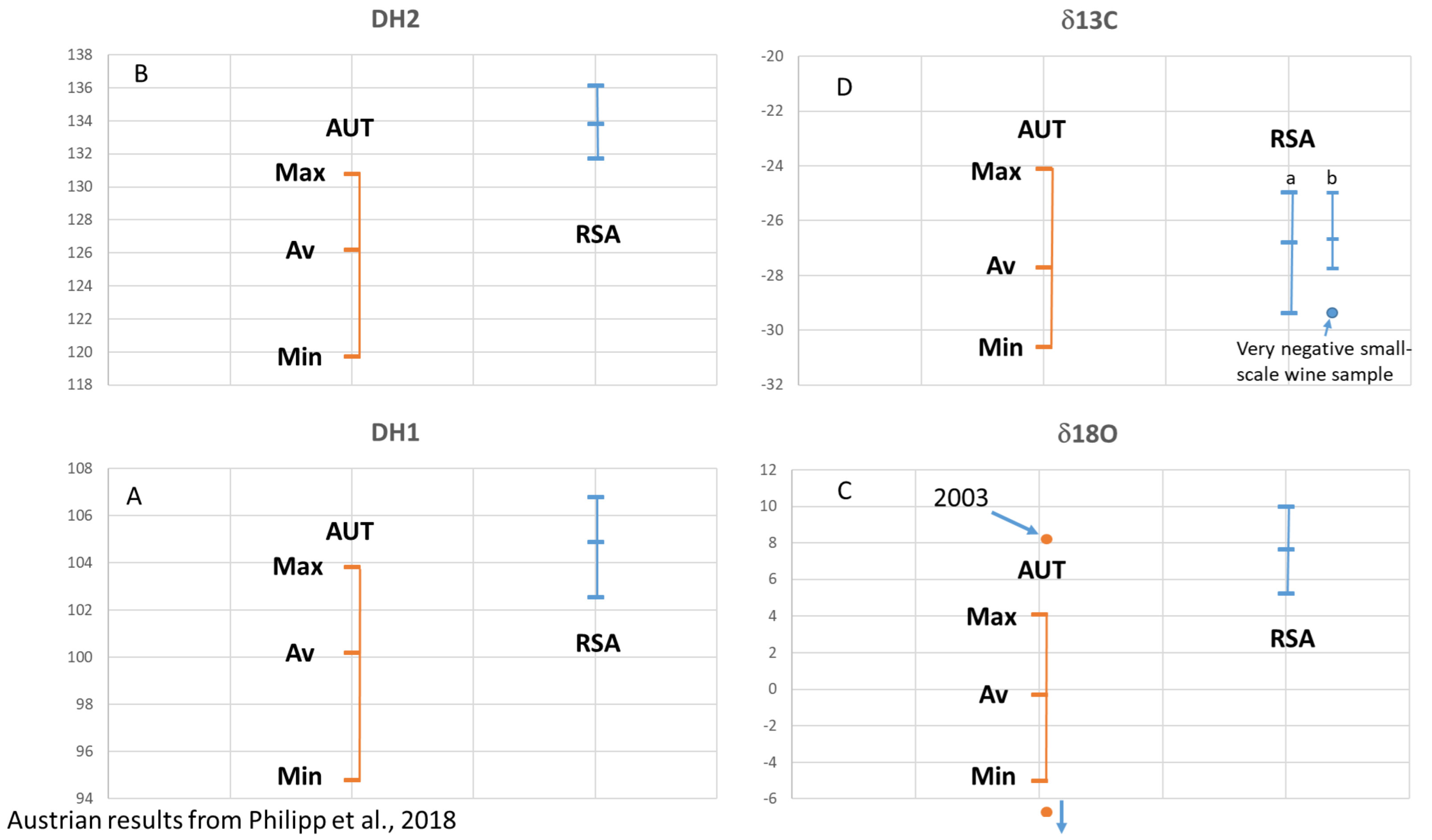
- Philipp, C.; Horacek, M.; Nauer, S.; Reitner, H.; Rosner, A.; Jaborek, C.; Guillou, C.; Patzl-Fischerleitner, E.; Eder, R. Stabilisotopendaten authentischer österreichischer Weine: Evaluierung des Potentials für den Herkunftsund Jahrgangsnachweis. Mitt. Klosterneubg. 2018, 68, 120–140. [Google Scholar]
- European Union. 2008: Verordnung (EG) Nr. 555/2008 DER KOMMISSION vom 27. Juni 2008 mit Durchführungsbestimmungen zur Verordnung (EG) Nr. 479/2008 des Rates über die gemeinsame Marktorganisation für Wein hinsichtlich der Stützungsprogramme, des Handels mit Drittländern, des Produktionspotenzials und der Kontrollen im Weinsektor. Off. J. Euro. Commun. 2008, L170, 1. [Google Scholar]
- Aghemo, C.; Albertino, A.; Gobetto, R.; Spanna, F. Correlation between isotopic and meteorological parameters in Italian wines: A localscale approach. J. Sci. Food Agric. 2011, 91, 2088–2094. [Google Scholar] [CrossRef]
- Camin, F.; Dordevic, N.; Wehrens, R.; Neteler, M.; Delucchi, L.; Postma, G.; Buydens, L. Climatic and geographical dependence of the H, C and O stable isotope ratios of Italian wine. Anal. Chim. Acta 2015, 853, 384–390. [Google Scholar] [CrossRef]
- Dutra, S.V.; Adami, L.; Marcon, A.R.; Carnieli, G.J.; Roani, C.A.; Spinelli, F.R.; Leonardelli, S.; Vanderlinde, R. Characterization of wines according the geographical origin by analysis of isotopes and minerals and the influence of harvest on the isotope values. Food Chem. 2013, 141, 2148–2153. [Google Scholar] [CrossRef]
- Fan, S.; Zhong, Q.; Gao, H.; Wang, D.; Li, G.; Huang, Z. Elemental profile and oxygen isotope ratio (δ 18 O) for verifying the geographical origin of Chinese wines. J. Food Drug Anal. 2018, 26, 1033–1044. [Google Scholar] [CrossRef] [Green Version]
- Heinrich, M.; Horacek, M.; Reitner, H. Do regional patterns and trends over time show in isotope data of Austrian wines? In Proceedings of the XI International Terroir Congress, Willamette Valley, OR, USA, 10–14 July 2016.
- Monakhova, Y.B.; Godelmann, R.; Hermann, A.; Kuballa, T.; Cannet, C.; Schäfer, H.; Spraul, M.; Rutledge, D.N. Synergistic effect of the simultaneous chemometric analysis of 1H NMR spectroscopic and stable isotope (SNIF-NMR, 18O, 13C) data: Application to wine analysis. Anal. Chim. Acta 2014, 833, 29–39. [Google Scholar] [CrossRef]
- Orellana, S.; Johansen, A.M.; Gazis, C. Geographic classification of U.S. Washington State wines using elemental and water isotope composition. Food Chem. X. 2019, 1, 100007. [Google Scholar] [CrossRef]
- Horacek, M.; Kolar, K.; Hola, M.; Tobolkova, B.; Vaculovic, T.; Philipp, C.; Marosanovic, B.; Mikes, O.; Polovka, M.; Lojovic, M.; et al. Investigation of geographic origin of wine from border regions: Potential limitations and possibilities of different analytical methods and combinations of methods to identify the correct side of the border. BIO Web Conf. 2019, 12, 02032. [Google Scholar] [CrossRef]
- Horacek, M.; Hola, M.; Tobolkova, B.; Kolar, K.; Vaculovic, T.; Mikes, O.; Marosanovic, B.; Philipp, C.; Lojovic, M.; Polovka, M.; et al. Investigation of geographic origin of wine from border regions: Results from investigation of two vintages. BIO Web Conf. 2019, 15, 02039. [Google Scholar] [CrossRef]
- Horacek, M.; Nives, O.; Magdas, A.; Wunderlin, D.; Sucur, S.; Maras, V.; Misurovic, A.; Eder, R.; Wyhlidal, S.; Cus, F.; et al. Isotope analysis (13C, 18O) of wine from Central and Eastern Europe and Argentina, 2008 and 2009 vintages: Differentiation of origin, environmental indications and variations within the countries. Front. Sustain. Food Syst. 2021, 5, 638941. [Google Scholar] [CrossRef]
- Horn, P.; Schaaf, P.; Holbach, B.; Hölzl, S.; Eschnauer, H. 87Sr/86Sr from rock and soil into vine and wine. Z. Lebensm. Unters. Forsch. 1993, 196, 407–409. [Google Scholar] [CrossRef]
- Victor, V.; Ross, S.; Karine, P.; André, P.; Jean-François, H.; David, W. Strontium Isotope Characterization of Wines from the Quebec (Canada) Terroir. Procedia Earth Planet. Sci. 2015, 13, 252–255. [Google Scholar] [CrossRef] [Green Version]
- Geană, E.-I.; Sandru, C.; Stanciu, V.; Ionete, R.E. Elemental Profile and 87Sr/86Sr Isotope Ratio as Fingerprints for Geographical Traceability of Wines: An Approach on Romanian Wines. Food Anal. Methods 2017, 10, 63–73. [Google Scholar] [CrossRef]
- Cellier, R.; Bérail, S.; Barre, J.; Epova, E.; Ronzani, A.-L.; Van Leeuwen, C.; Milcent, S.; Ors, P.; Donard, O.F.X. Specificity and Origin of the Stability of the Sr Isotopic Ratio in Champagne Wines. Molecules 2021, 26, 5104. [Google Scholar] [CrossRef] [PubMed]
- Goitom Asfaha, D.; Quetel, C.R.; Thomas, F.; Horacek, M.; Wimmer, B.; Heiss, G.; Dekant, C.; Deters-Itzelsberger, P.; Hölzl, S.; Rummel, S.; et al. Combining isotopic signatures of N(87Sr)/(86Sr) and light stable elements (C, N, O, S) with multi-elemental profiling for the authentication of provenance of European cereal samples. J. Cereal Sci. 2011, 53, 170–177. [Google Scholar] [CrossRef]
- Aguzzoni, A.; Bassi, M.; Pignotti, E.; Robatscher, P.; Scandellari, F.; Tirler, W.; Tagliavinia, M. Sr isotope composition of Golden Delicious apples in Northern Italy reflects the soil 87Sr/86Sr ratio of the cultivation area. J. Sci. Food Agric. 2020, 100, 3666–3674. [Google Scholar] [CrossRef]
- Horacek, M. Comment on: “The Provenance of Slovenian Milk Using 87Sr/86Sr Isotope Ratios” by Gregorcic et al., 2021: The need to consider geochemistry when interpreting Sr-isotopes. Foods 2022, 11, 564. [Google Scholar] [CrossRef]
- Horacek, M.; Klcova, L.; Hudcovicova, M.; Ondreickova, K.; Gubis, J.; Hölzl, S. Differentiation of apricots of different geographic origin by applying 87Sr/86Sr analysis: Potential and limitations. Foods 2022, 11, 2239. [Google Scholar] [CrossRef]
- Kokkinofta, R.; Fotakis, C.; Zervou, M.; Zoumpoulakis, P.; Savvidou, C.; Poulli, K.; Louka, C.; Economidou, N.; Tzioni, E.; Damianou, K.; et al. Isotopic and Elemental Authenticity Markers: A Case Study on Cypriot Wines. Food Anal. Methods 2017, 10, 3902–3913. [Google Scholar] [CrossRef]
- Leder, R.; Petric, I.V.; Jusup, J.; Banović, M. Geographical Discrimination of Croatian Wines by Stable Isotope Ratios and Multielemental Composition Analysis. Front. Nutr. 2021, 8, 625613. [Google Scholar] [CrossRef] [PubMed]
- Griboff, J.; Horacek, M.; Wunderlin, D.A.; Monferrán, M.V. Differentiation between Argentine and Austrian wines based on isotopic and multi-elemental composition. Front. Sustain. Food Syst. 2021, 5, 657412. [Google Scholar] [CrossRef]
- Van Der Linde, G.; Fischer, J.L.; Coetzee, P.P. Multi-element Analysis of South African Wines and their Provenance Soils by ICP-MS and their Classification according to Geographical Origin using Multivariate Statistics. S. Afr. J. Enol. Vitic. 2010, 31, 143–153. [Google Scholar] [CrossRef]
- Considine, J.; Frankish, E. A Complete Guide to Quality in Small-Scale Wine Making, 1st ed.; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Myburgh, P. Handbook for Irrigation of Wine Grapes in South Africa; Agricultural Research Council: Pretoria, South Africa, 2018; p. 309. ISBN 978-0-620-80402-8. [Google Scholar]
- De Wet, R.F.; West, A.G.; Harris, C. Seasonal variation in tap water δ2H and δ18O isotopes reveals two tap water worlds. Sci. Rep. 2020, 10, 13544. [Google Scholar] [CrossRef]
- Perini, M.; Failoni, A.; Simoni, M.; Tonon, A.; Camin, F. Influence of Fermentation Water on Stable Isotopic D/H Ratios of Alcohol Obtained from Concentrated Grape Must. Molecules 2020, 25, 3139. [Google Scholar] [CrossRef]
- Craig, H. Isotopic variations in meteoric waters. Science 1961, 133, 1702–1703. [Google Scholar] [CrossRef]
- Dansgaard, W. Stable isotopes in precipitation. Tellus A Dyn. Meteorol. Oceanogr. 2012, 6, 436–268. [Google Scholar] [CrossRef]
- Damiano, N.; Altieri, S.; Battipaglia, G.; De Micco, V. Comparing Methods for the Analysis of δ13C in Falanghina Grape Must from Different Pedoclimatic Conditions. Horticulturae 2022, 8, 226. [Google Scholar] [CrossRef]
- Camin, F.; Larcher, R.; Perini, M.; Bontempo, L.; Bertoldi, D.; Gagliano, G.; Versini, G. Characterisation of authentic Italian extra-virgin olive oils by stable isotope ratios of C, O and H and mineral composition. Food Chem. 2010, 118, 901–909. [Google Scholar] [CrossRef]
- Bagheri, B.; Philipp, C.; Horacek, M.; Bauer, F.; Setati, M. Distinction of microbial diversity in grape must of different grape varieties and regions from Austria and South Africa using Automated Ribosomal Intergenic Spacer Analysis. BIO Web Conf. 2019, 12, 02028. [Google Scholar] [CrossRef]
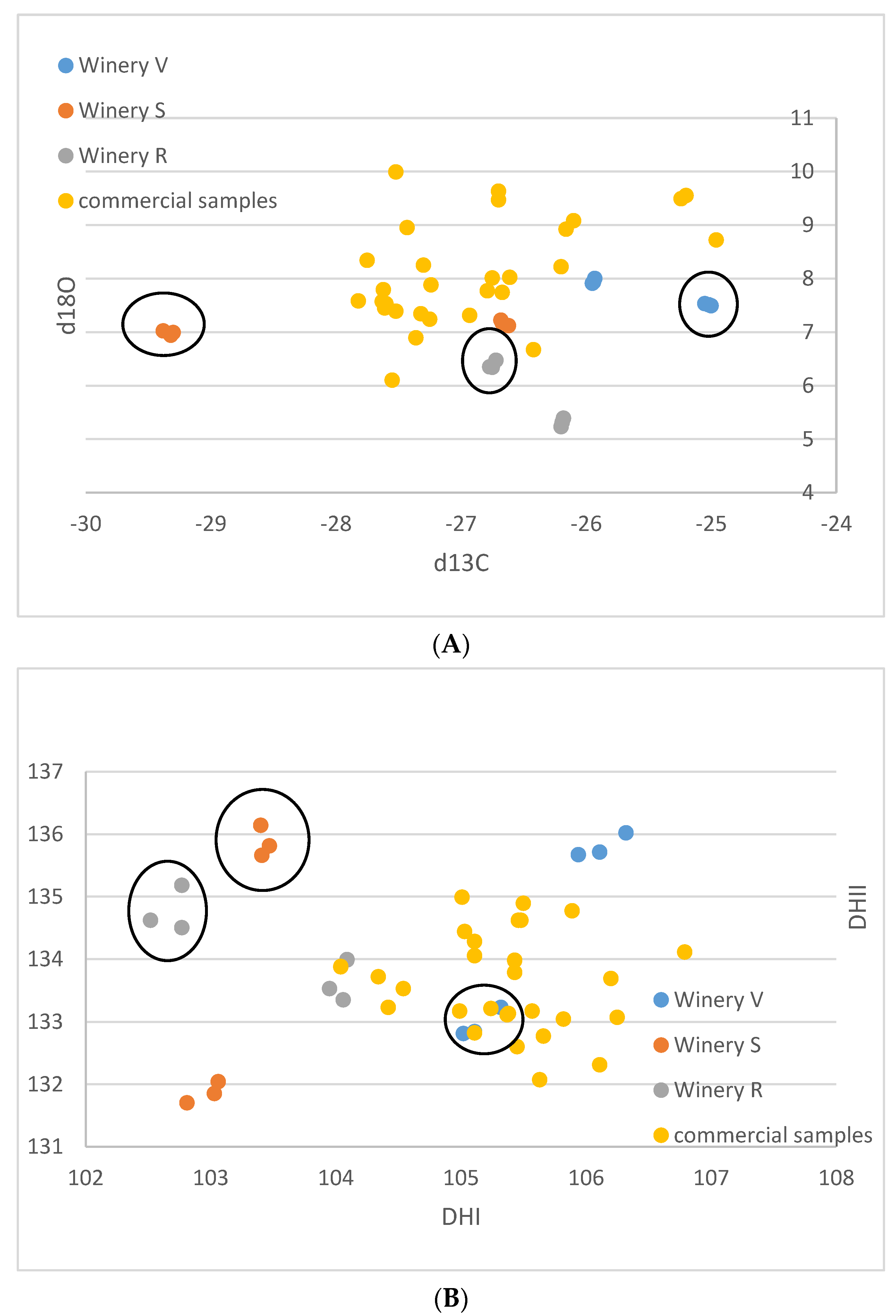
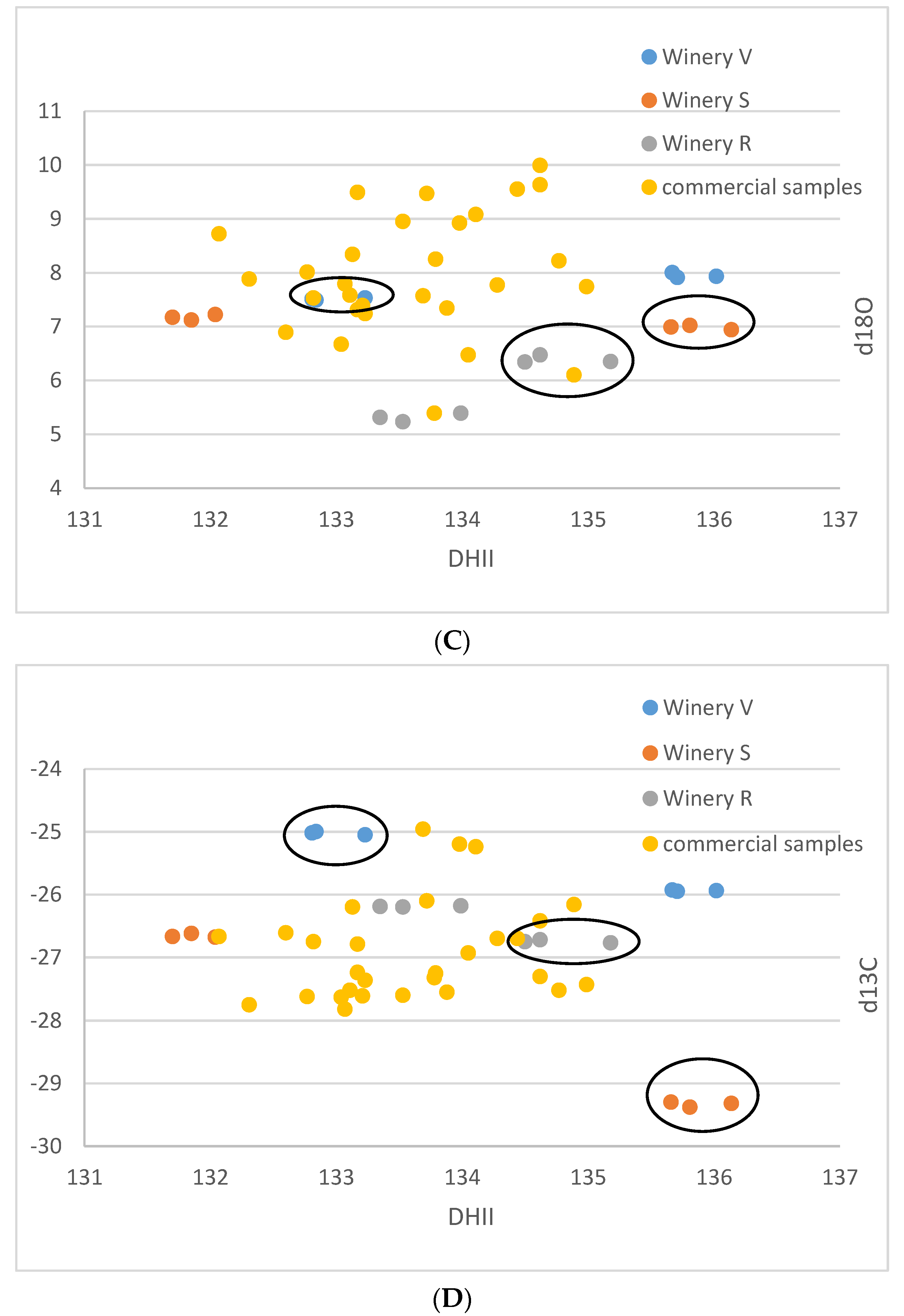
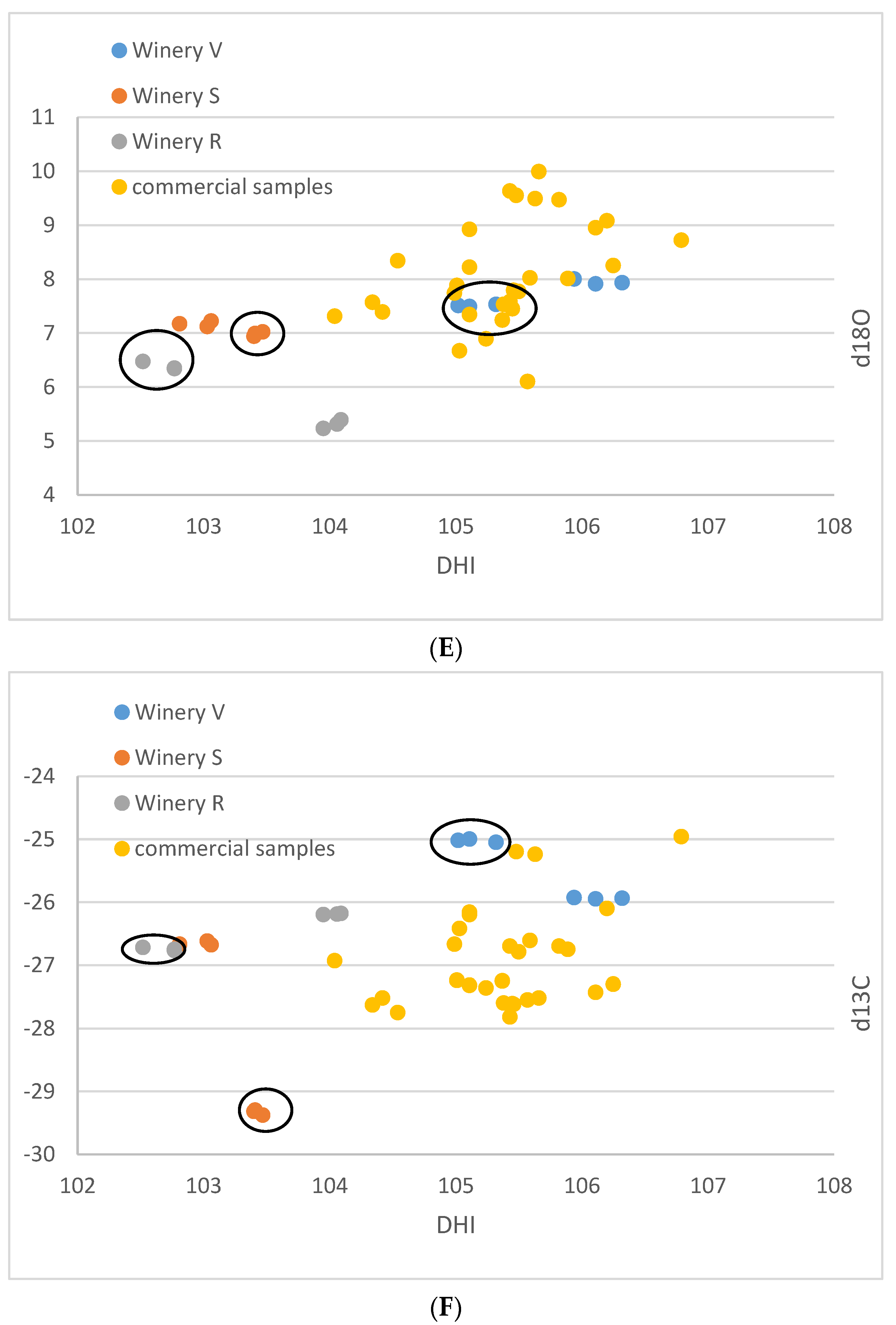


| Lab No. | Winery | Cultivar | Colour | DHI | DHII | d13C | d18O |
|---|---|---|---|---|---|---|---|
| 20050044 | V | SB | white | 105.3 | 133.2 | −25.1 | 7.5 |
| 20050045 | S | SB | white | 103.5 | 135.8 | −29.4 | 7.0 |
| 20050046 | S | C | white | 103.1 | 132.0 | −26.7 | 7.2 |
| 20050047 | V | C | white | 106.3 | 136.0 | −25.9 | 7.9 |
| 20050048 | S | SB | white | 103.4 | 136.1 | −29.3 | 6.9 |
| 20050049 | S | C | white | 103.0 | 131.9 | −26.6 | 7.1 |
| 20050050 | R | SB | white | 102.8 | 135.2 | −26.8 | 6.4 |
| 20050051 | R | SB | white | 102.8 | 134.5 | −26.8 | 6.3 |
| 20050052 | S | C | white | 102.8 | 131.7 | −26.7 | 7.2 |
| 20050053 | R | C | white | 104.1 | 133.4 | −26.2 | 5.3 |
| 20050054 | S | SB | white | 103.4 | 135.7 | −29.3 | 7.0 |
| 20050055 | R | C | white | 104.0 | 133.5 | −26.2 | 5.2 |
| 20050056 | V | SB | white | 105.0 | 132.8 | −25.0 | 7.5 |
| 20050057 | V | C | white | 105.9 | 135.7 | −25.9 | 8.0 |
| 20050058 | V | SB | white | 105.1 | 132.8 | −25.0 | 7.5 |
| 20050059 | R | C | white | 104.1 | 134.0 | −26.2 | 5.4 |
| 20050060 | R | SB | white | 102.5 | 134.6 | −26.7 | 6.5 |
| 20060003 | rose | 105.1 | 133.8 | −27.3 | 7.3 | ||
| 20060004 | rose | 104.0 | 134.1 | −26.9 | 7.3 | ||
| 20060005 | rose | 105.6 | 133.9 | −27.6 | 6.1 | ||
| 20060006 | white | 105.5 | 133.2 | −26.8 | 7.8 | ||
| 20060011 | red | 105.1 | 134.9 | −26.2 | 8.9 | ||
| 20060012 | red | 105.4 | 134.3 | −26.7 | 9.6 | ||
| 20060013 | rose | 105.5 | 134.0 | −25.2 | 9.6 | ||
| 20060014 | white | 105.0 | 134.6 | −26.4 | 6.7 | ||
| 20060015 | white | 105.8 | 134.4 | −26.7 | 9.5 | ||
| 20060016 | white | 104.3 | 133.0 | −27.6 | 7.6 | ||
| 20060018 | red | 106.2 | 133.7 | −26.1 | 9.1 | ||
| 20060019 | red | 106.8 | 133.7 | −25.0 | 8.7 | ||
| 20060020 | rose | 105.6 | 134.1 | −25.2 | 9.5 | ||
| 20060021 | rose | 105.0 | 132.1 | −26.7 | 7.7 | ||
| 20060022 | white | 105.0 | 133.2 | −27.2 | 7.9 | ||
| 20060023 | red | 106.1 | 135.0 | −27.4 | 9.0 | ||
| 20060024 | red | 104.5 | 132.3 | −27.8 | 8.3 | ||
| 20060025 | red | 105.4 | 133.5 | −27.6 | 7.5 | ||
| 20060026 | rose | 105.1 | 133.1 | −26.2 | 8.2 | ||
| 20060027 | white | 105.9 | 132.8 | −26.8 | 8.0 | ||
| 20060049 | red | 105.7 | 134.8 | −27.5 | 10.0 | ||
| 20060050 | red | 105.5 | 132.8 | −27.6 | 7.8 | ||
| 20060051 | red | 106.3 | 134.6 | −27.3 | 8.3 | ||
| 20060052 | rose | 105.4 | 133.1 | −27.8 | 7.6 | ||
| 20060061 | rose | 105.4 | 133.8 | −27.3 | 7.2 | ||
| 20060062 | white | 104.4 | 133.1 | −27.5 | 7.4 | ||
| 20060063 | white | 105.2 | 133.2 | −27.4 | 6.9 | ||
| 20060064 | white | 105.5 | 133.2 | −27.6 | 7.5 | ||
| 20060065 | white | 105.6 | 132.6 | −26.6 | 8.0 | ||
| 20060066 | V | C | white | 106.1 | 135.7 | −26.0 | 7.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horacek, M.; Nieuwoudt, H.; Bauer, F.F.; Bagheri, B.; Setati, M.E. Differentiation of Geographic Origin of South African Wines from Austrian Wines by IRMS and SNIF-NMR. Foods 2023, 12, 1175. https://doi.org/10.3390/foods12061175
Horacek M, Nieuwoudt H, Bauer FF, Bagheri B, Setati ME. Differentiation of Geographic Origin of South African Wines from Austrian Wines by IRMS and SNIF-NMR. Foods. 2023; 12(6):1175. https://doi.org/10.3390/foods12061175
Chicago/Turabian StyleHoracek, Micha, Helene Nieuwoudt, Florian F. Bauer, Bahareh Bagheri, and Mathabatha E. Setati. 2023. "Differentiation of Geographic Origin of South African Wines from Austrian Wines by IRMS and SNIF-NMR" Foods 12, no. 6: 1175. https://doi.org/10.3390/foods12061175
APA StyleHoracek, M., Nieuwoudt, H., Bauer, F. F., Bagheri, B., & Setati, M. E. (2023). Differentiation of Geographic Origin of South African Wines from Austrian Wines by IRMS and SNIF-NMR. Foods, 12(6), 1175. https://doi.org/10.3390/foods12061175







