δ34S and Geochemical Analyses for the Determination of, and Discrimination between, Salt Samples of Different Geographic Origin: A Feasibility Study
Abstract
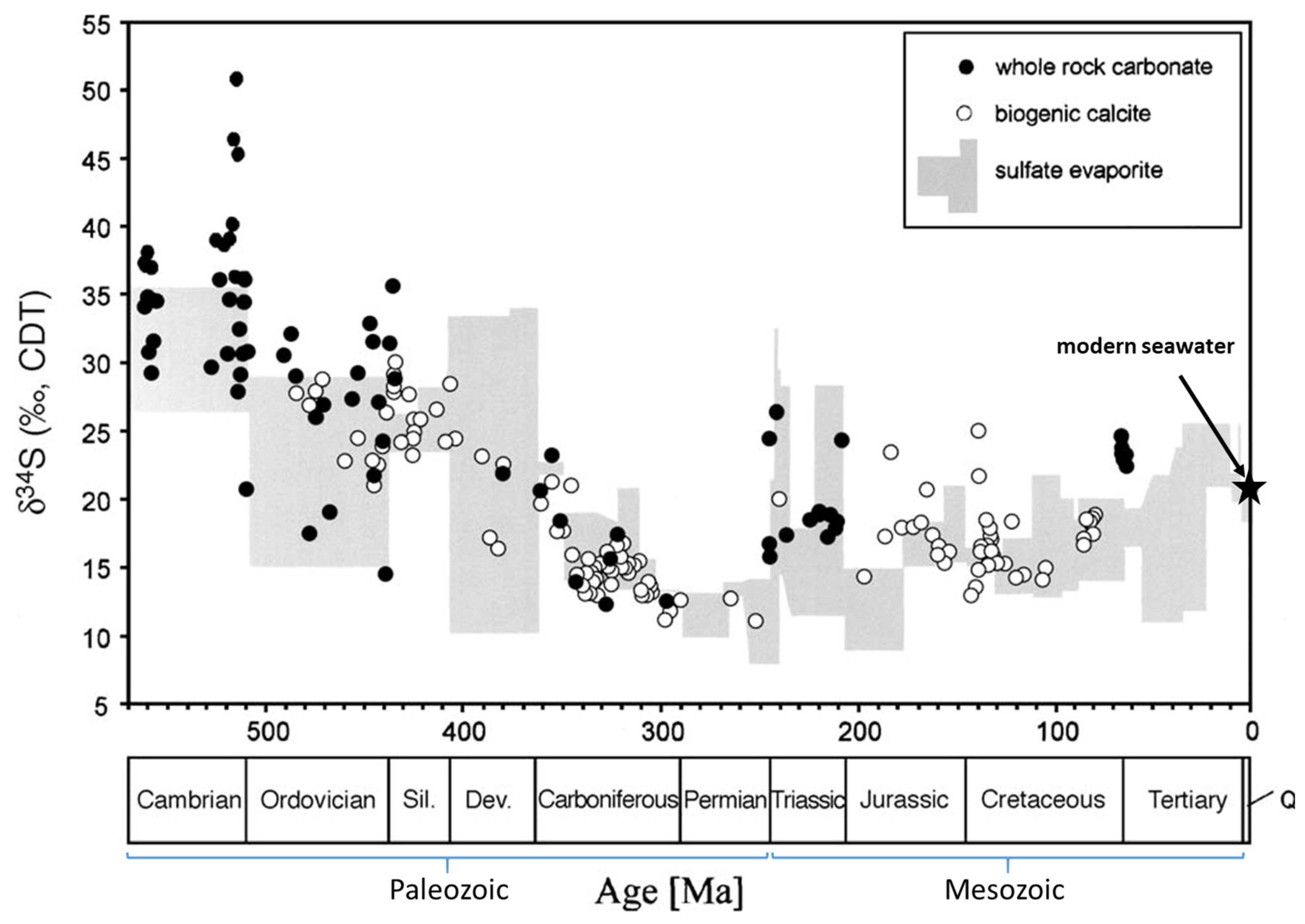
:1. Introduction
2. Materials and Methods
2.1. Sulfur Isotope Analysis
2.2. Trace Element Analysis
3. Results
3.1. δ34S
3.2. Element Concentrations
4. Discussion
4.1. δ34S-Results
4.2. Element Concentrations
5. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Camin, F.; Bontempo, L.; Heinrich, K.; Horacek, M.; Kelly, S.D.; Schlicht, C.; Thomas, F.; Monahan, F.J.; Hoogewerff, J.; Rossmann, A. Multi-element (H,C,N,S) stable isotope characteristics of lamb meat from different European regions. Anal. Bioanal. Chem. 2007, 389, 309–320. [Google Scholar] [CrossRef] [Green Version]
- Camin, F.; Larcher, R.; Nicolini, G.; Bontempo, L.; Bertoldi, D.; Perini, M.; Schlicht, C.; Schellenberg, A.; Thomas, F.; Heinrich, K.; et al. Isotopic and elemental data for tracing the origin of European olive oils. J. Agric. Food Chem. 2010, 58, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Schellenberg, A.; Chmielus, S.; Schlicht, C.; Camin, F.; Perini, M.; Bontempo, L.; Heinrich, K.; Kelly, S.D.; Rossmann, A.; Thomas, F.; et al. Multielement Stable Isotope Ratios (H, C, N, S) of Honey from different European Regions. Food Chem. 2010, 121, 770–777. [Google Scholar] [CrossRef]
- Horacek, M.; Min, J.-S.; Soja, G. Discrimination between ginseng from Korea and China by light stable isotope analysis. Anal. Chim. Acta 2010, 682, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Horacek, M.; Min, J.-S. Discrimination of Korean beef from beef of other origin by stable isotope measurements. Food Chem. 2010, 121, 517–520. [Google Scholar] [CrossRef]
- Goitom Asfaha, D.; Quetel, C.R.; Thomas, F.; Horacek, M.; Wimmer, B.; Heiss, G.; Dekant, C.; Deters-Itzelsberger, P.; Hölzl, S.; Rummel, S.; et al. Combining isotopic signatures of (87Sr)/(86Sr) and light stable elements (C, N, O, S) with multi-elemental profiling for the authentication of provenance of European cereal samples. J. Cereal Sci. 2011, 53, 170–177. [Google Scholar] [CrossRef]
- Horacek, M.; Hansel-Hohl, K.; Burg, K.; Soja, G.; Okello-Anyanga, W.; Fluch, S. Control of origin of sesame oil from various countries by stable isotope analysis and DNA based markers—A pilot study. PLoS ONE 2015, 10, e0123020. [Google Scholar] [CrossRef]
- Holser, W.T. Catastrophic chemical events in the history of the ocean. Nature 1977, 67, 403–407. [Google Scholar] [CrossRef]
- Claypool, G.E.; Holser, W.T.; Kaplan, I.R.; Sakai, H.; Zak, I. The age curves for sulfur and oxygen isotopes in marine sulfate and their mutual interpretation. Chem. Geol. 1980, 28, 199–260. [Google Scholar] [CrossRef]
- Cortecci, G.; Reyes, E.; Berti, G.; Casati, P. Sulfur and oxygen isotopes in Italian marine sulfates of Permian and Triassic ages. Chem. Geol. 1981, 34, 65–79. [Google Scholar] [CrossRef]
- Kampschulte, A.; Strauss, H. The sulfur isotopic evolution of Phanerozoic seawater based on the analysis of structurally substituted sulfate in carbonates. Chem. Geol. 2004, 204, 255–286. [Google Scholar] [CrossRef]
- Spötl, C.; Pak, E. A strontium and sulfur isotopic study of Permo-Triassic evaporites in the Northern Calcareous Alps, Austria. Chem. Geol. 1996, 131, 219–234. [Google Scholar] [CrossRef]
- Horacek, M.; Brandner, R.; Richoz, S.; Povoden, E. Lower Triassic sulphur isotope curve of marine sulphates from the Dolomites, N-Italy. Palaeogeogr. Palaleoclimatol. Palaecol. 2010, 290, 65–70. [Google Scholar] [CrossRef]
- Dufossé, L.; Donadio, C.; Valla, A.; Meile, J.-C.; Montet, D. Determination of speciality food salt origin by using 16S rDNA fingerprinting of bacterial communities by PCReDGGE: An application on marine salts produced in solar salterns from the French Atlantic Ocean. Food Control 2013, 32, 644–649. [Google Scholar] [CrossRef]
- Sharma, S.; Ambardar, S.; Bhagat, N.; Raj, S.; Trakroo, D.; Horacek, M.; Zouagui, R.; Sbabou, L.; Vakhlu, J. Microbiome fingerprint as biomarker for geographical origin and heredity in Crocus sativus: A Feasibility Study. Front. Sustain. Food Syst. 2021, 5, 688393. [Google Scholar] [CrossRef]
- Tchaikovsky, A.; Zitek, A.; Irrgeher, J.; Opper, C.; Scheiber, R.; Moder, K.; Congiu, L.; Prohaska, T. Chemometric tools for determining site-specific elemental and strontium isotopic fingerprints in raw and salted sturgeon caviar. Eur. Food Res. Technol. 2019, 245, 2515–2528. [Google Scholar] [CrossRef]
- Epova, E.N.; Bérail, S.; Zuliani, T.; Malherbe, J.; Sarthou, L.; Valiente, M.; Donard, O.F.X. 87Sr/86Sr isotope ratio and multielemental signatures as indicators of origin of European cured hams: The role of salt. Food Chem. 2018, 246, 313. [Google Scholar] [PubMed]
- Horacek, M.; Klcova, L.; Hudcovicova, M.; Ondreickova, K.; Gubis, J.; Hölzl, S. Differentiation of apricots of different geographic origin by applying 87Sr/86Sr analysis: Potential and limitations. Foods 2022, 11, 2239. [Google Scholar] [CrossRef] [PubMed]
- Horacek, M. Comment on: The Provenance of Slovenian Milk Using 87Sr/86Sr Isotope Ratios” by Gregorcic et al.; 2021: The need to consider geochemistry when interpreting Sr-isotopes. Foods 2022, 11, 564. [Google Scholar] [CrossRef]
- Mostafaii, G.R.; Moravveji, A.; Hajirostamloo, B.; Arani, M.H.; Dehghani, M.; Heidarinejad, Z.; Fakhri, Y.; Khaneghah, A.M. The concentration and risk assessment of potentially toxic elements (PTEs) in unrefined salt: A case study of Aran and Bidgol Lake, Iran. Int. J. Environ. Anal. Chem. 2022, 102, 1192–1204. [Google Scholar] [CrossRef]
- Horacek, M.; Cannavan, A. Isotope Fingerprints of Common and Tartary Buckwheat Grains and Milling Fractions: A Preliminary Study, by Sinkovic et al., 2022: Comment. Foods 2022, 11, 1414. [Google Scholar] [CrossRef] [PubMed]
- Lecuyer, C. Seawater Residence times of some elements of geochemical interest and the salinity of the oceans. Bull. Soc. Geol. Fr. 2016, 187, 245–260. [Google Scholar] [CrossRef]
- Wood, F.O.; Ralston, R.H.; Hills, J.M. Encyclopedia Britannica. 2023. Available online: https://www.britannica.com/science/salt/Occurrence (accessed on 21 March 2023).






| Sample No. | Sample Type and Origin |
|---|---|
| 159697 | Rock salt, Kalahari salt, South Africa |
| 159698 | Sea salt, Mauritius |
| 159699 | Sea salt Sardinia, Italy |
| 159700 | Sea salt, Savoia, Italy |
| 159701 | Table salt (refined), Tuscany, Italy |
| 159702 | Rock salt, Himalaya salt, Pakistan |
| 159703 | Sea salt, Korea |
| 159704 | Sea salt, New Zeeland |
| 159705 | Sea salt, Algarve, Portugal |
| 159706 | Sea salt, Ibiza, Spain |
| 155810-1 | Rock salt, Rotes Kernsalz, Rotsalzgebirge, Altaussee, “AA”-subsample 1, Austria |
| 155810-2 | Rock salt, Rotes Kernsalz, Rotsalzgebirge, Altaussee, “AA”-subsample 2 Austria |
| 155810-1-re-precipitated AA1 | |
| 155810-2-re-precipitated AA2 | |
| 155810 “AA“-subsample 3 milled in a corund mill, Austria | |
| 155811 “HA“-subsample 3 milled in a corund mill, Austria | |
| 155811-1 | Rock salt, Rötlichgraues Kernsalz, Rotsalzgebirge, Hallein, “HT”-subsample 1, Austria |
| 155811-2 | Rock salt, Rötlichgraues Kernsalz, Rotsalzgebirge, Hallein, “HT”-subsample 2, Austria |
| 155811-1-re-precipitated HT1 | |
| 155811-2-re-precipitated HT2 | |
| 155070 | Sea salt, Slovenia |
| 155599 | Dead Sea salt, Israel |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horacek, M. δ34S and Geochemical Analyses for the Determination of, and Discrimination between, Salt Samples of Different Geographic Origin: A Feasibility Study. Foods 2023, 12, 1572. https://doi.org/10.3390/foods12081572
Horacek M. δ34S and Geochemical Analyses for the Determination of, and Discrimination between, Salt Samples of Different Geographic Origin: A Feasibility Study. Foods. 2023; 12(8):1572. https://doi.org/10.3390/foods12081572
Chicago/Turabian StyleHoracek, Micha. 2023. "δ34S and Geochemical Analyses for the Determination of, and Discrimination between, Salt Samples of Different Geographic Origin: A Feasibility Study" Foods 12, no. 8: 1572. https://doi.org/10.3390/foods12081572
APA StyleHoracek, M. (2023). δ34S and Geochemical Analyses for the Determination of, and Discrimination between, Salt Samples of Different Geographic Origin: A Feasibility Study. Foods, 12(8), 1572. https://doi.org/10.3390/foods12081572







