Peanut Allergenicity: An Insight into Its Mitigation Using Thermomechanical Processing
Abstract
1. Introduction
2. Peanut Allergy: Prevalence, Persistence, and Severity
3. Peanut Allergens and Their Mechanisms of Action
3.1. Major Peanut Allergen Characteristics
3.2. Minor Peanut Allergen Characteristics
3.3. Cross-Reactivity
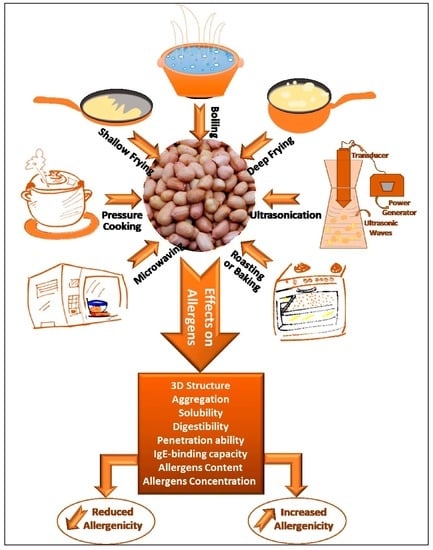
4. Thermomechanical Processing of Peanuts
4.1. Boiling
4.2. Roasting/Baking
4.3. Microwaving
4.4. Ultrasonication
4.5. Frying
4.6. High-Pressure Steaming/Autoclaving
4.7. Factors Affecting the Experimental Results of Treatments
4.8. Comparison between all Processing Methods
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bilaver, L.A.; Chadha, A.S.; Doshi, P.; O’Dwyer, L.; Gupta, R.S. Economic Burden of Food Allergy. Ann. Allergy Asthma Immunol. 2019, 122, 373–380.e1. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Liu, Y.; Liu, K.; Wang, S.; Liu, Q.; Lin, S. Gastrointestinal Fate of Food Allergens and Its Relationship with Allergenicity. Compr. Rev. Food Sci. Food Saf. 2022, 21, 3376–3404. [Google Scholar] [CrossRef] [PubMed]
- Sicherer, S.H.; Sampson, H.A. Food Allergy. J. Allergy Clin. Immunol. 2010, 125, S116–S125. [Google Scholar] [CrossRef] [PubMed]
- Virginia Carolinas Peanuts. The History of Peanuts. Available online: https://www.aboutpeanuts.com/all-about-peanuts/origin-history-of-peanuts (accessed on 2 February 2023).
- Sandefur, H.N.; McCarty, J.A.; Boles, E.C.; Matlock, M.D. Peanut Products as a Protein Source. In Sustainable Protein Sources; Elsevier: Amsterdam, The Netherlands, 2017; pp. 209–221. [Google Scholar] [CrossRef]
- Bonku, R.; Yu, J. Health Aspects of Peanuts as an Outcome of Its Chemical Composition. Food Sci. Hum. Wellness 2020, 9, 21–30. [Google Scholar] [CrossRef]
- Mora-Escobedo, R.; Hernández-Luna, P.; Joaquín-Torres, I.C.; Ortiz-Moreno, A.; Robles-Ramirez, M.D.C. Physicochemical Properties and Fatty Acid Profile of Eight Peanut Varieties Grown in Mexico. CyTA-J. Food 2015, 13, 300–304. [Google Scholar] [CrossRef]
- USFDA. Daily Value on the New Nutrition and Supplement Facts Labels; USFDA: Silver Spring, MD, USA, 2022.
- FSSAI. Food Safety and Standards Authority of India; FSSAI: New Delhi, India, 2020.
- Kumar, B.S.; Shankar, S.R.; Vasanthi, R.P.; Vishnuvardhan, K.M.; Purushotham, M. Comparative physio-chemical, proximate and mineral analysis on raw and roasted seeds of groundnut. Communications Plant Sci. 2013, 3, 25–29. [Google Scholar]
- Mupunga, I.; Mngqawa, P.; Katerere, D. Peanuts, Aflatoxins and Undernutrition in Children in Sub-Saharan Africa. Nutrients 2017, 9, 1287. [Google Scholar] [CrossRef]
- Manzanares, P.; Gandía, M.; Garrigues, S.; Marcos, J. Improving Health-Promoting Effects of Food-Derived Bioactive Peptides through Rational Design and Oral Delivery Strategies. Nutrients 2019, 11, 2545. [Google Scholar] [CrossRef]
- Tsai, C.-J. A Prospective Cohort Study of Nut Consumption and the Risk of Gallstone Disease in Men. Am. J. Epidemiol. 2004, 160, 961–968. [Google Scholar] [CrossRef]
- Jafari Azad, B.; Daneshzad, E.; Azadbakht, L. Peanut and Cardiovascular Disease Risk Factors: A Systematic Review and Meta-Analysis. Crit. Rev. Food Sci. Nutr. 2020, 60, 1123–1140. [Google Scholar] [CrossRef]
- Çiftçi, S.; Suna, G. Functional Components of Peanuts (Arachis hypogaea L.) and Health Benefits: A Review. Future Foods 2022, 5, 100140. [Google Scholar] [CrossRef]
- Naghshi, S.; Sadeghian, M.; Nasiri, M.; Mobarak, S.; Asadi, M.; Sadeghi, O. Association of Total Nut, Tree Nut, Peanut, and Peanut Butter Consumption with Cancer Incidence and Mortality: A Comprehensive Systematic Review and Dose-Response Meta-Analysis of Observational Studies. Adv. Nutr. 2021, 12, 793–808. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Yuan, J.; Cheng, Y.; Chen, M.; Zhang, G.; Wu, J. Selenomethionine-Dominated Selenium-Enriched Peanut Protein Ameliorates Alcohol-Induced Liver Disease in Mice by Suppressing Oxidative Stress. Foods 2021, 10, 2979. [Google Scholar] [CrossRef] [PubMed]
- Loh, W.; Tang, M. The Epidemiology of Food Allergy in the Global Context. Int. J. Environ. Res. Public Health 2018, 15, 2043. [Google Scholar] [CrossRef] [PubMed]
- Shah, F.; Shi, A.; Ashley, J.; Kronfel, C.; Wang, Q.; Maleki, S.J.; Adhikari, B.; Zhang, J. Peanut Allergy: Characteristics and Approaches for Mitigation. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1361–1387. [Google Scholar] [CrossRef]
- Lieberman, J.A.; Gupta, R.S.; Knibb, R.C.; Haselkorn, T.; Tilles, S.; Mack, D.P.; Pouessel, G. The Global Burden of Illness of Peanut Allergy: A Comprehensive Literature Review. Allergy 2021, 76, 1367–1384. [Google Scholar] [CrossRef]
- Gupta, R.S.; Warren, C.M.; Smith, B.M.; Jiang, J.; Blumenstock, J.A.; Davis, M.M.; Schleimer, R.P.; Nadeau, K.C. Prevalence and Severity of Food Allergies Among US Adults. JAMA Netw. Open 2019, 2, e185630. [Google Scholar] [CrossRef]
- Couratier, P.; Montagne, R.; Acaster, S.; Gallop, K.; Patel, R.; Vereda, A.; Pouessel, G. Allergy to Peanuts ImPacting Emotions And Life (APPEAL): The Impact of Peanut Allergy on Children, Adolescents, Adults and Caregivers in France. Allergy Asthma Clin. Immunol. 2020, 16, 86. [Google Scholar] [CrossRef]
- Smejkal, G.; Kakumanu, S.; Cannady-Miller, A. Increasing the Solubility and Recovery of Ara H3 Allergen from Raw and Roasted Peanut. In Nutrition in Health and Disease—Our Challenges Now and Forthcoming Time; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Masilamani, M.; Commins, S.; Shreffler, W. Determinants of Food Allergy. Immunol. Allergy Clin. N. Am. 2012, 32, 11–33. [Google Scholar] [CrossRef]
- Asai, Y.; Eslami, A.; van Ginkel, C.D.; Akhabir, L.; Wan, M.; Ellis, G.; Ben-Shoshan, M.; Martino, D.; Ferreira, M.A.; Allen, K.; et al. Genome-Wide Association Study and Meta-Analysis in Multiple Populations Identifies New Loci for Peanut Allergy and Establishes C11orf30/EMSY as a Genetic Risk Factor for Food Allergy. J. Allergy Clin. Immunol. 2018, 141, 991–1001. [Google Scholar] [CrossRef]
- Asai, Y.; Eslami, A.; van Ginkel, C.D.; Akhabir, L.; Wan, M.; Yin, D.; Ellis, G.; Ben-Shoshan, M.; Marenholz, I.; Martino, D.; et al. A Canadian Genome-Wide Association Study and Meta-Analysis Confirm HLA as a Risk Factor for Peanut Allergy Independent of Asthma. J. Allergy Clin. Immunol. 2018, 141, 1513–1516. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Wu, H.; Lu, Q. The Epigenetics of Food Allergy. In Epigenetics in Allergy and Autoimmunity; Springer: Singapore, 2020; pp. 141–152. [Google Scholar] [CrossRef]
- Cabanillas, B.; Jappe, U.; Novak, N. Allergy to Peanut, Soybean, and Other Legumes: Recent Advances in Allergen Characterization, Stability to Processing and IgE Cross-Reactivity. Mol. Nutr. Food Res. 2018, 62, 1700446. [Google Scholar] [CrossRef] [PubMed]
- Koppelman, S.J.; Jayasena, S.; Luykx, D.; Schepens, E.; Apostolovic, D.; de Jong, G.A.H.; Isleib, T.G.; Nordlee, J.; Baumert, J.; Taylor, S.L.; et al. Allergenicity Attributes of Different Peanut Market Types. Food Chem. Toxicol. 2016, 91, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Marsh, J.T.; Palmer, L.K.; Koppelman, S.J.; Johnson, P.E. Determination of Allergen Levels, Isoforms, and Their Hydroxyproline Modifications among Peanut Genotypes by Mass Spectrometry. Front. Allergy 2022, 3, 872714. [Google Scholar] [CrossRef] [PubMed]
- Geng, Q.; Zhang, Y.; Song, M.; Zhou, X.; Tang, Y.; Wu, Z.; Chen, H. Allergenicity of Peanut Allergens and Its Dependence on the Structure. Compr. Rev. Food Sci. Food Saf. 2023, 22, 1058–1081. [Google Scholar] [CrossRef]
- Blankestijn, M.A.; Knulst, A.C.; Knol, E.F.; Le, T.-M.; Rockmann, H.; Otten, H.G.; Klemans, R.J.B. Sensitization to PR-10 Proteins Is Indicative of Distinctive Sensitization Patterns in Adults with a Suspected Food Allergy. Clin. Transl. Allergy 2017, 7, 42. [Google Scholar] [CrossRef]
- Eiwegger, T.; Rigby, N.; Mondoulet, L.; Bernard, H.; Krauth, M.-T.; Boehm, A.; Dehlink, E.; Valent, P.; Wal, J.M.; Mills, E.N.C.; et al. Gastro-Duodenal Digestion Products of the Major Peanut Allergen Ara h 1 Retain an Allergenic Potential. Clin. Exp. Allergy 2006, 36, 1281–1288. [Google Scholar] [CrossRef]
- Jappe, U.; Schwager, C. Relevance of Lipophilic Allergens in Food Allergy Diagnosis. Curr. Allergy Asthma Rep. 2017, 17, 61. [Google Scholar] [CrossRef]
- Fuhrmann, V.; Huang, H.-J.; Akarsu, A.; Shilovskiy, I.; Elisyutina, O.; Khaitov, M.; van Hage, M.; Linhart, B.; Focke-Tejkl, M.; Valenta, R.; et al. From Allergen Molecules to Molecular Immunotherapy of Nut Allergy: A Hard Nut to Crack. Front. Immunol. 2021, 12, 742732. [Google Scholar] [CrossRef]
- Toomer, O.T. Nutritional Chemistry of the Peanut (Arachis hypogaea). Crit. Rev. Food Sci. Nutr. 2018, 58, 3042–3053. [Google Scholar] [CrossRef]
- Mittag, D.; Akkerdaas, J.; Ballmer-Weber, B.K.; Vogel, L.; Wensing, M.; Becker, W.-M.; Koppelman, S.J.; Knulst, A.C.; Helbling, A.; Hefle, S.L.; et al. Ara h 8, a Bet v 1–Homologous Allergen from Peanut, Is a Major Allergen in Patients with Combined Birch Pollen and Peanut Allergy. J. Allergy Clin. Immunol. 2004, 114, 1410–1417. [Google Scholar] [CrossRef] [PubMed]
- Koppelman, S.J.; Vlooswijk, R.A.A.; Knippels, L.M.J.; Hessing, M.; Knol, E.F.; van Reijsen, F.C.; Bruijnzeel-Koomen, C.A.F.M. Quantification of Major Peanut Allergens Ara h 1 and Ara h 2 in the Peanut Varieties Runner, Spanish, Virginia, and Valencia, Bred in Different Parts of the World. Allergy 2001, 56, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Shreffler, W.G.; Castro, R.R.; Kucuk, Z.Y.; Charlop-Powers, Z.; Grishina, G.; Yoo, S.; Burks, A.W.; Sampson, H.A. The Major Glycoprotein Allergen from Arachis hypogaea, Ara h 1, Is a Ligand of Dendritic Cell-Specific ICAM-Grabbing Nonintegrin and Acts as a Th2 Adjuvant In Vitro. J. Immunol. 2006, 177, 3677–3685. [Google Scholar] [CrossRef] [PubMed]
- Pele, M. Peanut Allergens. Rom. Biotechnol. Lett. 2010, 15, 5204–5212. [Google Scholar]
- Liu, C.; Sathe, S.K. Food Allergen Epitope Mapping. J. Agric. Food Chem. 2018, 66, 7238–7248. [Google Scholar] [CrossRef]
- Becker, W.M.; Petersen, A.; Jappe, U. Peanut Allergens: New Consolidated Findings on Structure, Characteristics and Allergom. Allergol. Select. 2018, 2, 67–79. [Google Scholar] [CrossRef]
- Chan, E.S.; Greenhawt, M.J.; Fleischer, D.M.; Caubet, J.-C. Managing Cross-Reactivity in Those with Peanut Allergy. J. Allergy Clin. Immunol. Pract. 2019, 7, 381–386. [Google Scholar] [CrossRef]
- Zhuang, Y.; Dreskin, S.C. Redefining the Major Peanut Allergens. Immunol. Res. 2013, 55, 125–134. [Google Scholar] [CrossRef]
- Sudharson, S.; Kalic, T.; Hafner, C.; Breiteneder, H. Newly Defined Allergens in the WHO/IUIS Allergen Nomenclature Database during 01/2019-03/2021. Allergy 2021, 76, 3359–3373. [Google Scholar] [CrossRef]
- Otsu, K.; Guo, R.; Dreskin, S.C. Epitope Analysis of Ara h 2 and Ara h 6: Characteristic Patterns of IgE-Binding Fingerprints among Individuals with Similar Clinical Histories. Clin. Exp. Allergy 2015, 45, 471–484. [Google Scholar] [CrossRef]
- Chen, X.; Negi, S.S.; Liao, S.; Gao, V.; Braun, W.; Dreskin, S.C. Conformational IgE Epitopes of Peanut Allergens Ara h 2 and Ara h 6. Clin. Exp. Allergy 2016, 46, 1120–1128. [Google Scholar] [CrossRef] [PubMed]
- Palladino, C.; Breiteneder, H. Peanut Allergens. Mol. Immunol. 2018, 100, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Zhang, Z.; Luo, W.; Hou, Y.; He, Y.; Chen, J.; Ji, K. Identification of Immunodominant IgE Epitopes of the Major House Dust Mite Allergen Der f 24. Int. J. Mol. Med. 2019, 44, 1888–1898. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fu, T.-J.; Howard, A.; Kothary, M.H.; McHugh, T.H.; Zhang, Y. Crystal Structure of Peanut (Arachis Hypogaea) Allergen Ara h 5. J. Agric. Food Chem. 2013, 61, 1573–1578. [Google Scholar] [CrossRef]
- Poole, A.; Song, Y.; Brown, H.; Hart, P.H.; Zhang, G. Cellular and Molecular Mechanisms of Vitamin D in Food Allergy. J. Cell. Mol. Med. 2018, 22, 3270–3277. [Google Scholar] [CrossRef]
- Pi, X.; Wan, Y.; Yang, Y.; Li, R.; Wu, X.; Xie, M.; Fu, G. Research Progress in Peanut Allergens and Their Allergenicity Reduction. Trends Food Sci. Technol. 2019, 93, 212–220. [Google Scholar] [CrossRef]
- Pi, X.; Yang, Y.; Sun, Y.; Cui, Q.; Wan, Y.; Fu, G.; Chen, H.; Cheng, J. Recent Advances in Alleviating Food Allergenicity through Fermentation. Crit. Rev. Food Sci. Nutr. 2022, 62, 7255–7268. [Google Scholar] [CrossRef]
- Zhang, T.; Shi, Y.; Zhao, Y.; Tang, G.; Niu, B.; Chen, Q. Boiling and Roasting Treatment Affecting the Peanut Allergenicity. Ann. Transl. Med. 2018, 6, 357. [Google Scholar] [CrossRef]
- Zhang, T.; Shi, Y.; Zhao, Y.; Wang, J.; Wang, M.; Niu, B.; Chen, Q. Different Thermal Processing Effects on Peanut Allergenicity. J. Sci. Food Agric. 2019, 99, 2321–2328. [Google Scholar] [CrossRef]
- Tian, Y.; Rao, H.; Zhang, K.; Tao, S.; Xue, W.T. Effects of Different Thermal Processing Methods on the Structure and Allergenicity of Peanut Allergen Ara h 1. Food Sci. Nutr. 2018, 6, 1706–1714. [Google Scholar] [CrossRef]
- Prodić, I.; Smiljanić, K.; Simović, A.; Radosavljević, J.; Ćirković Veličković, T. Thermal Processing of Peanut Grains Impairs Their Mimicked Gastrointestinal Digestion While Downstream Defatting Treatments Affect Digestomic Profiles. Foods 2019, 8, 463. [Google Scholar] [CrossRef]
- Meng, S.; Li, J.; Chang, S.; Maleki, S.J. Quantitative and Kinetic Analyses of Peanut Allergens as Affected by Food Processing. Food Chem. X 2019, 1, 100004. [Google Scholar] [CrossRef]
- Tao, B.; Bernardo, K.; Eldi, P.; Chegeni, N.; Wiese, M.; Colella, A.; Kral, A.; Hayball, J.; Smith, W.; Forsyth, K.; et al. Extended Boiling of Peanut Progressively Reduces IgE Allergenicity While Retaining T Cell Reactivity. Clin. Exp. Allergy 2016, 46, 1004–1014. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Huang, Y.; Tang, X.; Wang, H.; Li, B.; Meng, X.; Jiang, S. Effects of Different Cooking Methods on Peanut Allergenicity. Food Biosci. 2022, 47, 101757. [Google Scholar] [CrossRef]
- Novak, N.; Maleki, S.J.; Cuadrado, C.; Crespo, J.F.; Cabanillas, B. Interaction of Monocyte-Derived Dendritic Cells with Ara h 2 from Raw and Roasted Peanuts. Foods 2020, 9, 863. [Google Scholar] [CrossRef]
- Salve, A.R.; LeBlanc, J.G.; Arya, S.S. Effect of Processing on Polyphenol Profile, Aflatoxin Concentration and Allergenicity of Peanuts. J. Food Sci. Technol. 2021, 58, 2714–2724. [Google Scholar] [CrossRef] [PubMed]
- Cohen, C.; Zhao, W.; Beaudette, L.; Lejtenyi, D.; Jean-Claude, B.; Mazer, B. High-Pressure and Temperature Autoclaving of Peanuts Reduces the Proportion of Intact Allergenic Proteins. Authorea Prepr. 2021. [Google Scholar] [CrossRef]
- Đukić, T.; Smiljanić, K.; Mihailović, J.; Prodić, I.; Apostolović, D.; Liu, S.-H.; Epstein, M.M.; van Hage, M.; Stanić-Vučinić, D.; Ćirković Veličković, T. Proteomic Profiling of Major Peanut Allergens and Their Post-Translational Modifications Affected by Roasting. Foods 2022, 11, 3993. [Google Scholar] [CrossRef]
- Prodic, I.; Stanic-Vucinic, D.; Apostolovic, D.; Mihailovic, J.; Radibratovic, M.; Radosavljevic, J.; Burazer, L.; Milcic, M.; Smiljanic, K.; van Hage, M.; et al. Influence of Peanut Matrix on Stability of Allergens in Gastric-Simulated Digesta: 2S Albumins Are Main Contributors to the IgE Reactivity of Short Digestion-Resistant Peptides. Clin. Exp. Allergy 2018, 48, 731–740. [Google Scholar] [CrossRef]
- di Stasio, L.; Tranquet, O.; Picariello, G.; Ferranti, P.; Morisset, M.; Denery-Papini, S.; Mamone, G. Comparative Analysis of Eliciting Capacity of Raw and Roasted Peanuts: The Role of Gastrointestinal Digestion. Food Res. Int. 2020, 127, 108758. [Google Scholar] [CrossRef]
- Wang, S.; Sun, X.; Wang, M.; Deng, Z.; Niu, B.; Chen, Q. Effect of Roasted Peanut Allergen Ara h 3 Protein on the Sensitization of Caco-2 Cells. J. Sci. Food Agric. 2021, 101, 5325–5336. [Google Scholar] [CrossRef] [PubMed]
- Cabanillas, B.; Maleki, S.J.; Cheng, H.; Novak, N. Differences in the Uptake of Ara h 3 from Raw and Roasted Peanut by Monocyte-Derived Dendritic Cells. Int. Arch. Allergy Immunol. 2018, 177, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Corzo-Martínez, M.; Villamiel, M.; Moreno, F.J. Impact of High-Intensity Ultrasound on Protein Structure and Functionality during Food Processing. In Ultrasound in Food Processing; John Wiley & Sons, Ltd.: Chichester, UK, 2017; pp. 417–436. [Google Scholar] [CrossRef]
- Li, H.; Yu, J.; Ahmedna, M.; Goktepe, I. Reduction of Major Peanut Allergens Ara h 1 and Ara h 2, in Roasted Peanuts by Ultrasound Assisted Enzymatic Treatment. Food Chem. 2013, 141, 762–768. [Google Scholar] [CrossRef]
- Meng, S. Quantitative Analysis of Allergens in Peanut Varieties and Assessment of Effects of Food Processing on Peanut Allergens. Ph.D. Thesis, 2018. 3696. Available online: https://scholarsjunction.msstate.edu/td/3696 (accessed on 2 November 2022).
- Bavaro, S.L.; di Stasio, L.; Mamone, G.; de Angelis, E.; Nocerino, R.; Canani, R.B.; Logrieco, A.F.; Montemurro, N.; Monaci, L. Effect of Thermal/Pressure Processing and Simulated Human Digestion on the Immunoreactivity of Extractable Peanut Allergens. Food Res. Int. 2018, 109, 126–137. [Google Scholar] [CrossRef]
- Zhang, J.; Hong, Y.; Cai, Z.; Huang, B.; Wang, J.; Ren, Y. Simultaneous Determination of Major Peanut Allergens Ara H1 and Ara H2 in Baked Foodstuffs Based on Their Signature Peptides Using Ultra-Performance Liquid Chromatography Coupled to Tandem Mass Spectrometry. Anal. Methods 2019, 11, 1689–1696. [Google Scholar] [CrossRef]
- Faisal, S.; Zhang, J.; Meng, S.; Shi, A.; Li, L.; Wang, Q.; Maleki, S.J.; Adhikari, B. Effect of High-Moisture Extrusion and Addition of Transglutaminase on Major Peanut Allergens Content Extracted by Three Step Sequential Method. Food Chem. 2022, 385, 132569. [Google Scholar] [CrossRef]
- Bavaro, S.L.; Orlando, A.; de Angelis, E.; Russo, F.; Monaci, L. Investigation on the Allergen Profile of the Soluble Fraction of Autoclaved Peanuts and Its Interaction with Caco-2 Cells. Food Funct. 2019, 10, 3615–3625. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Z.; Li, K.; Li, X.; Yang, A.; Tong, P.; Chen, H. Allergenicity Assessment on Thermally Processed Peanut Influenced by Extraction and Assessment Methods. Food Chem. 2019, 281, 130–139. [Google Scholar] [CrossRef]
- Cuadrado, C.; Cabanillas, B.; Pedrosa, M.M.; Muzquiz, M.; Haddad, J.; Allaf, K.; Rodriguez, J.; Crespo, J.F.; Burbano, C. Effect of Instant Controlled Pressure Drop on IgE Antibody Reactivity to Peanut, Lentil, Chickpea and Soybean Proteins. Int. Arch. Allergy Immunol. 2011, 156, 397–404. [Google Scholar] [CrossRef]
- Vicente, F.; Sanchiz, A.; Rodríguez-Pérez, R.; Pedrosa, M.; Quirce, S.; Haddad, J.; Besombes, C.; Linacero, R.; Allaf, K.; Cuadrado, C. Influence of Instant Controlled Pressure Drop (DIC) on Allergenic Potential of Tree Nuts. Molecules 2020, 25, 1742. [Google Scholar] [CrossRef]
- Takács, K.; Guillamon, E.; Pedrosa, M.M.; Cuadrado, C.; Burbano, C.; Muzquiz, M.; Haddad, J.; Allaf, K.; Maczó, A.; Polgár, M.; et al. Study of the Effect of Instant Controlled Pressure Drop (DIC) Treatment on IgE-Reactive Legume-Protein Patterns by Electrophoresis and Immunoblot. Food Agric. Immunol. 2014, 25, 173–185. [Google Scholar] [CrossRef]
- Rao, H.; Tian, Y.; Fu, W.; Xue, W. In Vitro Digestibility and Immunoreactivity of Thermally Processed Peanut. Food Agric. Immunol. 2018, 29, 989–1001. [Google Scholar] [CrossRef]


| Allergen Superfamily | Allergen Name | Molecular Weight (in kDa) | Resistance to Heat and Digestion | Interaction with Water | |
|---|---|---|---|---|---|
| Cupin (Vicilin-type, 7 S globulin) | Ara h 1 | 64 | Resistant | Hydrophilic | |
| Prolamin (2 S albumin–conglutin) | Ara h 2 | 17 | Resistant | Hydrophilic | |
| Ara h 6 | 15 | Resistant | |||
| Ara h 7 | 15 | - | |||
| Cupin (11 S globulin–glycinin) | Ara h 3.01 | 60 | Resistant | Hydrophilic | |
| Ara h 3.02 | 37 (fragment) | ||||
| Profilin | Ara h 5 | 15 | Minimal resistance | Amphipathic | |
| Bet v 1 (PR-10 protein) | Ara h 8 | 17 | Minimal resistance | Hydrophilic | |
| Prolamin (Non-specific lipid-transfer protein nsLTP) | Type 1 | Ara h 9 | 9.8 | Resistant | Hydrophobic |
| Type 2 | Ara h 16 | 8.5 | |||
| Type 1 | Ara h 17 | 11 | |||
| Glycosyl transferase (oleosin) | Ara h 10 | 16 | Minimal resistance | Hydrophobic | |
| Ara h 11 | 14 | ||||
| Ara h 14 | 17.5 | ||||
| Ara h 15 | 17 | ||||
| Scorpion toxin-like knottin (defensin) | Ara h 12 | 8 (reducing), 12 (non-reducing) | - | Hydrophilic | |
| Ara h 13 | 8 (reducing), 11 (non-reducing) | ||||
| Cyclophilin | Ara h 18 (pan-allergens) | 21 | Minimal resistance | Hydrophilic | |
| Allergen Name | Function | Cross-Reactivity | IgE-Binding Potential | Clinical Relevance |
|---|---|---|---|---|
| Ara h 1 | Seed storage protein | Brazil nut, cashew, hazelnut, peanut, walnut, soybean, lupin, peas, chickpea, lentil | 33 to 65% | Severe systemic reaction up to anaphylaxis |
| Ara h 2 | Brazil nut, cashew, hazelnut, walnut, soybean, chickpea | 42 to 100% | ||
| Ara h 3.01 Ara h 3.02 | Brazil nut, cashew, hazelnut, walnut, soybean, lupin, pea, chickpea, lentil | 16 to 50% | ||
| Ara h 5 | Regulator of cellular processes Actin-binding protein Transport across membrane Cytoskeletal dynamics | Brazil nut, cashew, hazelnut, walnut, soybean, lupin, lentil | 3% to 24% (in birch pollen-allergic people) | No or local clinical reaction Pollen food allergy syndrome |
| Ara h 6 | Seed storage protein | Soybean, chickpea | 85% to 92% | Severe systemic reaction up to anaphylaxis |
| Ara h 7 | - | 43% to 80% | - | |
| Ara h 8 | Stress mechanism Plant defense | Other PR-10 allergens, soybean | 2.4% to 49% (in birch pollen-allergic people) | Local clinical reaction Mild oropharyngeal reaction |
| Ara h 9 | Lipid transfer across membrane Stress mechanism Plant defense | Chestnut, almond, peach, Rosaceae family, pear, plum, cherry, strawberry, lentil, sunflower, bean, pea | - | Systemic reaction |
| Ara h 10 | Structural proteins: oil bodies | Other soy and buck wheat group | - | Local clinical reaction |
| Ara h 11 | - | - | - | |
| Ara h 12 | Plant defense | - | - | - |
| Ara h 13 | - | - | - | |
| Ara h 14 | Structural proteins: oil bodies | - | - | - |
| Ara h 15 | - | - | - | |
| Ara h 16 | Lipid transfer across the membrane Stress mechanism Plant defense | Pollen, olive pollen, most respiratory allergens | - | - |
| Ara h 17 | ||||
| Ara h 18 | Peptidyl–prolyl cis–trans isomerase | Pollen, olive pollen, most respiratory allergens | 87% POS IgE-binding for r Ara h 18 | Local and temporary clinical reaction |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haidar, E.; Lakkis, J.; Karam, M.; Koubaa, M.; Louka, N.; Debs, E. Peanut Allergenicity: An Insight into Its Mitigation Using Thermomechanical Processing. Foods 2023, 12, 1253. https://doi.org/10.3390/foods12061253
Haidar E, Lakkis J, Karam M, Koubaa M, Louka N, Debs E. Peanut Allergenicity: An Insight into Its Mitigation Using Thermomechanical Processing. Foods. 2023; 12(6):1253. https://doi.org/10.3390/foods12061253
Chicago/Turabian StyleHaidar, Elissa, Jack Lakkis, Marc Karam, Mohamed Koubaa, Nicolas Louka, and Espérance Debs. 2023. "Peanut Allergenicity: An Insight into Its Mitigation Using Thermomechanical Processing" Foods 12, no. 6: 1253. https://doi.org/10.3390/foods12061253
APA StyleHaidar, E., Lakkis, J., Karam, M., Koubaa, M., Louka, N., & Debs, E. (2023). Peanut Allergenicity: An Insight into Its Mitigation Using Thermomechanical Processing. Foods, 12(6), 1253. https://doi.org/10.3390/foods12061253