1. Introduction
High-field, high-resolution
1H nuclear magnetic resonance (NMR) analysis is extensively applied in lipidomics, together with determinations of the full composition and molecular nature of many culinary oil products (including the saturation and unsaturation status of major fatty acids (FAs) present, for example), their longevities, and even geographic origins [
1]. More recently, it has also been quite widely employed for the specific determination of toxic lipid oxidation products (LOPs) in these products, including a series of aldehydes which are formed in these commonly employed cooking oils during high-temperature frying practices [
2,
3,
4]. Furthermore, additional studies have extensively documented the potential toxicological hazards that these aldehydic species may present, along with their overall public health implications [
5,
6,
7]. Indeed, these aldehydes, especially the α,β-unsaturated classes, are highly chemically reactive, and they form relatively stable, latent source adducts with many critical biomolecules in vivo, for example selected proteins and DNA. Such DNA damage renders these toxins mutagenic, genotoxic and, at least in some cases, carcinogenic. Consistently, Weng et al. reported that aldehydes represent the dominant carcinogens present in tobacco cigarette smoke [
8]. The causal links between reactive aldehyde species and non-communicable chronic disease (NCD) risks in humans are therefore of much pertinent importance, and to date there is much evidence available indicating associations between the consumption of fried foods and the development and progression of a range of serious NCDs, for example, coronary heart diseases [
9] and prostate cancer [
10]. Further studies, e.g., that reported in [
11], have established associations between human exposure to Chinese-style cooking fumes, which contain high levels of toxic aldehydes such as acrolein, and the risk of developing lung cancer. Moreover, it has been demonstrated that aldehydic toxins are readily transferred to foods from the oils in which they are fried; these foods typically include potato chips, beef patties, and fried chicken, which are frequently consumed by humans [
12]. However, these levels were found to be significantly lower in fried potato chips than they were in the frying oil itself (only ca. 5% or so in mol/kg units), and this is attributable to only a small amount of the food mass being accounted for by uptake of aldehyde-rich oil (typically 10–15% (
w/w) [
13]), and their known chemical reactions with different classes of food biomolecules. A range of other factors such as frying oil FA compositions and frying duration are also relevant.
To date, high-field (HF) NMR spectroscopy has been successfully utilised for the rapid multicomponent analysis of quite a wide variety of LOPs present in thermally stressed frying oils, which have included primary conjugated hydroperoxydiene isomers, their secondary fragmentation products (particularly saturated and unsaturated aldehydes), and epoxy-fatty acids, for example [
4,
14]. Moreover, the use of two-dimensional correlation spectroscopies, both homo- and heteronuclear, has been invaluable for confirming provisional LOP assignments made in 1D spectra [
15]. Notably, our research group was the very first to report these applications and advantages as early as 1994 [
16]. Such advances have recently led to the development of methods for the analysis of such LOPs in fried foods, approaches which feature a key lipid extraction stage [
12].
Notwithstanding, the use of both medium-field (MF, with 300–400 MHz operating frequencies) and HF
1H NMR spectroscopy for the simultaneous multicomponent analysis of aldehydic and further LOPs in such matrices is reliant upon a number of operational necessities such as the institutional accessibility of such expensive instruments, which are dependent on bulky cryogenically cooled superconducting magnets, the frequent use of high volumes of deuterated solvents such as deuterochloroform (CDCl
3) for sample preparation purposes, and requirements for the professional inputs of highly specialised operational technical staff, along with the availability of those with specialist spectral interpretational skills. Moreover, the high costs of such large HF facilities (e.g., with 500–700 MHz operating frequencies), together with their stringent demands for high volumes of cryogenic cooling gases [
16], limits their applications in many academic institutions, or within the commercial sector. Comparatively, low-field (LF) ‘benchtop’ NMR instruments benefit from considerably lower power requirements, permanent magnets, and virtual portability. Thus, LF NMR analysis shows great promise for applications within industrial cooking oil production, or even restaurant settings, for the direct ‘on-site’ detection and determination of edible oil quality, together with screens for toxic LOPs generated within during the use of such frying media.
Currently, such LF NMR spectrometers are emerging, cost-effective and portable alternatives to their HF counterparts [
17]. Indeed, recent studies have demonstrated the growing applications of LF NMR spectroscopy to numerous analytical sectors, including ‘point-of-care’ medical diagnostics [
17], forensic science, synthetic chemical reaction monitoring [
18], and versatile approaches towards chemical education [
19]. Furthermore, selected hyperpolarisation techniques have been applied to specific investigations [
20]. The research landscape of developing applications for LF NMR has also been shown to offer major advantages in the detection of vegetable oil adulteration [
21,
22], together with both one- (1D) and two-dimensional (2D) NMR approaches for probing and determining edible oil authenticities [
23].
Previously, preliminary studies of the LF NMR detection and analysis of aldehyde species in used and reused cooking oils has only been documented by Grootveld et al. (2014) [
24,
25], wherein detectable levels of selected aldehyde classes were observed in heated sunflower oil samples, and olive oil collected from a ‘real-life’ restaurant site. In the current study, we report the further exploration and development of LF NMR techniques for evaluating frying oil qualities. In particular, we consider the analytical reliability and reproducibility of LF
1H NMR determinations of aldehydic LOPs in oils heated according to shallow frying practices, and also limits for their detection and quantification. Comparisons of these analytical data to those acquired at MF strength are also made. We also consider the potential health risk status of these products through the reliable quantification of aldehydic LOP toxins. Overall, this LF NMR analysis technology highlights great promise for application at industrial food product manufacturing sites or restaurants for the detection and determination of these agents.
2. Materials and Methods
2.1. Reagents and 1H NMR Solvents
All materials, including deuterochloroform (CDCl3) and aldehyde calibration standards, were purchased from Sigma-Aldrich Chemical Co. (UK), unless otherwise stated; the CDCl3 product contained the 1H NMR reference standard tetramethylsilane (TMS) at an added level of 1.00% (v/v) (equivalent to 73.45 mmol/L on consideration of its density of 0.648 g/mL). NMR tubes were purchased from Norell (Morganton, NC, USA), and EppendorfTM microcentrifuge tubes were obtained from Fisher Scientific Ltd. (Loughborough, UK).
2.2. Culinary Oil Products and Their FA Compositions
Four culinary oils of differing triacylglycerol FA compositions were studied. These comprised a mixed-origin refined olive oil (MOO), soybean oil (SBO), rapeseed oil (RSO) and chia seed oil (CSO). With the exception of the SBO, which originated from a US retail source, all these products were purchased from reputable retail outlets based in the UK. The acylglycerol contents of FA classes present in the oils analysed were calculated using major acylglycerol resonance intensities from 400 MHz
1H NMR spectra of the unheated control (0 min heating time-point) samples, according to the methodological equations reported in Ref. [
4].
Samples were stored under dark conditions at ambient temperature to diminish photodegradative peroxidation during storage periods prior to analysis. The lipid content profiles of each oil analysed, which are represented as % (w/w) saturated (SAT), monounsaturated (MUFA) and polyunsaturated (PUFA) FA contents, were found to be MOO: 16% SAT, 76% MUFA and 8% (w/w) PUFA; SBO: 16% SAT, 24% MUFA and 60% (w/w) PUFA, the latter including 5% omega-3 FAs (predominantly linolenoylglycerols); RSO: 5% SAT, 67% MUFA and 28% (w/w) PUFA, the latter including 7% (w/w) omega-3 FAs (predominantly linolenoylglycerols); CSO: 9% SAT, 9% MUFA and 82% (w/w) PUFA, the latter including 65% (w/w) omega-3 FAs (again predominantly as linolenoylglycerols). For the SBO, RSO and CSO products, omega-3 FAs were analysed via electronic integration of their characteristic linolenoylglycerol-distinctive δ = 0.95 ppm triplet resonance in 400 MHz spectra acquired on this oil.
Preliminary experiments conducted also featured a commercially available sample of a UK refined sunflower oil (SFO) product. This oil was found to have an FA content of 10% SAT, 31% MUFA and 59% PUFA.
2.3. Aldehydic LOP Calibration Standards
Aldehyde calibration standards were prepared using n-hexanal and trans-2-octenal in deuterochloroform (CDCl3) solution, and increasing concentrations of these analyte solutions were added to unheated (control) olive oil (MOO product) samples. These preparations were conducted by adding a 0.20 mL aliquot of each aldehyde calibration solution (ranging from 0.00 to 54.98 mmol/L for n-hexanal, and 0.00 to 40.40 mmol/L for trans-2-octenal), 0.20 mL of unheated olive oil, 0.20 mL of additional CDCl3 solvent, and 0.10 and 0.06 mL of solutions of the lipid-soluble antioxidant 2,5-di-tert-butylhydroquinone (2,5-DTBHQ) and the secondary 1H NMR chemical shift reference and internal standard 1,3,5-trichlorobenzene (TCB) (67.0 and 19.8 mmol/L, respectively), both in CDCl3. The densities of these aldehydes were accounted for when adding or diluting µL volumes of each. For experiments in which the signal-to-noise (STN) ratio was determined and evaluated, a corresponding series of aldehyde calibration standards were prepared in a medium without any added oil, and in these cases the 0.20 mL unheated olive oil constituent was replaced with an equivalent volume of additional CDCl3.
2.4. H NMR Analysis
1H NMR analysis of prepared aldehyde calibration standard solutions, and control and thermally stressed oil samples, was performed on a 60 MHz Magritek Spinsolve Benchtop system operating at a frequency of 61.67 MHz. Spectra were acquired using a 1D Proton+ sequence. Parameters employed for these analyses were 32K data points; 128 scans; acquisition time 6.4 s; repetition time 10 s; and a pulse angle of 90° (the total sample acquisition duration was ~21 min). These spectra were also acquired on a MF 400 MHz Bruker Avance AV400 NMR spectrometer (Leicester School of Pharmacy, De Montfort University, Leicester, UK) operating at a frequency of 399.93 MHz. For this facility, spectral acquisition parameters were 32K data points; 128 scans with 2 dummy scans; 3 µs pulses, spectral width 8278 Hz; and a receiver gain setting of 14.8.
2.5. Purity of Aldehyde Calibrations Standards Used
Reference
1H NMR spectra of these aldehyde standards revealed that they contained 17 and 5 mol% of their corresponding carboxylic acid oxidation products, i.e., hexanoic acid and
trans-2-octenoic acid, for
n-hexanal and
trans-2-octenal, respectively, and this was again considered when preparing their calibration standard solutions; purities reported by their manufacturer were 98 and 94% (
w/w), respectively. The identities of these carboxylic acid oxidation products were confirmed by the observation of their characteristic
1H NMR resonances in the aldehyde spectra acquired, i.e., that of the α-CH
2 protons of hexanoic acid (
t, δ = 2.354 ppm,
J = 7.67 Hz) and those of the 2- and 3-positon olefinic protons in
trans-2-octenoic acid (i.e.,
dt, δ = 5.831 ppm,
J = 16.0, 1.3 Hz, and
m, δ = 6.660 ppm,
J = 15.9, 7.0 Hz, respectively) [
26]. The concentrations and contaminating mol % values of these oxidation products in these standard solutions were determined via electronic integration of these resonances, together with the corresponding, more prominent signals arising from their parent aldehydes.
2.6. H NMR Determination of Different Classes of Aldehydes
Aldehyde concentrations were determined by electronic integration of their characteristic 1H NMR -CHO function resonances, and normalising these data to those of the total acylglycerol terminal-CH3 function resonance (δ = 0.82–1.11 ppm) of total FAs from the added unheated olive oil co-calibrant at both 60 and 400 MHz operating frequencies, so that aldehyde concentrations are reported as mmol aldehyde per mol of total FA (mmol/mol FA units). Aldehydic LOP levels in control and thermally stressed culinary oil products were then determined via reference to the calibration plots shown in the Results Section below. However, it should be noted that for the purpose of comparative evaluations, aldehydes were simply classified as either total saturated or total α,β-unsaturated, since it was not possible to effectively resolve superimposing resonances of individual sub-classes of these analyte species within their overall saturated and α,β-unsaturated classes at LF strength (60 MHz).
2.7. Calibration and Bland–Altman Dominance Plots of Authentic Standard Aldehyde Solutions in Combined CDCl3/Culinary Oil Media
Calibration and Bland–Altman style dominance plots of the
1H NMR-determined concentrations of the saturated and α,β-unsaturated aldehydes
n-hexanal and
trans-2-octenal, respectively, involved matched analysis sample datasets, with determinations made on these two analytes at both 60 and 400 MHz operating frequencies. Units for these determinations, which were conducted in the combined CDCl
3/culinary oil medium, were mmol aldehyde/mol total acylglycerol FA. For these plots, Dixon’s test was applied to detect any potential outlier samples, but none were found. Similarly, all determinations which were found to have none detectable (nd, specifically values below the specified lower limit of detection (i.e., <LLOD) at both operating frequencies utilised were removed from the datasets. As recommended [
27], corresponding
1H NMR profiles of blank samples, which were prepared as described above but with CDCl
3 in place of culinary oils, were acquired, and their ’noise’ intensities at the appropriate δ values were included in these calibration plots. Spectra were acquired on replicate (n = 3) preparations of such blank samples for these purposes.
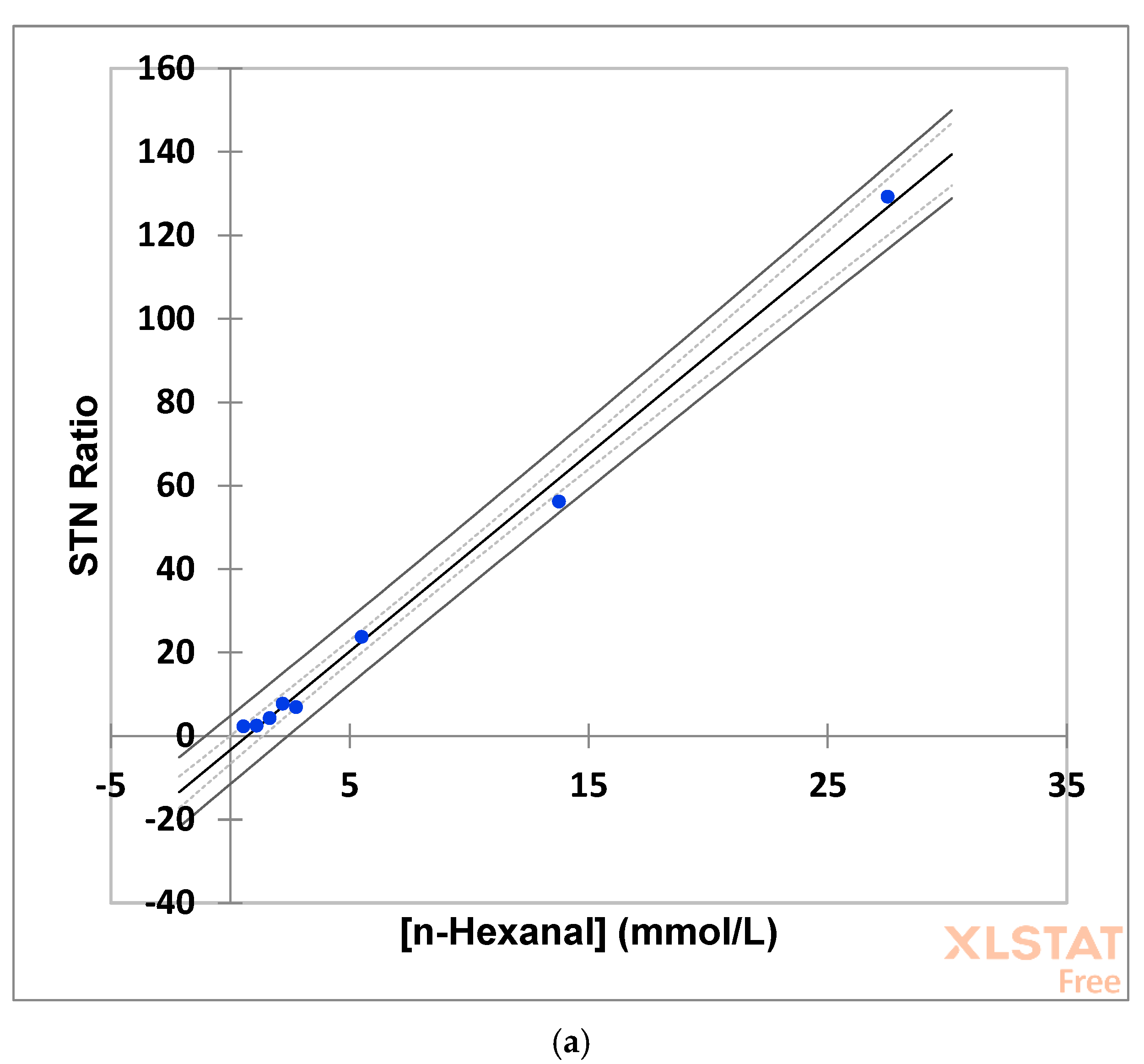
2.8. Estimation of Signal-to-Noise Ratios, and Lower Limits of Detection and Quantification Values, for Saturated and α,β-Unsaturated Aldehyde Analyte Solution Calibrants in Neat CDCl3 and Combined CDCl3/Culinary Oil Media
STN ratios for total saturated and α,β-unsaturated aldehydes were monitored by LF
1H NMR analysis of the
n-hexanal and
trans-2-octenal calibrant standards for each of these two aldehyde classes, and which were fully resolvable and quantifiable at this field strength, were determined using built-in software scripts within the
MestreNova software package (Feliciano Barrera 9B—Bajo, 15,706 Santiago de Compostela, Spain). The signal-free region used to calculate the noise level was δ = 10.50–11.50 ppm, and Equation (1) was employed to derive the standard deviation of this value (
Noise SD), where N = number of datapoints within the signal-free region; Y
i = value of each digital point in the spectrum; and Y
m = mean value of the digital points within that region. Mean STN ratio values were then computed as the ratio of the aldehyde resonance peak heights to that of the
Noise SD value.
Lower limits of detection and quantification (LLOD and LLOQ, respectively) were estimated as three-times and ten-times the mean STN ratio values (3(STN) and 10(STN), respectively).
2.9. Thermal Stressing of Culinary Oil Products According to Laboratory-Simulated Shallow Frying Episodes
Each culinary oil evaluated was exposed to laboratory-simulated shallow frying episodes (LSSFEs) for periods of 0–90 min as previously described in Ref. [
12], although for the experiments outlined here, samples were collected for
1H NMR analysis at the 0, 30, 60 and 90 min time-points. A total of n = 3 replicate samples for each culinary oil tested and each collection time-point were obtained. Volumes (6.00 mL) of each culinary oil were placed in air-dried 250 mL glass beakers within a thermostatted silicon oil bath, which was heated to 180 °C in the presence of atmospheric O
2 according to our LSSFEs in order to simulate shallow frying conditions. Aliquots (0.25 mL) of these oils were sampled for
1H NMR analysis at each of the above time-points.
2.10. Sample Collection and Preparation for 1H NMR Analysis
Aliquots (0.20 mL) of all oil samples collected were transferred to 1.5 mL microcentrifuge tubes, and then 0.40 mL of CDCl3 containing 1% (v/v) TMS as a chemical shift reference (δ = 0.00 ppm), and 0.06 and 0.10 mL volumes of CDCl3 solutions of TCB (67.0 mmol/L) and the chain-breaking antioxidant 2,5-DTBHQ (19.8 mmol/L), respectively, were added. 2,5-DTBHQ was included in these preparations in order to prevent any artefactual peroxidation of culinary oil unsaturated FAs (UFAs) during periods of sample preparation and storage prior to analysis. Subsequently, samples were vortexed thoroughly and transferred to 5 mm diameter Norell NMR tubes for 1H NMR analysis.
1H NMR analysis of these oil samples was performed at both 60 and 400 MHz operating frequencies as described in
Section 2.4 2.11. ANOVA Model for the Statistical Analysis of Experimental Datasets
Univariate statistical chemometrics analysis was conducted to detect any significant differences between the mean replicate values of both total saturated and total α,β-unsaturated aldehydic LOPs for each oil product evaluated, each LSSFE sampling time-point (60 versus 90 min) and for each instrumental operating frequency (60 versus 400 MHz) by an analysis-of-variance (ANOVA) model. The experimental design employed comprised a three-factor model with the fixed effects of culinary oil product (O
i), LSSFE sampling time-point (T
j) and spectrometer operating frequency (F
k). Also incorporated in the designs were first-order oil product × sampling time-point, oil product × spectrometer operating frequency, and sampling time-point × spectrometer operating frequency interaction effects (OT
ij, OF
ik and TF
jk, respectively). The mathematical model for this design is displayed in Equation (2), where y
ijkl represents each replicate aldehyde concentration, μ the overall sample mean aldehyde concentration in the absence of any possible explanatory sources of variation, and e
ijkl fundamental error. An ANOVA rather than an analysis-of-covariance (ANCOVA) model was selected for this analysis since the latter approach is dependent on linear relationships between response variables (aldehyde concentrations) and the quantitative sampling time-point covariable considered, and this was clearly not the case for the dataset acquired in this study, since it is well known that all such relationships are sigmoidal (S-shaped) and not linear [
12]. Software module options utilised for the performance of these ANOVA models were those available from
XLSTAT2020 (Addinsoft, Paris, France). Post hoc evaluations of the different oil products tested involved comparisons of their mean values using the Bonferroni test.
Subsequent to application of this model, if all first-order interaction terms were found not to be statistically significant (as indeed they were for the total unsaturated but not the saturated aldehyde outcome variables), they were removed from the model, and their variance contributions were then transferred to that of the error term for the re-testing of the main factor effects only (Equation (3)).
Any missing data from the above ANOVA models were estimated by replacement with group mean or mode values, followed by reduction of error mean square degrees-of-freedom values by the number of such replacements made accordingly.
2.12. Computational Simulations of the 1H NMR Spectra of Solution-Phase Triacylglycerols
Simulated
1H NMR spectra of triacylglycerols, and that of their glycerol backbone, were obtained using
Bruker TopSpin NMR-SIM software. Chemicals shift and coupling constant values, and example
1H NMR spectra, were first obtained from the
Human Metabolome Database (HMBD) [
26]. A 400 MHz reference spectrum of glycerol in hexadeuterated dimethylsulphoxide (d6-DMSO), was first simulated, and these data were then applied and optimised to simulate the experimental spectra of a typical triacylglycerol species at both 60 and 400 MHz operating frequencies.
4. Discussion
This study demonstrated that LF (60 MHz)
1H NMR analysis could detect and quantify secondary total saturated and unsaturated aldehydic LOP concentrations in thermally stressed cooking oils as a function of high-temperature LSSFE exposure time, albeit most especially at thermal stressing time-points of 60 and 90 min However, in the case of MUFA-rich oils, such as the MOO product explored here, results obtained at LF were hampered by limitations of such analysis using this operating frequency, predominantly by the lowered sensitivity of this technique and the superimposition of resonances usually resolved at MF strength, along with increased levels of spectral noise. Indeed, for the purpose of making comparative analytical evaluations between results obtained on the 60 and 400 MHz spectrometers in this study, we had no option but to combine aldehyde contents within two classification groups: total saturated and total α,β-unsaturated types. This approach was adopted despite some partial resolution between
trans-2-alkenal and
trans,trans-alka-2,4-dienal resonances in all oils investigated (
Figure 2). However, the absolute concentrations of these two different α,β-unsaturated aldehydes could, at least in principle, be quantified on LF spectrometers through the application of suitable spectral deconvolution strategies.
As previously reported, thermally stressed MUFA-rich oils produce greater relative amounts of
trans-2-alkenals and only minor relative levels of all other α,β-unsaturated aldehyde species [
1,
2,
3,
4,
12]. However, in MF spectra acquired, resonances assignable to virtually all these unsaturated aldehydes, including isomeric alka-2,4-dienals, are observed in spectra acquired on such heated oils, and may be individually included in the resonance integration values for quantification purposes, as shown in
Figure 3. However, in LF spectra, the
trans,trans- isomer signal (δ = 9.52 ppm,
d) is not sufficiently resolved from that of
trans-2-alkenals (δ = 9.48 ppm,
d), whereas levels of the
cis,trans-isomer (δ = 9.63 ppm,
d) generally fall below the LLOQ threshold value specified, and this served as a limitation of the technique. Therefore, these more minor aldehydic products are not accurately represented by LF
1H NMR data, and therefore a difference in total α,β-unsaturated aldehyde content between MF and LF analyses would be expected. This is indeed the case, with total α,β-unsaturated aldehyde concentrations in heated culinary oils ranging from 10 to 30% higher at the 400 MHz operating frequency (
Figure 9b and
Figure 10b). Also expected was the evolution of a more diverse range of α,β-unsaturated aldehyde compound signals in the MF spectra acquired at the extended heating periods featured (>60 min). Notably, these resonances, e.g., those of oxygenated aldehydes such as 4,5-diepoxy- and 4-hydroxy/4-hydroperoxy-trans-2-alkenals, and that of malondialdehyde, may also arise from the thermally induced oxidation and/or degradation of pre-formed alka-2,4-dienal species [
12].
The higher levels of isomeric alka-2,4-dienals observed in thermally stressed PUFA-rich oils arise from the thermo-oxidative fragmentation of higher concentrations of their specific CHPD precursors, and not of HPMs from the primary MUFA peroxidation stage (the latter yielding only
trans-2-alkeanls and
n-alkanals on degradation). Indeed, higher total concentrations of α,β-unsaturated aldehydic species derived from PUFA-rich oils are more readily observable at LF than only
trans-2-alkenals arising from MUFAs (
Figure 10b).
Interestingly, the analytical precision of the LF α,β-unsaturated aldehyde concentration data was found to be similar to those acquired at MF operating frequency. Indeed, ‘between-replicate’ CV values determined at 60 MHz predominantly ranged from 0.4 to 13.4%, and those at 400 MHz ranged from 1.2 to 12.1%. However, for saturated aldehyde species, estimated LF CV values were found to be not as precise, whereas those at MF were predominantly <7.0%. Additionally, the CSO product displayed a less than favourable level of reproducibility for both total saturated and α,β-unsaturated aldehydes determined at the 90 min heating time-point using the LF spectrometer.
However, CSO is certainly not recommended for cooking or frying purposes anyway in view of its very high ω-3 FA content. Indeed, this oil would be expected to lead to higher levels of aldehydic LOPs than those observed in oils with high linoleoylglycerol contents (e.g., natural sunflower and corn oils); this appears to be coupled with proportionate increases in their ‘within- and between-assay’ analytical variabilities. Furthermore, the potentially higher volatilities of aldehydic LOPs arising in linolenoylglycerol-rich oils may also contribute towards this higher variance (the inclusion of this unusual oil in this study was primarily to determine the capability of LF NMR analysis to identify and determine differential classes of aldehydic LOPs arising from the specific peroxidation of omega-3 rather than omega-6 PUFAs).
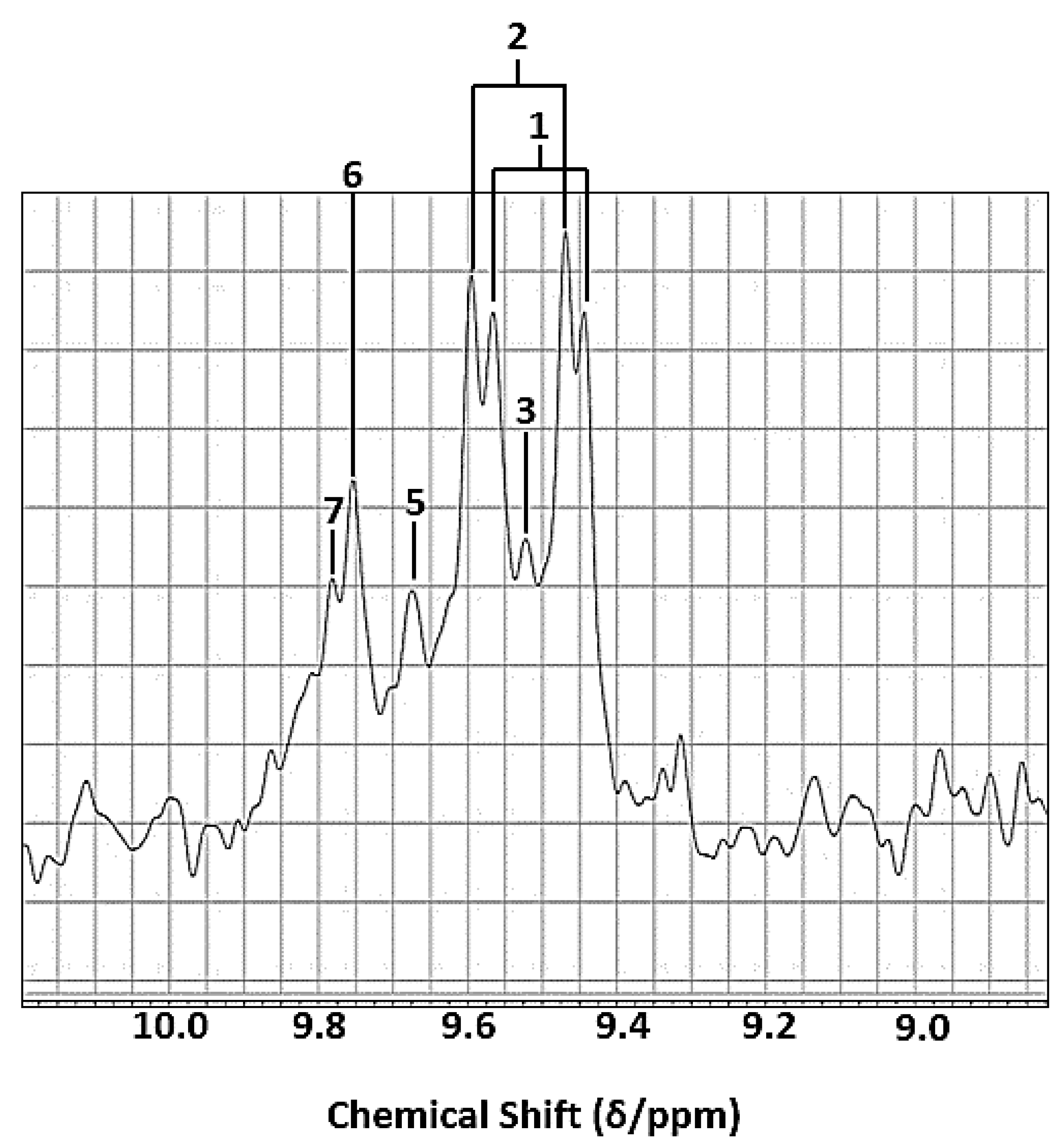
The STN ratios and LLOQ values of aldehydic-CHO function proton resonances determined here are, unfortunately, restricted by the fact that only a single proton gives rise to their signals observed at 60 MHz (δ = 9.4–9.9 ppm), which are split into doublets or triplets for α,β-unsaturated and saturated n-alkanals, respectively. Unfortunately, the use of alternative 1H NMR resonances for these aldehydes is not possible, since that of the terminal-CH3 group of all aldehydes is obscured by very intense, major bulk lipid ones, as are those of their chain-(CH2)n- groups; the STN values of the latter are also limited by their complex coupling patterns. Similarly, the use of olefinic proton resonances of α,β-unsaturated aldehydes for QNMR purposes at LF strength is also restricted by such complex coupling patterns.
As previously described, the potential toxicological effects of dietary LOPs are likely expended by their uptake by foods fried at high-temperatures in cooking oils, followed by the consumption of such fried foods, e.g., potato chips and fried chicken, etc., by humans [
7,
12]. Analytical estimates of the three major classes of aldehydic LOPs in potato chips were reported to be 121 ± 33, 157 ± 43 and 126 ± 25 µmol/kg for total n-alkanals,
trans-2- alkenals and alka-(
trans,trans)-2,4-dienals, respectively, using
1H NMR analysis [
12]. The mean
trans,trans-alka-2,4-dienal level found was comparable to that determined for the single
trans,trans-deca-2,4-dienal analyte alone by Boskou et al. [
30] in French fries fried in a domestic deep-fryer at 170 °C (65 µmol/kg). It has been shown that fried food aldehyde levels are predominantly dependent on their uptake of LOP-containing frying oils during frying practices; this uptake may range from a few % to as high as 30% (
w/w), however [
7]. If we assume that this uptake level is 10% (
w/w), a typical value for UK fast-food restaurants [
13], then the above values would be ca. 10-fold higher in the oil itself, and would be equivalent to contents of 0.37, 0.49 and 0.39 mmol Aldehyde per mol Of oil FA for
n-alkanals,
trans-2-alkenals and
trans,trans-alka-2,4-dienals, respectively, all of these values being lower than, but approaching, our LLOQ values for the analysis of cooking oil aldehydes by the LF benchtop NMR spectrometer employed here (
Table 4). However, the total level of unsaturated aldehydes in these potato chip samples would be 0.88 mmol/mol of uptaken FA, a value which is indeed above our LLOQ threshold limit for determinations made at 60 MHz; although saturated aldehydes would not be validly quantifiable at this operating frequency, they would be detectable since the value of 0.37 mmol/mol FA remains above our LLOD limit, which is 0.19 mmol/mol FA for
n-hexanal (
Table 4). Our previously reported MF or HF
1H NMR analysis of aldehydic LOPs in French fry samples relies on a CDCl
3 extraction process, and modifications to this protocol, such as the use of larger quantities of food samples for this purpose, a process giving rise to greater volumes of oils extracted therefrom, would, of course, yield lower LLOQ values for analysis at LF strength. However, it has been reported that the fried food aldehyde contents are markedly lower than those anticipated from the percentage oil uptake value alone [
14], and this is likely to be attributable to their chemical consumption through Maillard or Michael addition reactions with food amino acids, peptides and proteins, and/or acetal and ketal formation on reaction with certain food carbohydrates. Indeed, it is well known that such aldehydes have a high level of reactivity with a range of biomolecules present in many biosystems. Fried food contents of aldehydic LOPs will, of course, also be strikingly dependent on a wide range of further factors, for example frying temperatures and fried food exposure times, the potential recycling of reused oils, frying type (i.e., shallow versus deep-frying episodes), frying oil MUFA, PUFA and ω-3 FA contents, and potentially also their lipid-soluble antioxidant contents, etc.
Therefore, in summary, LF NMR analysis has the capacity to monitor
n-alkanals (saturated aldehydes) in thermally stressed culinary oils at detectable levels of 0.19 mmol/mol FA (equivalent to 0.66 mmol/L), and quantifiable at levels of 0.65 mmol/mol FA (equivalent to 2.21 mmol/L) when analysed in an olive oil co-calibrant placed in CDCl
3 solution. Moreover, it was determined that
trans-2-alkenal species were detectable at levels of 0.18 mmol/mol FA (equivalent to 0.63 mmol/L), with quantifiable levels of 0.62 mmol/mol FA (equivalent to 2.10 mmol/L) in an olive oil/CDCl
3 medium. Similar LLOD and LLOQ estimates were found for both aldehyde calibrants when analysed in a ‘neat’ CDCl
3 solution medium alone (
Table 4).
Of further interest, this technological LF NMR development was also found to be valuable for the detection and quantification of precursors of the aldehydic LOPs monitored, specifically CHPDs and HPMs, as demonstrated in
Figure 11. Results arising from the LF NMR assessment of the generation of these precursors, and the dependence of their culinary oil concentrations on LSSFE heating time, will be made available in a follow-up paper shortly.
This LF NMR analysis option is also valuable for determining the FA and unsaturation status of culinary oils in general, as shown in
Figure 1. One interesting feature of these major lipid component H NMR profiles was differences in the spectral appearance of selected triacylglycerol multiplet resonances, and this is ascribable to the previously reported ‘roofing’ effect which occurs when scalar
J-coupled signals appear just a few Hz away from each other in spectra acquired, as indeed they are in LF (60 MHz) spectra.
In principle, near-portable, non-stationary LF NMR spectrometers could be employed for the purpose of directly monitoring toxic aldehydic LOPs, together with their lipid hydroperoxide precursors, in a range of culinary frying oils at commercial food production and manufacturing sites, or alternatively at large outlets of global fast-food restaurant chains. Indeed, analysis is rapid, and the skillset required for the operation and management of such devices is far simpler and appealing than that required for institutionally based MF or HF spectrometers; indeed, with the exception of a succinct level of training, no previous specialist operator knowledge is required.
5. Limitations of the Study
Although results obtained herein have demonstrated the ability of LF benchtop NMR spectrometers to detect several different classes of aldehydic LOP species in oil samples, unfortunately quantification of these species is limited to total saturated and total α,β-unsaturated aldehydes in view of an insufficient resolution of the
1H NMR resonances of these LOPs, most notably that involving the latter class of these analytes. Veritably, LF NMR analysis does suffer from a number of limitations, including signal convolution which leads to issues of both identification and quantification for individual unsaturated aldehyde species. Furthermore, because of the reduced resolution and increased background noise of such instruments, the magnitude of and demands for satisfactory LLOD and LLOQ parameters were somewhat greater than those which are usually mandatory for QNMR approaches employed on MF and HF NMR facilities. Moreover, the analytical sensitivity threshold of the LF NMR technique for our analysis protocol (
Table 4) also served as a further limitation; this involved the analysis of only a 0.20 mL aliquot of culinary oil in a total NMR tube volume of 0.760 mL. However, pilot experiments have revealed that we may increase this analytical volume of oil sample up to 0.40 mL, and therefore the LLOD thresholds determined here would be decreased 2-fold if that were the case. Unfortunately, larger increments in proportionate sample volume restrict signal resolution and hence the analysis of aldehydes and other LOPs in view of the resulting high viscosity of the analyte solution medium.
Data available in
Figure 9b and
Figure 10b revealed that for both the 60 and 90 min heating time-points, mean total saturated aldehyde concentrations determined in PUFA-rich oils at an operating frequency of 60 MHz were 79 and 89% of that determined at 400 MHz for the CSO and RSO products, respectively, but only 62% for the SBO one (these ‘between-oil’ differences in analyte receptivity were also manifested by the observation of a statistically significant first-order oil × spectrometer operating frequency interaction effect for this aldehyde classification). For total α,β-unsaturated aldehyde levels, however, corresponding values were 83, 69 and 81% for the CSO, RSO and SBO products, respectively (MOO 71%). Therefore, it is important that this current restriction should be recognised by analysts and researchers, and perhaps the application of a suitable ‘correction factor’ should be considered to overcome this issue.
It should also be noted that saturated aldehydic LOPs (mainly two types of NMR-distinguishable n-alkanals) are generated at much lower concentrations than the α,β-unsaturated aldehyde classifications, and for this study, it was found that the former class were only ca. 20% of the total aldehyde levels determined by the LF spectrometer at the 90 min heating time-point. These diminished concentrations will also restrict their determination at an operating frequency of only 60 MHz.
Another consequence of the LF
1H NMR monitoring of aldehydes in culinary oils, heated or otherwise, is that the total amounts of aldehydic LOPs liberated from the peroxidation of UFAs is not a direct reflection of those found in these oil products following exposure to thermal stressing periods. Indeed, a significant proportion of these toxins have boiling-points below standard frying temperature (now ranging from 160 to 180 °C), with some, such as acrolein (the lowest α,β-unsaturated aldehyde homologue), exceedingly so [
14]. Hence, a quite substantial fraction of the total amount of aldehydic LOPs evolved from these frying oil ‘reaction’ media are in the volatile, i.e., gaseous form, and due caution should be exercised by humans to avoid the inhalation of these toxins during frying or cooking exercises, be they commercial or domestic. Therefore, oil aldehyde contents only serve as incomplete reflections of the total amounts generated.
Since our LF 1H NMR-determined aldehyde concentrations were segmented into total saturated and α,β-unsaturated classifications, variance contributions towards these from structurally more complex LOPs would be expected to expand with increasing complexity level, most notably for PUFA-rich oils which have been exposed to prolonged heating durations. Ultimately, saturated aldehydes were only observed at quantifiable levels in the LF NMR spectra of heated oil samples from the 60 min, but not the 30 min, time-point, although not all oils thermally stressed in this manner for a 60 min period gave sample aldehyde levels higher than the LLOQ threshold level determined here. Despite this, LF-NMR analysis shows promise as an appropriate approach for detecting and quantifying total aldehydes in oils that have been heated for durations which are ≥60 min, and putatively less so for samples with higher oil contents present in analyte media solutions.