Quantification of Free Short-Chain Fatty Acids in Raw Cow Milk by Gas Chromatography-Mass Spectrometry
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Sample Preparation
2.3. Standards Preparation
2.4. GC-MS Analysis Procedure
2.5. Methodological Validation
2.6. Statistical Analysis
3. Results and Discussion
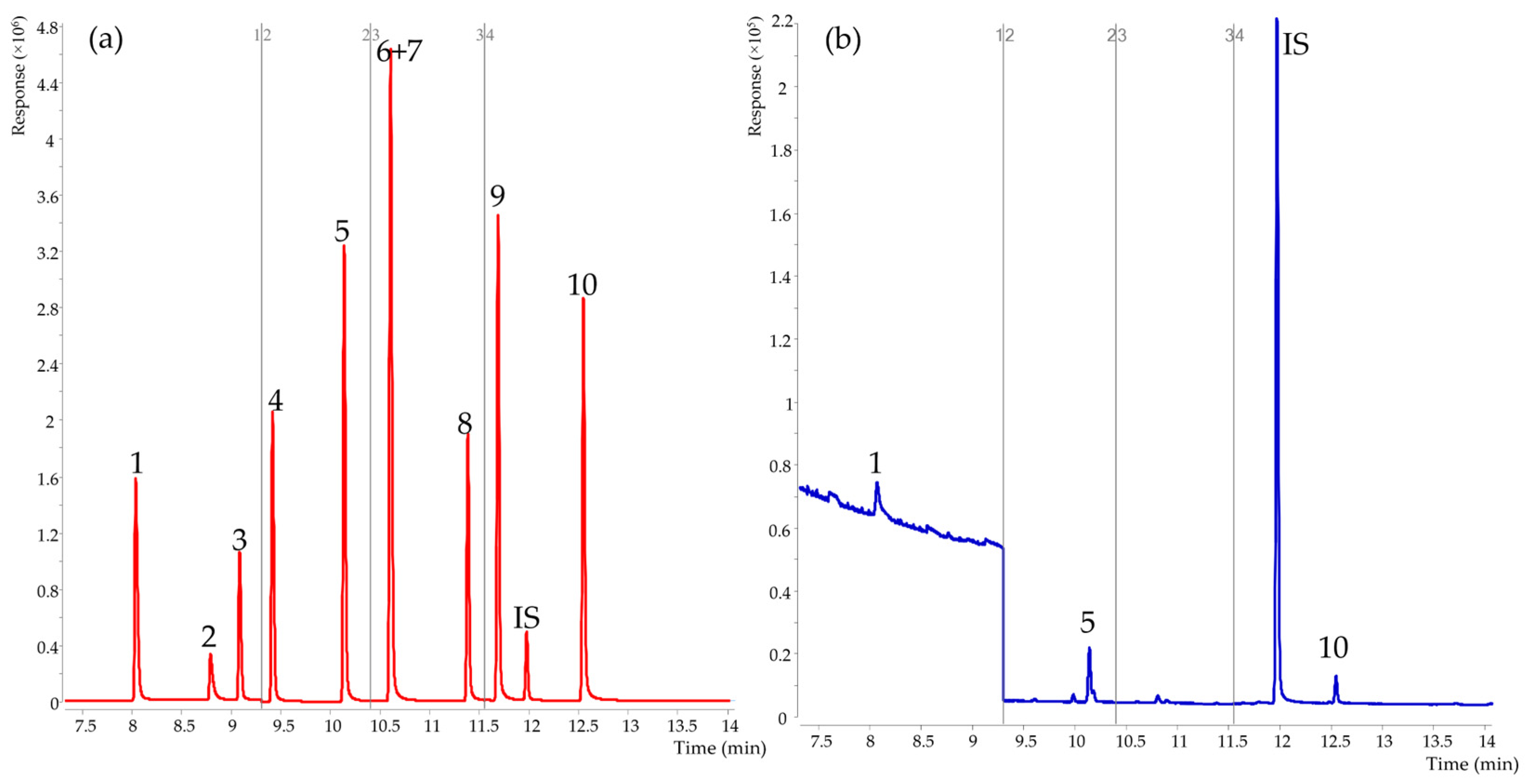
3.1. Optimization of Chromatographic Conditions
3.2. Methodological Validation
3.3. Content of FSCFAs in Raw Cow Milk
3.4. Comparison of Previous GC-MS Methods with the Method Reported Here
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ren, G.; Cheng, G.; Wang, J. Understanding the role of milk in regulating human homeostasis in the context of the COVID-19 global pandemic. Trends Food Sci. Technol. 2021, 107, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Genkinger, J.M.; Wang, M.; Li, R.; Albanes, D.; Anderson, K.E.; Bernstein, L.; van den Brandt, P.A.; English, D.R.; Freudenheim, J.L.; Fuchs, C.S.; et al. Dairy products and pancreatic cancer risk: A pooled analysis of 14 cohort studies. Ann. Oncol. 2014, 25, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Wang, W.J.; Zhang, F.; Shao, Z.P.; Guo, L. Formation of the oxidized flavor compounds at different heat treatment and changes in the oxidation stability of milk. Food Sci. Nutr. 2019, 7, 238–246. [Google Scholar] [CrossRef]
- Bendall, J.G. Aroma compounds of fresh milk from New Zealand cows fed different diets. J. Agric. Food Chem. 2001, 49, 4825–4832. [Google Scholar] [CrossRef] [PubMed]
- Clarke, H.J.; Griffin, C.; Rai, D.K.; O’Callaghan, T.F.; O’Sullivan, M.G.; Kerry, J.P.; Kilcawley, K.N. Dietary compounds influencing the sensorial, volatile and phytochemical properties of bovine milk. Molecules 2019, 25, 26. [Google Scholar] [CrossRef]
- Deeth, H.C. Lipoprotein lipase and lipolysis in milk. Int. Dairy J. 2006, 16, 555–562. [Google Scholar] [CrossRef]
- Dan, T.; Jin, R.; Ren, W.; Li, T.; Chen, H.; Sun, T. Characteristics of milk fermented by streptococcus thermophilus MGA45-4 and the profiles of associated volatile compounds during fermentation and storage. Molecules 2018, 23, 878. [Google Scholar] [CrossRef]
- McSweeney, P.L.H.; Fox, P.F. Advanced Dairy Chemistry; Springer Science and Business Media: New York, NY, USA, 2009; Volume 3, pp. 631–690. [Google Scholar]
- González-Córdova, A.F.; Vallejo-Cordoba., B. Detection and prediction of hydrolytic rancidity in milk by multiple regression analysis of short-chain free fatty acids determined by solid phase microextraction gas chromatography and quantitative flavor intensity assessment. J. Agric. Food Chem. 2003, 51, 7127–7131. [Google Scholar] [CrossRef]
- Kara, U.; Sert, D. The use of microfiltration technique in the production of skim milk powder: The effect of milk transport conditions on the microbiological and physicochemical properties of milk and milk powders. Int. J. Dairy Technol. 2022, 75, 438–447. [Google Scholar] [CrossRef]
- Agus, A.; Clement, K.; Sokol, H. Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut 2021, 70, 1174–1182. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Backhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Bosch, C.; Boorman, E.; Zunszain, P.A.; Mann, G.E. Short-chain fatty acids as modulators of redox signaling in health and disease. Redox Biol. 2021, 47, 102165. [Google Scholar] [CrossRef]
- Wei, Y.; Chang, L.; Hashimoto, K. Molecular mechanisms underlying the antidepressant actions of arketamine: Beyond the NMDA receptor. Mol. Psychiatry 2022, 27, 559–573. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-F.; Ren, S.-C.; Tang, G.; Wu, C.; Chen, X.; Tang, X.-Q. Short-chain fatty acids in blood pressure, friend or foe. Chin. Med. J. 2021, 134, 2393–2394. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xu, H.; Tu, X.; Gao, Z. The role of short-shain fatty acids of gut microbiota origin in hypertension. Front. Microbiol. 2021, 12, 730809. [Google Scholar] [CrossRef] [PubMed]
- Stinson, L.F.; Gay, M.C.L.; Koleva, P.T.; Eggesbø, M.; Johnson, C.C.; Wegienka, G.; Toit, E.d.; Shimojo, N.; Munblit, D.; Campbell, D.E.; et al. Human milk from atopic mothers has lower levels of short chain fatty acids. Front. Immunol. 2020, 11, 1427. [Google Scholar] [CrossRef]
- Nan, Z.; Yanan, G.; Weishu, Z.; Di, M.; Allan, W.W. Short chain fatty acids produced by colonizing intestinal commensal bacterial interaction with expressed breast milk are anti-inflammatory in human immature enterocytes. PLoS ONE 2020, 15, e0229283. [Google Scholar] [CrossRef]
- Canfora, E.E.; Jocken, J.W.; Blaak, E.E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 2015, 11, 577–591. [Google Scholar] [CrossRef]
- Frost, G.; Sleeth, M.L.; Sahuri-Arisoylu, M.; Lizarbe, B.; Cerdan, S.; Brody, L.; Anastasovska, J.; Ghourab, S.; Hankir, M.; Zhang, S.; et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat. Commun. 2014, 5, 3611. [Google Scholar] [CrossRef]
- Mannion, D.T.; Furey, A.; Kilcawley, K.N. Development and validation of a novel free fatty acid butyl ester gas chromatography method for the determination of free fatty acids in dairy products. J. Agric. Food Chem. 2018, 67, 499–506. [Google Scholar] [CrossRef]
- Collomb, M.; Malke, P.; Spahni, M.; Sieber, R.; Butikofer, U. Gas chromatographic determination of free fatty acids in cheese: Precision of the method and influence of seasons on lipolysis in different Swiss cheeses. Mitt. Aus Lebensm. Und Hyg. 2003, 94, 212–229. [Google Scholar]
- Kwak, H.S.; Jung, C.S.; Seok, J.S.; Ahn, J. Cholesterol removal and flavor development in cheddar cheese. Asian-Australas. J. Anim. Sci. 2003, 16, 409–416. [Google Scholar] [CrossRef]
- Saha, S.; Day-Walsh, P.; Shehata, E.; Kroon, P.A. Development and validation of a LC-MS/MS technique for the analysis of short chain fatty acids in tissues and biological fluids without derivatisation using isotope labelled internal standards. Molecules 2021, 26, 6444. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, Z.; Bath, C.; Marett, L.; Pryce, J.; Rochfort, S. Optimised method for short-chain fatty acid profiling of bovine milk and serum. Molecules 2022, 14, 436. [Google Scholar] [CrossRef]
- Amer, B.; Nebel, C.; Bertram, H.C.; Mortensen, G.; Hermansen, K.; Dalsgaard, T.K. Novel method for quantification of individual free fatty acids in milk using an in-solution derivatisation approach and gas chromatography-mass spectrometry. Int. Dairy J. 2013, 32, 199–203. [Google Scholar] [CrossRef]
- Jiang, Z.Z.; Liu, Y.N.; Zhu, Y.; Yang, J.; Sun, L.L.; Chai, X.; Wang, Y.F. Characteristic chromatographic fingerprint study of short-chain fatty acids in human milk, infant formula, pure milk and fermented milk by gas chromatography-mass spectrometry. Int. J. Food Sci. Nutr. 2016, 67, 632–640. [Google Scholar] [CrossRef]
- Prentice, P.M.; Schoemaker, M.H.; Vervoort, J.; Hettinga, K.; Lambers, T.T.; Van Tol, E.A.F.; Acerini, C.L.; Olga, L.; Petry, C.J.; Hughes, I.A.; et al. Human milk short-chain fatty acid composition is associated with adiposity outcomes in infants. J. Nutr. 2019, 149, 716–722. [Google Scholar] [CrossRef]
- Iverson, J.L.; Sheppard, A.J. Butyl ester preparation for gas-liquid chromatographic determination of fatty acids in butter. J. Assoc. Off. Anal. Chem. 1977, 60, 284–288. [Google Scholar] [CrossRef]
- Manoni, M.; Di Lorenzo, C.; Ottoboni, M.; Tretola, M.; Pinotti, L. Comparative proteomics of milk fat globule membrane (MFGM) proteome across species and lactation stages and the potentials of MFGM fractions in infant formula preparation. Foods 2020, 9, 1251. [Google Scholar] [CrossRef]
- ICH Expert Working Group. ICH Harmonized Tripartite Guideline, Validation of Analytical Procedures: Text and Metodology Q2; U.S. Department of Health and Human Services, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research: Geneva, Switzerland, 2005.
- Rahman, M.N.; Diantini, A.; Fattah, M.; Barliana, M.I.; Wijaya, A. A highly sensitive, simple, and fast gas chromatography-mass spectrometry method for the quantification of serum short-chain fatty acids and their potential features in central obesity. Anal. Bioanal. Chem. 2021, 413, 6837–6844. [Google Scholar] [CrossRef]
- Eberhart, B.L., 2nd; Wilson, A.S.; O’Keefe, S.J.D.; Ramaboli, M.C.; Nesengani, L.T. A simplified method for the quantitation of short-chain fatty acids in human stool. Anal. Biochem. 2021, 612, 114016. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.; Guang-Yu, F.; Tong, L.; Feng, Z. Determination of short-chain fatty acids in well and rock salt by headspace gas chromatography-mass spectrometry. Chin. J. Anal. Chem. 2019, 2, 244–248. [Google Scholar] [CrossRef]
- Fox, P.F.; Lowe, T.U.; McSweeney, P.L.H.; O’Mahony, J.A. Dairy Chemistry and Biochemistry; Springer International: Cham/Heidelberg, Germany; New York, NY, USA; Dordrecht, The Netherlands; London, UK; Cham, Switzerland, 2015. [Google Scholar]
- Liu, Z.; Rochfort, S.; Cocks, B. Milk lipidomics: What we know and what we don’t. Prog. Lipid Res. 2018, 71, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.-H.; Choong, Y.-M. A rapid gas chromatographic method for direct determination of short-chain (C2–C12) volatile organic acids in foods. Food Chem. 2001, 75, 101–108. [Google Scholar] [CrossRef]
| Compounds | Window No. | Start Time (Min) | Retention Time (Min) | Quantitative Ion (m/z) | Qualitative Ion (m/z) | Dwell Time (ms) | ||
|---|---|---|---|---|---|---|---|---|
| C2:0 | 1 | 4.5 | 8.077 | 43 | 60 | 28 | 45 | 10 |
| C1:0 | 8.900 | 46 | 43 | 28 | 29 | |||
| C3:0 | 9.119 | 74 | 28 | 45 | 57 | |||
| iso C4:0 | 2 | 9.3 | 9.452 | 43 | 73 | 88 | 60 | 10 |
| C4:0 | 10.172 | 60 | 73 | 43 | 88 | |||
| iso C5:0/anteiso C5:0 | 3 | 10.5 | 10.641 | 43 | 87 | 60 | 74 | 10 |
| C5:0 | 11.416 | 60 | 43 | 55 | 74 | |||
| iso C6:0 | 4 | 11.6 | 11.720 | 74 | 87 | 43 | 55 | 10 |
| C6:0 | 12.579 | 60 | 43 | 73 | 55 | |||
| IS 1 | 12.006 | 60 | 43 | 87 | 55 | |||
| Compounds | Regression Equation | Linearity Range (μg mL−1) | R2 1 | LOD (μg mL−1) 1 | LOQ (μg mL−1) 1 |
|---|---|---|---|---|---|
| C2:0 | y = 0.4641x + 0.0292 | 1~200 | 0.9992 | 0.375 | 1.250 |
| C1:0 | y = 0.2505x + 0.0041 | 1~200 | 0.9998 | 0.068 | 0.227 |
| C3:0 | y = 0.5629x + 0.0265 | 1~200 | 0.9990 | 0.050 | 0.167 |
| iso C4:0 | y = 0.8367x + 0.0358 | 1~200 | 0.9992 | 0.100 | 0.333 |
| C4:0 | y = 1.7464x + 0.0627 | 1~200 | 0.9994 | 0.064 | 0.213 |
| iso C5:0/anteiso C5:0 | y = 0.3631x + 0.0225 | 1~200 | 0.9990 | 0.140 | 0.465 |
| C5:0 | y = 1.2557x + 0.0364 | 1~200 | 0.9996 | 0.107 | 0.357 |
| iso C6:0 | y = 1.6426x + 0.0808 | 1~200 | 0.9995 | 0.167 | 0.556 |
| C6:0 | y = 1.1568x + 0.0337 | 1~200 | 0.9997 | 0.171 | 0.571 |
| Compounds | Spiked Level | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 µg mL−1 | 50 µg mL−1 | 200 µg mL−1 | ||||||||||
| R 1 | % CV | % RSD 1 1 | %RSD 2 1 | R | % CV | % RSD 1 | %RSD2 | R | % CV | % RSD 1 | % RSD2 | |
| C2:0 | 116.26 | 6.35 | 3.17 | 1.52 | 103.01 | 1.40 | 1.21 | 1.66 | 98.81 | 2.32 | 7.23 | 0.99 |
| C1:0 | 126.42 | 3.61 | 7.94 | 8.83 | 89.91 | 10.40 | 3.47 | 8.04 | 104.21 | 2.81 | 8.68 | 2.63 |
| C3:0 | 86.75 | 3.53 | 1.58 | 1.29 | 91.76 | 2.45 | 1.32 | 0.56 | 89.38 | 12.15 | 2.19 | 3.78 |
| iso C4:0 | 86.35 | 3.94 | 1.29 | 2.85 | 96.67 | 2.18 | 1.56 | 0.85 | 97.07 | 2.29 | 2.27 | 1.07 |
| C4:0 | 88.10 | 2.75 | 1.68 | 2.19 | 94.04 | 2.07 | 1.02 | 0.95 | 97.14 | 1.73 | 1.73 | 0.71 |
| iso C5:0/anteiso C5:0 | 85.62 | 5.43 | 1.73 | 6.79 | 102.35 | 2.23 | 1.45 | 1.32 | 98.99 | 1.92 | 3.93 | 1.22 |
| C5:0 | 88.97 | 3.09 | 1.42 | 3.36 | 94.94 | 1.41 | 1.01 | 1.34 | 98.86 | 1.51 | 2.92 | 3.15 |
| iso C6:0 | 86.46 | 9.64 | 1.50 | 9.09 | 97.02 | 2.62 | 1.49 | 2.58 | 99.95 | 2.72 | 5.22 | 1.97 |
| C6:0 | 88.11 | 3.97 | 0.92 | 3.78 | 95.86 | 1.77 | 1.64 | 1.71 | 99.54 | 1.98 | 3.69 | 1.21 |
| C2:0 TAG 2 | ND | ND | / | / | ND | ND | / | / | ND | ND | / | / |
| C4:0 TAG | ND | ND | / | / | ND | ND | / | / | ND | ND | / | / |
| C6:0 TAG | ND | ND | / | / | ND | ND | / | / | ND | ND | / | / |
| Compounds | Content (µg mL−1) | % of Total FSCFAs |
|---|---|---|
| C2:0 | 3.51 | 23.99 |
| C1:0 | ND 1 | 0.00 |
| C3:0 | ND | 0.00 |
| iso C4:0 | ND | 0.00 |
| C4:0 | 6.40 | 43.77 |
| iso C5:0/anteiso C5:0 | ND | 0.00 |
| C5:0 | ND | 0.00 |
| iso C6:0 | ND | 0.00 |
| C6:0 | 4.72 | 32.24 |
| Sample | Sample Preparation | Instrument Method | Quantification | Samples FSCFAs Species | Reference | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Derivatization | IS | Reagents | Preparation Time | Run Time | FSCFAs Number | LOQ (μg mL−1) | ||||
| cow milk | yes | \ | Boron trifluoride butanol | long | 34.67 min | 2 | 20 | external quantification | C4:0, C6:0 | [21] |
| human milk | no | 2-ethylbutyric acid | hydrochloric acid/ethanol | short | 14 min | 6 | \ | semi-quantitative | C1:0, C2:0, C3:0, iso C4:0, C4:0, C6:0 | [27] |
| human milk | no | \ | \ | short | 15 min | 1 | \ | external quantification | C4:0 | [28] |
| cow milk | yes | Isotope | ethyl chloroformate, ethanol, pyridine | long | 50 min | 2 | 0.067 | IS quantitative | C4:0, C6:0 | [26] |
| cow milk | no | 1,3-butanediol | \ | short | 28.5 min | 7 | \ | relative response factor | C2:0, C3:0, iso C5:0, C6:0 | [37] |
| cow milk | no | anteiso C6:0 | hydrochloric acid/ethanol | short | 26 min | 10 | 0.167–1.250 | IS quantitative | C2:0, C4:0, C6:0 | current |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Wang, F.; Chen, M.; Wang, J.; Zhang, Y. Quantification of Free Short-Chain Fatty Acids in Raw Cow Milk by Gas Chromatography-Mass Spectrometry. Foods 2023, 12, 1367. https://doi.org/10.3390/foods12071367
Wu X, Wang F, Chen M, Wang J, Zhang Y. Quantification of Free Short-Chain Fatty Acids in Raw Cow Milk by Gas Chromatography-Mass Spectrometry. Foods. 2023; 12(7):1367. https://doi.org/10.3390/foods12071367
Chicago/Turabian StyleWu, Xufang, Fengen Wang, Meiqing Chen, Jiaqi Wang, and Yangdong Zhang. 2023. "Quantification of Free Short-Chain Fatty Acids in Raw Cow Milk by Gas Chromatography-Mass Spectrometry" Foods 12, no. 7: 1367. https://doi.org/10.3390/foods12071367
APA StyleWu, X., Wang, F., Chen, M., Wang, J., & Zhang, Y. (2023). Quantification of Free Short-Chain Fatty Acids in Raw Cow Milk by Gas Chromatography-Mass Spectrometry. Foods, 12(7), 1367. https://doi.org/10.3390/foods12071367







