Improving the Yield of Feruloyl Oligosaccharides from Rice Bran through Enzymatic Extrusion and Its Mechanism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
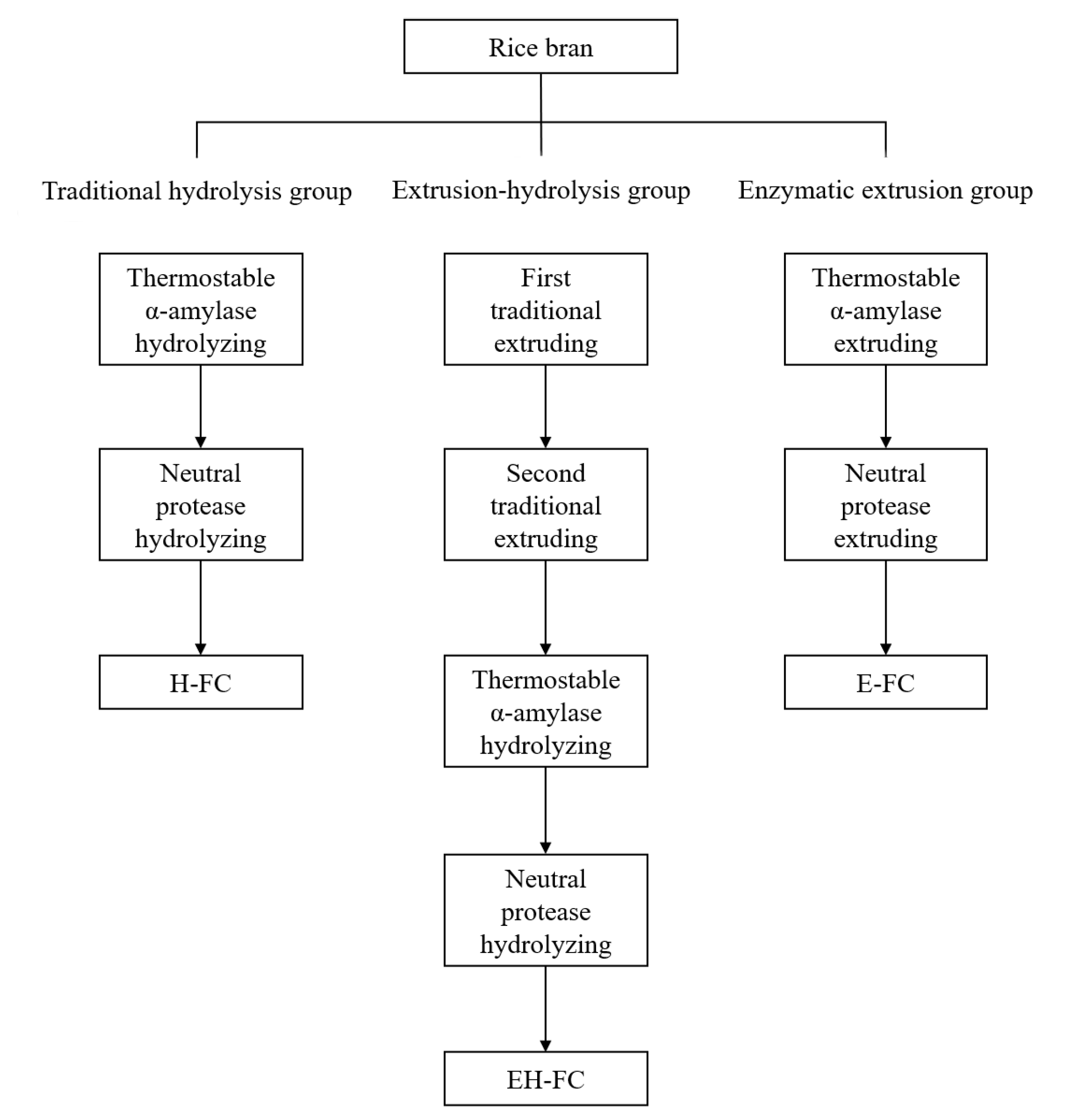
2.2. Pre-Treatment of Rice Bran
2.3. Xylanase Hydrolysis of Fiber Concentrates
2.4. Purification of FOs
2.5. Composition Analysis
2.6. Light Microscopy Analysis
2.7. Scanning Electron Microscopy (SEM) Analysis
2.8. Characterization of FOs
2.9. Optimization of Enzymatic Extrusion
2.10. Statistical Analysis
3. Results and Discussion
3.1. Composition of Rice Bran and Fiber Concentrates
3.2. The Yields of Ferulic Acid and Pentose in FOs
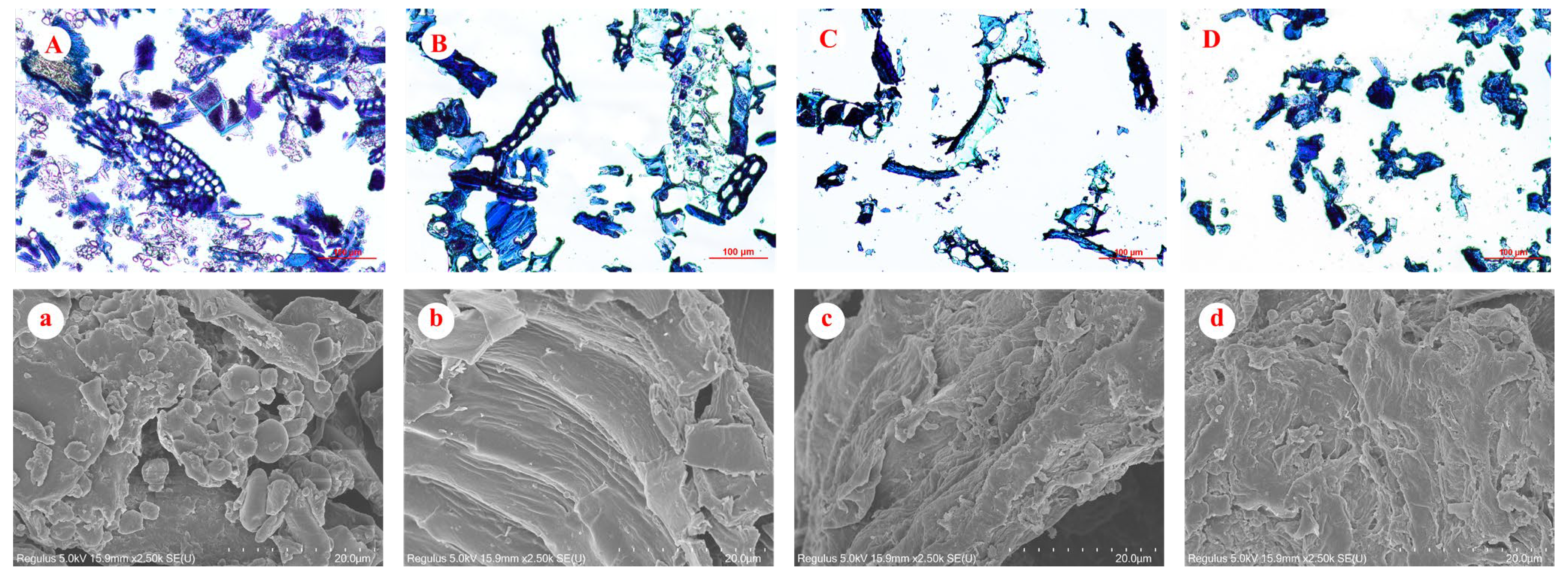
3.3. Microstructure of Rice Bran and Fiber Concentrates
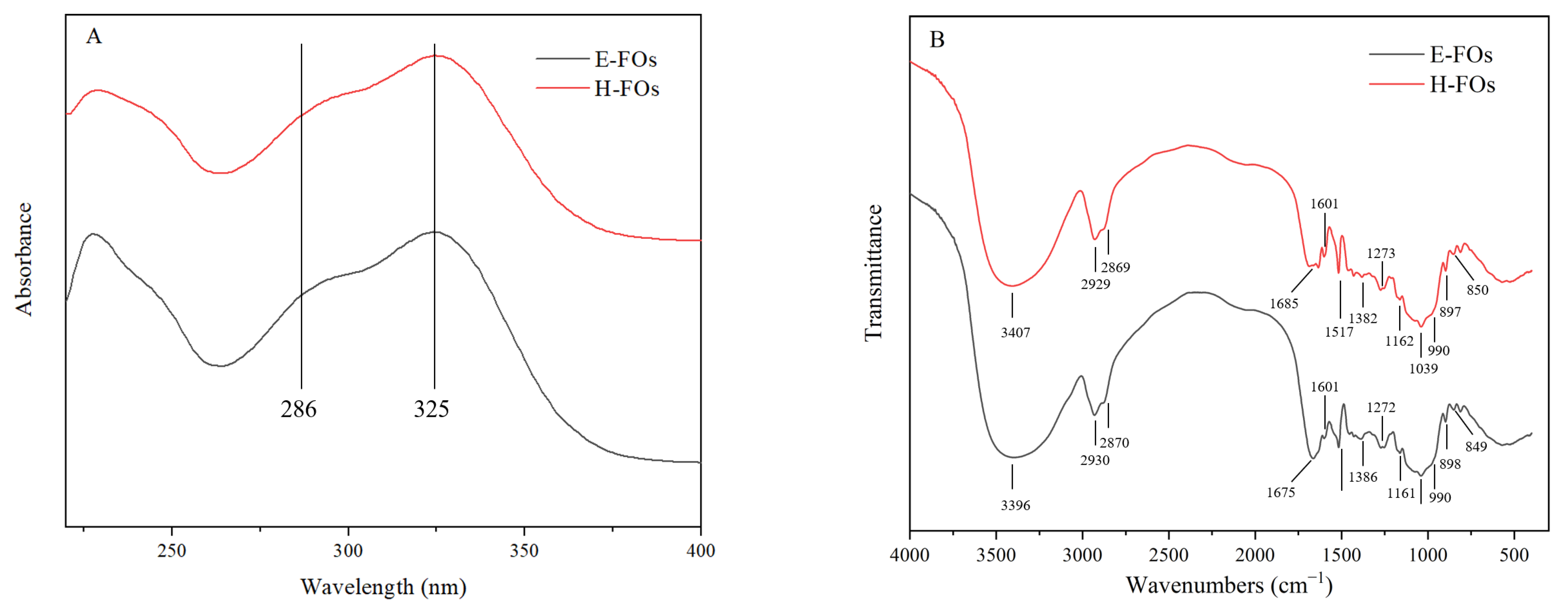
3.4. Characterization of Purified FOs from H-FC and E-FC
3.5. Optimization of Enzymatic Extrusion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Alan, P.A.; Ofelia, R.S.; Patricia, T.; Maribel, R.R. Cereal bran and wholegrain as a source of dietary fibre: Technological and health aspects. Int. J. Food Sci. Nutr. 2012, 63, 882–892. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Bai, J.; Fan, M.; Li, T.; Li, Y.; Qian, H.; Wang, L.; Zhang, H.; Qi, X.; Rao, Z. Cereal-derived arabinoxylans: Structural features and structure–activity correlations. Trends Food Sci. Technol. 2020, 96, 157–165. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Y.; Yang, L.; Peng, X.; Zheng, J.; Ou, S. Effect of maize bran feruloylated oligosaccharides on the formation of endogenous contaminants and the appearance and textural properties of biscuits. Food Chem. 2018, 245, 974–980. [Google Scholar] [CrossRef]
- Saeed, F.; Pasha, I.; Anjum, F.M.; Sultan, M.T. Arabinoxylans and arabinogalactans: A comprehensive treatise. Crit. Rev. Food Sci. Nutr. 2011, 51, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; Abraham, T.E. Ferulic acid: An antioxidant found naturally in plant cell walls and feruloyl esterases involved in its release and their applications. Crit. Rev. Biotechnol. 2004, 24, 59–83. [Google Scholar] [CrossRef]
- Schendel, R.R.; Becker, A.; Tyl, C.E.; Bunzel, M. Isolation and characterization of feruloylated arabinoxylan oligosaccharides from the perennial cereal grain intermediate wheat grass (Thinopyrum intermedium). Carbohydr. Res. 2015, 407, 16–25. [Google Scholar] [CrossRef]
- Zhao, W.; Chen, H.; Wu, L.; Ma, W.; Xie, Y. Antioxidant properties of feruloylated oligosaccharides of different degrees of polymerization from wheat bran. Glycoconj. J. 2018, 35, 547–559. [Google Scholar] [CrossRef]
- Yuan, X.P.; Wang, J.; Yao, H.Y. Feruloyl oligosaccharides stimulate the growth of Bifidobacterium bifidum. Anaerobe 2005, 11, 225–229. [Google Scholar] [CrossRef]
- Fang, H.Y.; Chen, Y.K.; Chen, H.H.; Lin, S.Y.; Fang, Y.T. Immunomodulatory effects of feruloylated oligosaccharides from rice bran. Food Chem. 2012, 134, 836–840. [Google Scholar] [CrossRef]
- Gottschalk, L.M.F.; Oliveira, R.A.; Bon, E.P.d.S. Cellulases, xylanases, β-glucosidase and ferulic acid esterase produced by Trichoderma and Aspergillus act synergistically in the hydrolysis of sugarcane bagasse. Biochem. Eng. J. 2010, 51, 72–78. [Google Scholar] [CrossRef]
- Rose, D.J.; Inglett, G.E. Production of feruloylated arabinoxylo-oligosaccharides from maize (Zea mays) bran by microwave-assisted autohydrolysis. Food Chem. 2010, 119, 1613–1618. [Google Scholar] [CrossRef]
- Hromadkova, Z.; Kost’alova, Z.; Ebringerova, A. Comparison of conventional and ultrasound-assisted extraction of phenolics-rich heteroxylans from wheat bran. Ultrason. Sonochem. 2008, 15, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.P.; Wang, J.; Yao, H.Y. Production of feruloyl oligosaccharides from wheat bran insoluble dietary fibre by xylanases from Bacillus subtilis. Food Chem. 2006, 95, 484–492. [Google Scholar] [CrossRef]
- Zeng, Z.; Li, Y.; Yang, R.; Liu, C.; Hu, X.; Luo, S.; Gong, E.; Ye, J. The relationship between reducing sugars and phenolic retention of brown rice after enzymatic extrusion. J. Cereal Sci. 2017, 74, 244–249. [Google Scholar] [CrossRef]
- Xu, E.; Campanella, O.H.; Ye, X.; Jin, Z.; Liu, D.; BeMiller, J.N. Advances in conversion of natural biopolymers: A reactive extrusion (REX)–enzyme-combined strategy for starch/protein-based food processing. Trends Food Sci. Technol. 2020, 99, 167–180. [Google Scholar] [CrossRef]
- Komolprasert, V.; Ofoli, R.Y. A dispersion model for predicting the extent of starch liquefaction by Bacillus licheniformis α-amylase during reactive extrusion. Biotechnol. Bioeng. 1991, 37, 681–690. [Google Scholar] [CrossRef]
- Duque, A.; Doménech, P.; Álvarez, C.; Ballesteros, M.; Manzanares, P. Study of the bioprocess conditions to produce bioethanol from barley straw pretreated by combined soda and enzyme-catalyzed extrusion. Renew. Energy 2020, 158, 263–270. [Google Scholar] [CrossRef]
- De Pilli, T.; Legrand, J.; Giuliani, R.; Derossi, A.; Severini, C. Effect of Processing Variables and Enzymatic Activity on Wheat Flour Dough Extruded Under Different Operating Conditions. Food Technol. Biotechnol. 2010, 47, 404. [Google Scholar]
- Li, K.Y.; Lai, P.; Lu, S.; Fang, Y.T.; Chen, H.H. Optimization of acid hydrolysis conditions for feruloylated oligosaccharides from rice bran through response surface methodology. J. Agric. Food Chem. 2008, 56, 8975–8978. [Google Scholar] [CrossRef]
- Saulnier, L.; Vigouroux, J.; Thibault, J.-F. Isolation and partial characterization of feruloylated oligosaccharides from maize bran. Carbohydr. Res. 1995, 272, 241–253. [Google Scholar] [CrossRef]
- Douglas, S.G. A rapid method for the determination of pentosans in wheat flour. Food Chem. 1981, 7, 139–145. [Google Scholar] [CrossRef]
- Dang, T.T.; Vasanthan, T. Modification of rice bran dietary fiber concentrates using enzyme and extrusion cooking. Food Hydrocoll. 2019, 89, 773–782. [Google Scholar] [CrossRef]
- Linko, P.; Hakulin, S.; Linko, Y.Y. Extrusion cooking of barley starch for the production of glucose syrup and ethanol. J. Cereal Sci. 1983, 1, 275–284. [Google Scholar] [CrossRef]
- Baks, T.; Kappen, F.H.J.; Janssen, A.E.M.; Boom, R.M. Towards an optimal process for gelatinisation and hydrolysis of highly concentrated starch–water mixtures with alpha-amylase from B. licheniformis. J. Cereal Sci. 2008, 47, 214–225. [Google Scholar] [CrossRef]
- Esposito, F.; Arlotti, G.; Maria Bonifati, A.; Napolitano, A.; Vitale, D.; Fogliano, V. Antioxidant activity and dietary fibre in durum wheat bran by-products. Food Res. Int. 2005, 38, 1167–1173. [Google Scholar] [CrossRef]
- Acosta-Estrada, B.A.; Gutierrez-Uribe, J.A.; Serna-Saldivar, S.O. Bound phenolics in foods, a review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Xu, E.; Wu, Z.; Jiao, A.; Long, J.; Li, J.; Jin, Z. Dynamics of rapid starch gelatinization and total phenolic thermomechanical destruction moderated via rice bio-extrusion with alpha-amylase activation. RSC Adv. 2017, 7, 19464–19478. [Google Scholar] [CrossRef] [Green Version]
- Santala, O.; Nordlund, E.; Poutanen, K. Use of an extruder for pre-mixing enhances xylanase action on wheat bran at low water content. Bioresour. Technol. 2013, 149, 191–199. [Google Scholar] [CrossRef]
- Coda, R.; Katina, K.; Rizzello, C.G. Bran bioprocessing for enhanced functional properties. Curr. Opin. Food Sci. 2015, 1, 50–55. [Google Scholar] [CrossRef]
- Bunzel, M.; Allerdings, E.; Sinwell, V.; Ralph, J.; Steinhart, H. Cell wall hydroxycinnamates in wild rice (Zizania aquatica L.) insoluble dietary fibre. Eur. Food Res. Technol. 2002, 214, 482–488. [Google Scholar] [CrossRef]
- Truong, K.T.P.; Rumpagaporn, P. Oligosaccharides preparation from rice bran arabinoxylan by two different commercial endoxylanase enzymes. J. Nutr. Sci. Vitaminol. 2019, 65, S171–S174. [Google Scholar] [CrossRef] [Green Version]
- Schendel, R.R.; Meyer, M.R.; Bunzel, M. Quantitative profiling of feruloylated arabinoxylan side-chains from graminaceous cell walls. Front. Plant Sci. 2015, 6, 1249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lefsih, K.; Delattre, C.; Pierre, G.; Michaud, P.; Aminabhavi, T.M.; Dahmoune, F.; Madani, K. Extraction, characterization and gelling behavior enhancement of pectins from the cladodes of Opuntia ficus indica. Int. J. Biol. Macromol. 2016, 82, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yuan, X.; Sun, B.; Cao, Y.; Tian, Y.; Wang, C. On-line separation and structural characterisation of feruloylated oligosaccharides from wheat bran using HPLC-ESI-MSn. Food Chem. 2009, 115, 1529–1541. [Google Scholar] [CrossRef]
- Robert, P.; Marquis, M.; Barron, C.; Guillon, F.; Saulnier, L. FT-IR investigation of cell wall polysaccharides from cereal grains. Arabinoxylan infrared assignment. J. Agric. Food Chem. 2005, 53, 7014–7018. [Google Scholar] [CrossRef]
- Chaikumpollert, O. Structural elucidation of hemicelluloses from Vetiver grass. Carbohydr. Polym. 2004, 57, 191–196. [Google Scholar] [CrossRef]
- Redgwell, R.J.; Curti, D.; Robin, F.; Donato, L.; Pineau, N. Extrusion-induced changes to the chemical profile and viscosity generating properties of citrus fiber. J. Agric. Food Chem. 2011, 59, 8272–8279. [Google Scholar] [CrossRef]
- Jeon, S.J.; Singkhornart, S.; Ryu, G.H. The effect of extrusion conditions on water-extractable arabinoxylans from corn fiber. Prev. Nutr. Food Sci. 2014, 19, 124–127. [Google Scholar] [CrossRef] [Green Version]
- Fadel, A.; Plunkett, A.; Ashworth, J.; Mahmoud, A.M.; Ranneh, Y.; El Mohtadi, M.; Li, W. The effect of extrusion screw-speed on the water extractability and molecular weight distribution of arabinoxylans from defatted rice bran. J. Food Sci. Technol. 2018, 55, 1201–1206. [Google Scholar] [CrossRef]


| Samples | Starch | Protein | Ferulic Acid | Pentose |
|---|---|---|---|---|
| Rice bran | 10.30 ± 0.14 a | 16.19 ± 0.21 a | 0.46 ± 0.02 c | 26.09 ± 0.64 d |
| H-FC | 0.21 ± 0.01 c | 9.07 ± 0.09 d | 0.71 ± 0.03 b | 52.06 ± 2.91 a |
| EH-FC | 0.54 ± 0.01 b | 10.54 ± 0.12 c | 0.72 ± 0.01 b | 37.06 ± 1.61 c |
| E-FC | 0.32 ± 0.03 c | 11.64 ± 0.18 b | 0.78 ± 0.02 a | 39.40 ± 1.97 b |
| Samples | The Yield of Purified FOs (%) | The Ferulic Acid Content (%) |
|---|---|---|
| H-FOs | 4.25 ± 0.29 b | 7.66 ± 1.68 a |
| E-FOs | 5.79 ± 0.09 a | 7.54 ± 0.29 a |
| Sample | Starch | Protein | Ferulic Acid | Pentose |
|---|---|---|---|---|
| H-FC (control) | 0.21 ± 0.01 c | 9.07 ± 0.09 d | 0.71 ± 0.03 b | 55.43 ± 2.91 a |
| 30/50 * | 0.33 ± 0.01 bc | 13.36 ± 0.36 b | 0.76 ± 0.01 ab | 41.38 ± 2.27 a |
| 30/100 | 0.39 ± 0.02 a | 12.69 ± 0.03 c | 0.72 ± 0.02 cd | 41.29 ± 0.66 a |
| 30/150 | 0.43 ± 0.01 a | 13.99 ± 0.02 a | 0.71 ± 0.03 d | 39.45 ± 1.77 ab |
| 40/50 | 0.32 ± 0.03 bc | 11.64 ± 0.18 e | 0.78 ± 0.02 ab | 41.42 ± 1.21 a |
| 40/100 | 0.31 ± 0.03 c | 12.31 ± 0.08 d | 0.80 ± 0.02 a | 41.17 ± 2.76 a |
| 40/150 | 0.35 ± 0.03 b | 12.89 ± 0.09 c | 0.75 ± 0.01 bc | 36.46 ± 1.66 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, F.; Hu, X.; Wang, Y.; Luo, S.; Liu, C. Improving the Yield of Feruloyl Oligosaccharides from Rice Bran through Enzymatic Extrusion and Its Mechanism. Foods 2023, 12, 1369. https://doi.org/10.3390/foods12071369
Deng F, Hu X, Wang Y, Luo S, Liu C. Improving the Yield of Feruloyl Oligosaccharides from Rice Bran through Enzymatic Extrusion and Its Mechanism. Foods. 2023; 12(7):1369. https://doi.org/10.3390/foods12071369
Chicago/Turabian StyleDeng, Fenghong, Xiuting Hu, Yueru Wang, Shunjing Luo, and Chengmei Liu. 2023. "Improving the Yield of Feruloyl Oligosaccharides from Rice Bran through Enzymatic Extrusion and Its Mechanism" Foods 12, no. 7: 1369. https://doi.org/10.3390/foods12071369




