Burn Defect and Phenol Prediction for Flavoured Californian-Style Black Olives Using Digital Sensors
Abstract
:1. Introduction
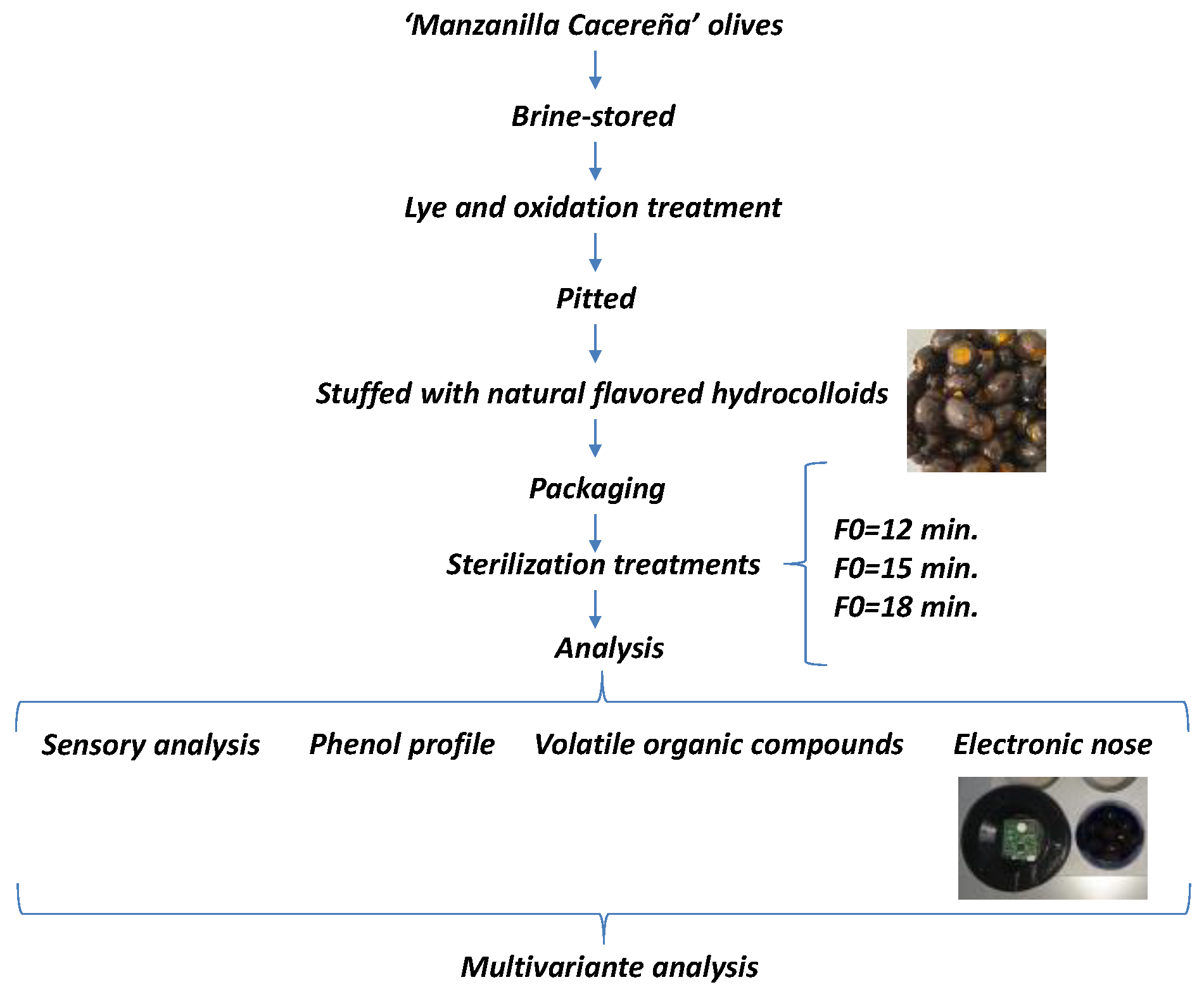
2. Materials and Methods
2.1. Reagents
2.2. Raw Material
2.3. Californian-Style Black Olive Production Process
2.4. Hydrocolloid Preparation
2.5. Accumulated Lethality Values
2.6. Analysis
2.6.1. Sensory Analysis
2.6.2. Phenol Profile Analysis
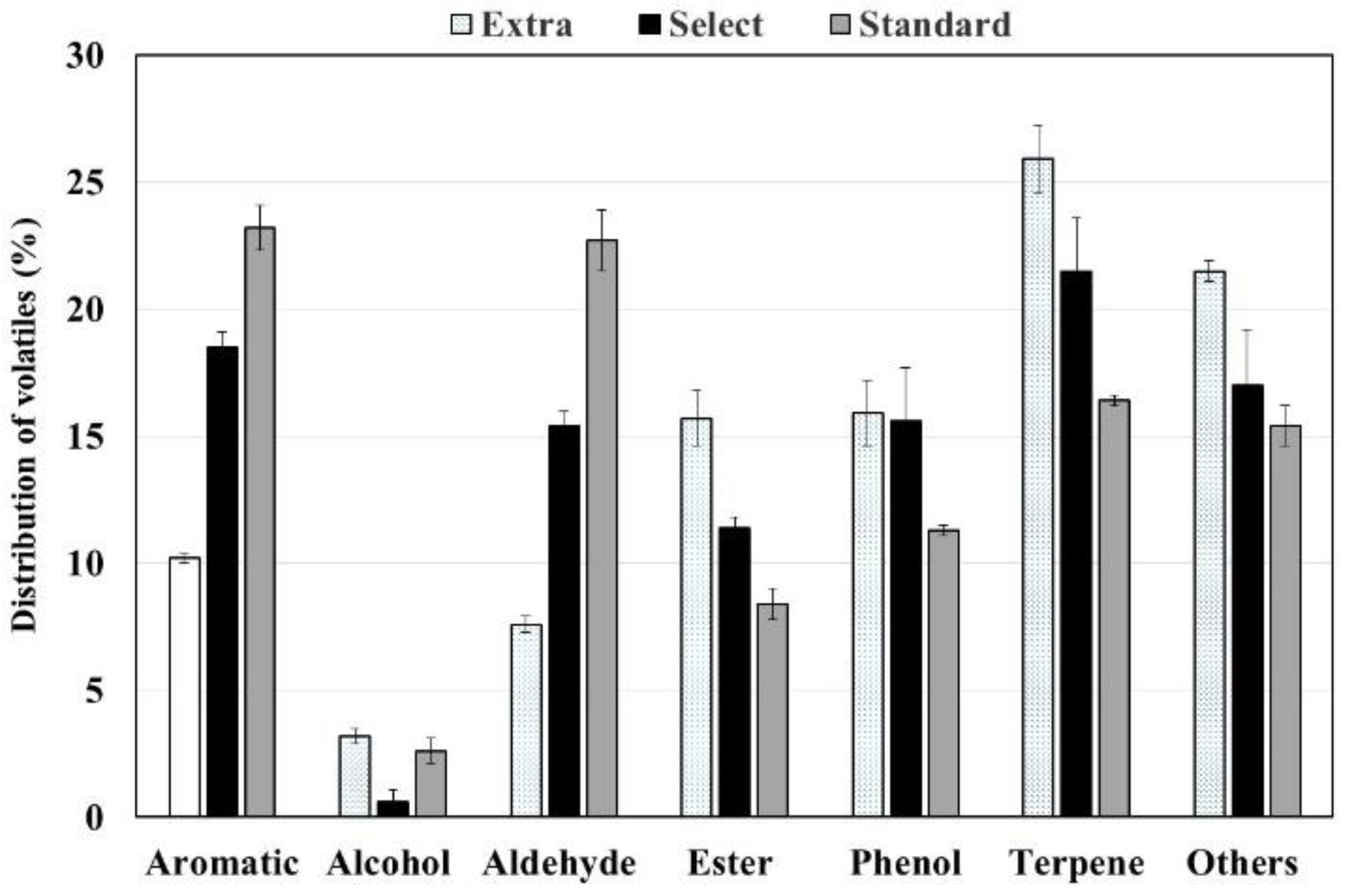
2.6.3. Volatile Organic Compounds
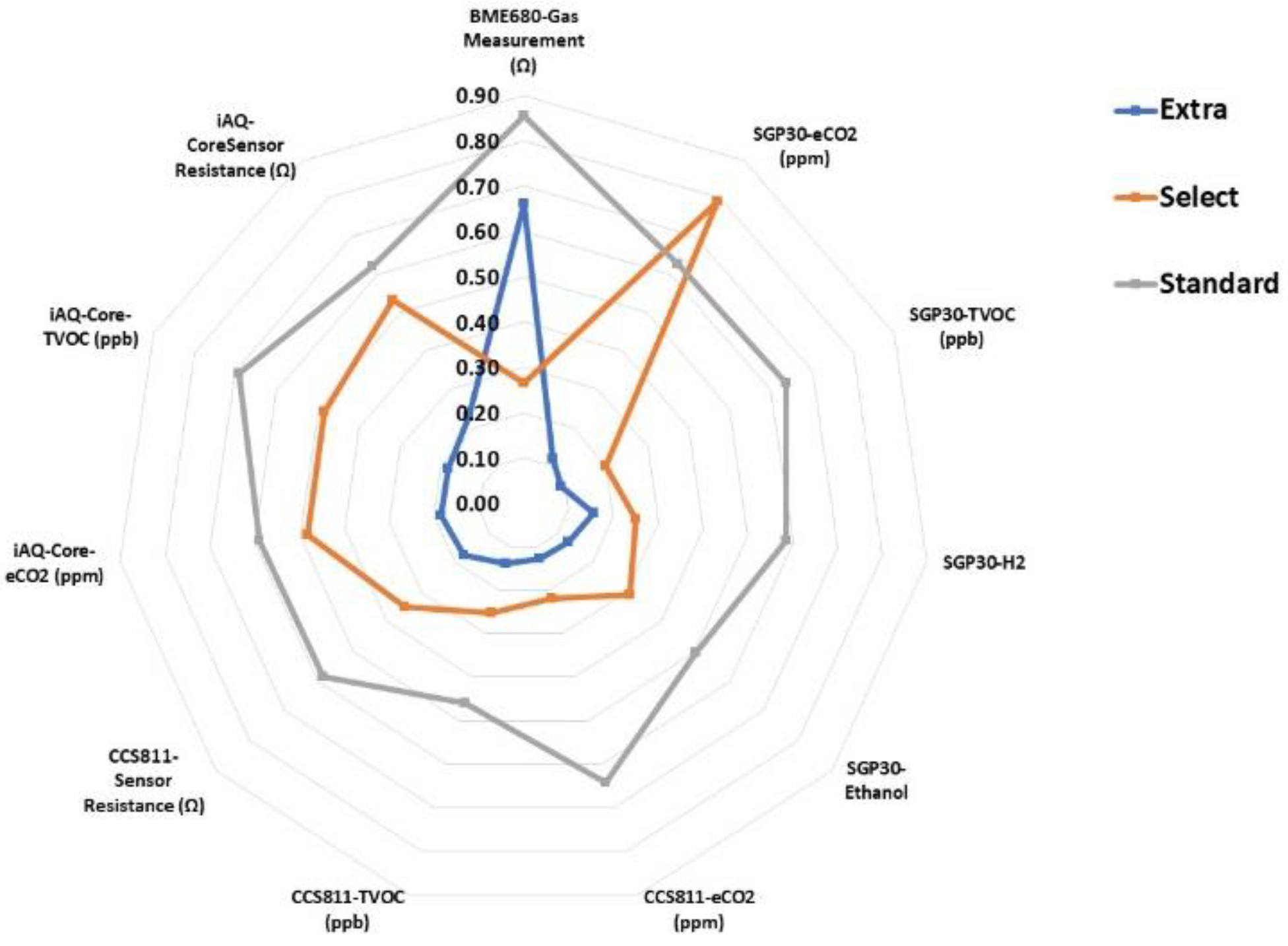
2.6.4. Electronic Device
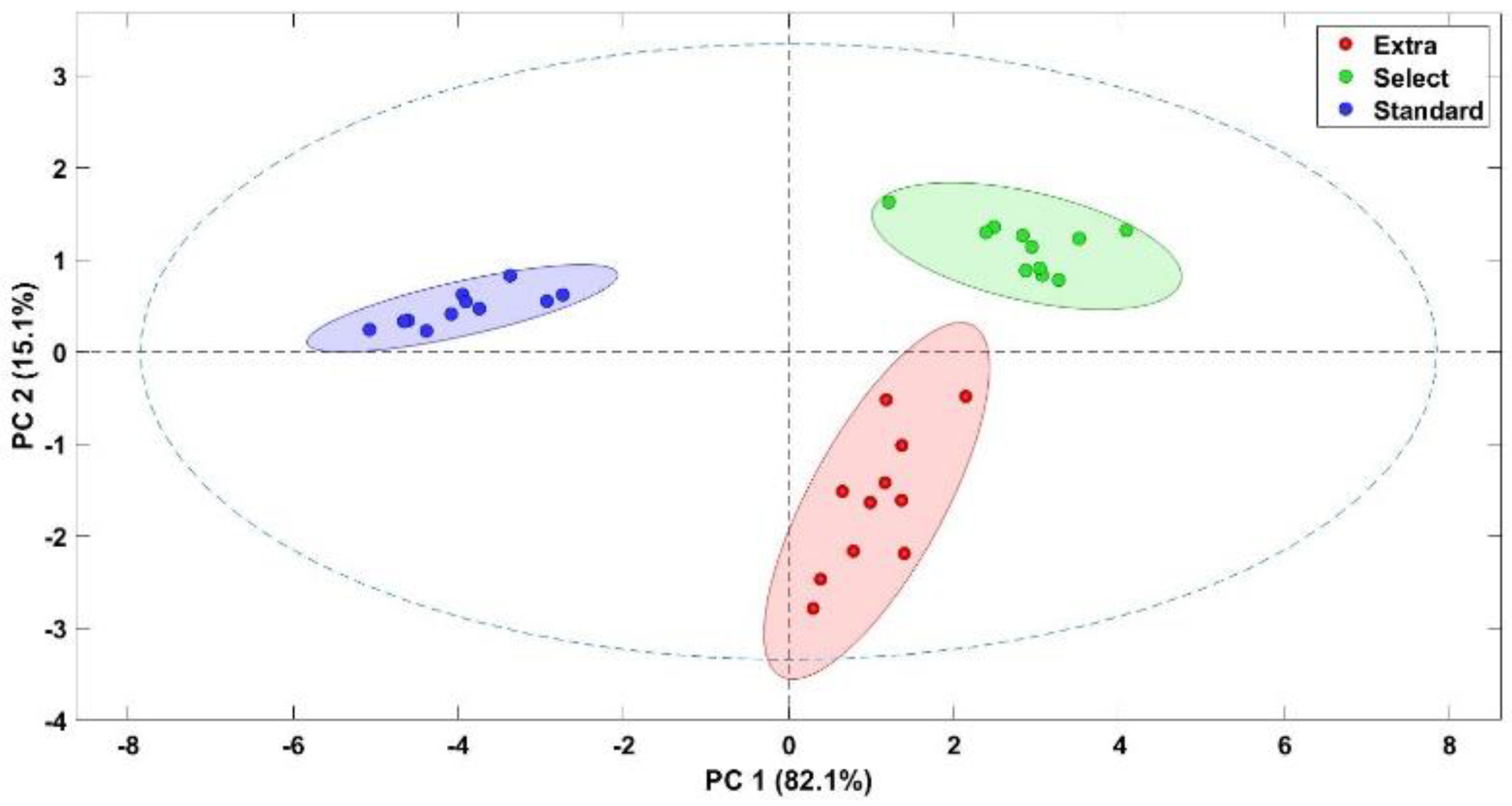
2.7. Multivariate Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Sensory Odour Characteristics of Californian-Style Black Olives Stuffed with Hydrocolloids
3.2. Phenol Profiles of Californian-Style Black Olives Stuffed with Hydrocolloids
3.3. Discrimination of Olive Aroma Categories Using an Electronic Device
3.4. Aromas of Californian-Style Black Olives Stuffed with Hydrocolloids
3.5. Burn Defect and Phenol Profile Quantification Using an Electronic Device
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rocha, J.; Borges, N.; Pinho, O. Table olives and health: A review. J. Nutr. Sci. 2020, 9, e57. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, R.; Martín-Tornero, E.; Lozano, J.; Fernández, A.; Arroyo, P.; Meléndez, F.; Martín-Vertedor, D. Electronic nose application for the discrimination of sterilization treatments applied to Californian-style black olive varieties. J. Sci. Food Agric. 2022, 102, 2232–2241. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Gómez, A.H.; García García, P.; Rejano Navarro, L. Elaboration of table olives. Grasas Aceites 2006, 57, 86–94. [Google Scholar] [CrossRef] [Green Version]
- Sánchez, R.; Martín-Tornero, E.; Lozano, J.; Arroyo, P.; Meléndez, F.; Martín-Vertedor, D. Evaluation of the olfactory pattern of black olives stuffed with flavored hydrocolloids. LWT 2022, 163, 113556. [Google Scholar] [CrossRef]
- Tang, S.; Avena-Bustillos, R.J.; Lear, M.; Sedej, I.; Holstege, D.M.; Friedman, M.; McHugh, T.H.; Wang, S.C. Evaluation of thermal processing variables for reducing acrylamide in canned black ripe olives. J. Food Eng. 2016, 191, 124–130. [Google Scholar] [CrossRef] [Green Version]
- Martín-Vertedor, D.; Rodrigues, N.; Marx, Í.M.; Veloso, A.C.; Peres, A.M.; Pereira, J.A. Impact of thermal sterilization on the physicochemical-sensory characteristics of Californian-style black olives and its assessment using an electronic tongue. Food Control 2020, 117, 107369. [Google Scholar] [CrossRef]
- Royal Degree 679/2016; Norma de Calidad de las Aceitunas de Mesa. Boletín Oficial del Estado: Marid, Spain, 2016; pp. 88525–88533.
- International Olive Council (IOC). Method Sensory Analysis of Table Olives. 2021. COI/OT/MO No 1/Rev.2 November 2021. Available online: https://www.internationaloliveoil.org (accessed on 5 February 2022).
- Sánchez, R.; Fernández, A.; Martín-Tornero, E.; Meléndez, F.; Lozano, J.; Martín-Vertedor, D. Application of Digital Olfaction for Table Olive Industry. Sensors 2022, 22, 5702. [Google Scholar] [CrossRef]
- Lodolini, E.M.; Cabrera-Bañegil, M.; Fernández, A.; Delgado-Adámez, J.; Ramírez, R.; Martín-Vertedor, D. Monitoring of Acrylamide and Phenolic Compounds in Table Olive after High Hydrostatic Pressure and Cooking Treatments. Food Chem. 2019, 286, 250–259. [Google Scholar] [CrossRef]
- Zhang, K.; Cheng, J.; Hong, Q.; Dong, W.; Chen, X.; Wu, G.; Zhang, Z. Identification of changes in the volatile compounds of robusta coffee beans during drying based on HS-SPME/GC-MS and E-nose analyses with the aid of chemometrics. LWT 2022, 161, 113317. [Google Scholar] [CrossRef]
- Gómez, A.H.; Wang, J.; Hu, G.; Pereira, A.G. Monitoring storage shelf life of tomato using electronic nose technique. J. Food Eng. 2008, 85, 625–631. [Google Scholar] [CrossRef]
- Álvarez-Ortí, M.; Pardo, J.E.; Cascos, G.; Sánchez, R.; Lozano, J.; Martín-Vertedor, D. E-Nose Discrimination of Almond Oils Extracted from Roasted Kernels. Nutrients 2023, 15, 130. [Google Scholar] [CrossRef]
- Martínez-García, R.; Moreno, J.; Bellincontro, A.; Centioni, L.; Puig-Pujol, A.; Peinado, R.A.; Mauricio, J.C.; García-Martínez, T. Using an electronic nose and volatilome analysis to differentiate sparkling wines obtained under different conditions of temperature, ageing time and yeast formats. Food Chem. 2021, 334, 127574. [Google Scholar] [CrossRef]
- Cabrera-Bañegil, M.; Schaide, T.; Manzano, R.; Delgado-Adámez, J.; Durán-Merás, I.; Martín-Vertedor, D. Optimization and validation of a rapid liquid chromatography method for determination of the main polyphenolic compounds in table olives and in olive paste. Food Chem. 2017, 233, 164–173. [Google Scholar] [CrossRef]
- Arroyo, P.; Meléndez, F.; Suárez, J.I.; Herrero, J.L.; Rodríguez, S.; Lozano, J. Electronic Nose with Digital Gas Sensors Connected via Bluetooth to a Smartphone for Air Quality Measurements. Sensors 2020, 20, 786. [Google Scholar] [CrossRef] [Green Version]
- Wold, S.; Esbensen, K.; Geladi, P. Principal component analysis. Chemom. Intell. Lab. Syst. 1987, 2, 37–52. [Google Scholar] [CrossRef]
- Barker, M.; Rayens, W. Partial least squares for discrimination. J. Chemometr. 2003, 17, 166–173. [Google Scholar] [CrossRef]
- Lodolini, E.M.; Fernández, A.; Morales-Sillero, A.; Mendiano, A.; Martín-Vertedor, D. Influence of pre-harvest calcium applications on table olive characteristics during Spanish-style elaboration process. Sci. Hortic. 2023, 308, 111577. [Google Scholar] [CrossRef]
- Marx, Í.M.; Rodrigues, N.; Dias, L.G.; Veloso, A.C.; Pereira, J.A.; Drunkler, D.A.; Peres, A.M. Quantification of table olives’ acid, bitter and salty tastes using potentiometric electronic tongue fingerprints. LWT-Food Sci. Technol. 2017, 79, 394–401. [Google Scholar] [CrossRef] [Green Version]
- Fernández, A.; Muñoz, J.M.; Martín-Tornero, E.; Martínez, M.; Martín-Vertedor, D. Acrylamide mitigation in Californian-style olives after thermal and baking treatments. J. Food Compos. Anal. 2022, 108, 104423. [Google Scholar] [CrossRef]
- Martín-Tornero, E.; Sánchez, R.; Lozano, J.; Martínez, M.; Arroyo, P.; Martín-Vertedor, D. Characterization of Polyphenol and Volatile Fractions of Californian-Style Black Olives and Innovative Application of E-Nose for Acrylamide Determination. Foods 2021, 10, 2973. [Google Scholar] [CrossRef]
- Perestrelo, R.; Silva, C.; Silva, P.; Câmara, J.S. Global volatile profile of virgin olive oils flavoured by aromatic/medicinal plants. Food Chem. 2017, 227, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Montero-Fernández, I.; Marcía-Fuentes, J.A.; Cascos, G.; Saravia-Maldonado, S.A.; Lozano, J.; Martín-Vertedor, D. Masking Effect of Cassia grandis Sensory Defect with Flavoured Stuffed Olives. Foods 2022, 11, 2305. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Tang, K.; Xu, Y.; Chen, S. Characterization of the potent odorants in Tibetan Qingke Jiu by sensory analysis, aroma extract dilution analysis, quantitative analysis and odor activity values. Food Res. Int. 2020, 137, 109349. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, R.; Martín-Tornero, E.; Lozano, J.; Boselli, E.; Arroyo, P.; Meléndez, F.; Martín-Vertedor, D. E-Nose discrimination of abnormal fermentations in Spanish-Style Green Olives. Molecules 2021, 26, 5353. [Google Scholar] [CrossRef]
- Ordoñez Araque, R.; Barat, J.M. Evaluación de un Sistema de Enmascaramiento de Olor de Muestras de Ajo, Mediante un Sistema de Nariz Electrónica. Rev. Politécnica 2017, 40, 13–19. [Google Scholar]
- Qiao, Y.; Xie, B.; Zhang, Y.; Pan, S. Characterization of aroma active compounds in blood orange juice by solid phase microextraction and gas chromatography-mass spectrometry-olfactometry. Chin. J. Chromatogr. 2008, 26, 509–514. [Google Scholar]
- Ruiz, C.; Tunarosa, F.; Martínez, J.; Stashenko, E. Estudio comparativo por GC-MS de metabolitos secundarios volátiles de dos quimiotipos de Lippia origanoides HBK, obtenidos por diferentes técnicas de extracción. Sci. Tech. 2007, 1. [Google Scholar] [CrossRef]
- Debabhuti, N.; Mukherjee, S.; Neogi, S.; Sharma, P.; Sk, U.H.; Maiti, S.; Poddar Sarkar, M.; Tudu, B.; Bhattacharyya, N.; Bandyopadhyay, R. A study of vegetable oil modified QCM sensor to detect β-pinene in Indian cardamom. Talanta 2022, 236, 122837. [Google Scholar] [CrossRef]
- Cedron Fernández, M.T. Estudio analítico de compuestos volátiles en vino. Caracterización quimiometría de distintas denominaciones de origen. Ph.D. Thesis, Universidad de La Rioja, Logroño, Spain, 2004. ISBN 84-689-0190-0190-3. [Google Scholar]
- Wang, Y.; Kays, S.J. Contribution of Volatile Compounds to the Characteristic Aroma of Baked ‘Jewel’ Sweetpotatoes. J. Am. Soc. Hortic. Sci. 2000, 125, 638–643. [Google Scholar] [CrossRef] [Green Version]
- Martínez, P.; Flores, S.M.; Páez, J.R.; Sánchez, M.G. Análisis de Monómero Residual en Envases de Poliestireno Grado Alimenticio. Química Hoy 2012, 2, 5–8. [Google Scholar] [CrossRef]
- Kandyala, R.; Raghavendra, S.P.C.; Rajasekharan, S.T. Xylene: An overview of its health hazards and preventive measures. J. Oral. Maxillofac. Pathol. 2010, 14, 1. [Google Scholar] [CrossRef] [Green Version]
- McRae, J.F.; Mainland, J.D.; Jaeger, S.R.; Adipietro, K.A.; Matsunami, H.; Newcomb, R.D. Genetic variation in the odorant receptor OR2J3 is associated with the ability to detect the “grassy” smelling odor, cis-3-hexen-1-ol. Chem. Senses 2012, 37, 585–593. [Google Scholar] [CrossRef] [Green Version]
- Sánchez, R.; Pérez-Nevado, F.; Martillanes, S.; Montero-Fernández, I.; Lozano, J.; Martín-Vertedor, D. Machine olfaction discrimination of Spanish-style green olives inoculated with spoilage mold species. Food Control 2023, 147, 109600. [Google Scholar] [CrossRef]
- Butzenlechner, M.; Rossmann, A.; Schmidt, H.L. Assignment of bitter almond oil to natural and synthetic sources by stable isotope ratio analysis. J. Agric. Food Chem. 1989, 37, 410–412. [Google Scholar] [CrossRef]
- Brodnitz, M.H.; Pascale, J.V.; Van Derslice, L. Flavor components of garlic extract. J. Agric. Food Chem. 1971, 19, 273–275. [Google Scholar] [CrossRef]
- Zhu, G.; Xiao, Z.; Zhou, R.; Lei, D. Preparation and simulation of a taro flavor. Chin. J. Chem. Eng. 2015, 23, 1733–1735. [Google Scholar] [CrossRef]
- Cui, S.; Wang, J.; Yang, L.; Wu, J.; Wang, X. Qualitative and quantitative analysis on aroma characteristics of ginseng at different ages using E-nose and GC–MS combined with chemometrics. J. Pharm. Biomed. Anal. 2015, 102, 64–77. [Google Scholar] [CrossRef]
- Sánchez, R.; Boselli, E.; Fernández, A.; Arroyo, P.; Lozano, J.; Martín-Vertedor, D. Determination of the Masking Effect of the ‘Zapateria’ Defect in Flavoured Stuffed Olives Using E-Nose. Molecules 2022, 27, 4300. [Google Scholar] [CrossRef]
- López-López, A.; Cortés-Delgado, A.; de Castro, A.; Sánchez, A.H.; Montaño, A. Changes in volatile composition during the processing and storage of black ripe olives. Food Res. Int. 2019, 125, 108568. [Google Scholar] [CrossRef]
- Sabatini, N.; Marsilio, V. Volatile compounds in table olives (Olea Europaea L., Nocellara del Belice cultivar). Food Chem. 2008, 107, 1522–1528. [Google Scholar] [CrossRef]
- Rodríguez, J.; Durán, C.; Reyes, A. Electronic nose for quality control of Colombian coffee through the detection of defects in “Cup Tests”. Sensors 2009, 10, 36–46. [Google Scholar] [CrossRef] [PubMed]


| Extra | Select | Standard | ||
|---|---|---|---|---|
| Extra | --- | p < 0.05 n = 5 100% | p < 0.05 n = 5 100% | Extra |
| Select | p < 0.05 n = 5 100% | --- | p > 0.05 n = 5 85% | Select |
| Standard | p < 0.05 n = 5 100% | p > 0.05 n = 5 89% | --- | Standard |
| Variety | DPP Classification | Positive Aroma | Burn Defect |
|---|---|---|---|
| “Manzanilla Sevillana” | Extra | 4.3 ± 0.5 a | 2.1 ± 0.2 c |
| Select | 3.2 ± 0.5 b | 4.1 ± 0.3 b | |
| Standard | 1.9 ± 0.3 c | 6.1 ± 0.4 a |
| Phenolic Profile (mg·100 g−1) | Extra | Select | Standard |
|---|---|---|---|
| Hydroxytyrosol | 1594.5 ± 76.7 b,C | 1104.1 ± 17.4 b,B | 551.8 ± 26.2 b,A |
| Tyrosol | 405.7 ± 7.1 c,C | 235.9 ± 19.3 c,B | 127.0 ± 6.3 c,A |
| Procyanidin B1 | 53.8 ± 3.9 e,C | 33.0 ± 6.6 e,B | 15.5 ± 1.0 e,A |
| Vanillic acid | 8.8 ± 0.6 h | n.d. | n.d. |
| (-)-Epicatechin | 5.5 ± 0.1 i | n.d. | n.d. |
| Oleuropein | 311.3 ± 12.0 d,C | 185.9 ± 8.9 d,B | 72.3 ± 1.5 d,A |
| Luteolin-7-O-glucoside | 7.9 ± 0.7 h,B | 4.9 ± 0.3 h,A | n.d. |
| Apigenin-7-O | 10.1 ± 0.6 g,B | 7.4 ± 0.2 g,A | n.d. |
| Verbascoside | 11.5 ± 1.2 g,B | 8.9 ± 0.4 g,A | n.d. |
| p-Coumaric acid | 28.1 ± 1.6 f,B | 19.7 ± 0.8 f,A | n.d. |
| Σ phenols | 2437.4 ± 15.7 a,C | 1599.9 ± 6.7 a,B | 766.6 ± 30.4 a,A |
| Predicted Class | |||
|---|---|---|---|
| Real Class | Extra | Select | Standard |
| Extra | 33.3 | 9.0 | 0 |
| Select | 0 | 24.3 | 0 |
| Standard | 0 | 0 | 33.3 |
| RT | DPP Classification | |||
|---|---|---|---|---|
| (min) | Extra | Select | Standard | |
| 2,4-Dimethylhexane | 6.7 | 0.5 ± 0.0 | 2.7 ± 0.5 | 4.8 ± 0.1 |
| 3-Methylpyridine | 10.0 | n.d. | 2.6 ± 0.6 | 6.1 ± 0.2 |
| p-Xylene | 10.2 | 5.1 ± 0.1 | 3.7 ± 2.4 | 1.7 ± 0.2 |
| Styrene | 11.3 | 5.3 ± 0.3 | 4.1 ± 0.4 | 3.3 ± 0.3 |
| Benzaldehyde | 15.3 | 2.5 ± 0.4 | 12.8 ± 2.0 | 21.0 ± 2.5 |
| Beta-pinene | 15.5 | 9.4 ± 0.5 | 7.2 ± 1.5 | 5.0 ± 0.8 |
| 4-Ethenylpyridine | 15.6 | 1.6 ± 0.1 | 6.7 ± 1.2 | 13.3 ± 0.4 |
| 3-Hexen-1-ol, acetate | 17.5 | 3.9 ± 0.4 | 2.5 ± 0.2 | 1.8 ± 0.4 |
| Acetic acid, hexyl ester | 17.9 | 2.2 ± 0.2 | 1.9 ± 0.2 | 1.3 ± 0.2 |
| p-Cymene | 18.1 | 10.3 ± 0.6 | 7.9 ± 0.3 | 6.1 ± 0.1 |
| Gamma-terpinene | 19.9 | 11.6 ± 0.4 | 10.3 ± 0.2 | 7.1 ± 0.2 |
| Diallyl disulphide | 21.1 | 12.1 ± 0.3 | 6.5 ± 0.4 | 2.5 ± 0.4 |
| Octan-1-ol | 21.2 | 1.7 ± 0.5 | n.d. | n.d. |
| Beta-farnesene | 23.1 | 1.6 ± 0.1 | 2.6 ± 0.1 | 5.8 ± 0.1 |
| Cyclohexanecarboxylic acid, ethyl ester | 24.1 | 6.6 ± 1.2 | 4.8 ± 1.3 | 4.1 ± 1.2 |
| Creosol | 27.2 | 19.2 ± 0.8 | 18.4 ± 2.3 | 12.5 ± 1.1 |
| Copaene | 35.3 | 6.5 ± 1.1 | 5.3 ± 0.4 | 3.7 ± 1.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cascos, G.; Barea-Ramos, J.D.; Montero-Fernández, I.; Ruiz-Canales, A.; Lozano, J.; Martín-Vertedor, D. Burn Defect and Phenol Prediction for Flavoured Californian-Style Black Olives Using Digital Sensors. Foods 2023, 12, 1377. https://doi.org/10.3390/foods12071377
Cascos G, Barea-Ramos JD, Montero-Fernández I, Ruiz-Canales A, Lozano J, Martín-Vertedor D. Burn Defect and Phenol Prediction for Flavoured Californian-Style Black Olives Using Digital Sensors. Foods. 2023; 12(7):1377. https://doi.org/10.3390/foods12071377
Chicago/Turabian StyleCascos, Gema, Juan Diego Barea-Ramos, Ismael Montero-Fernández, Antonio Ruiz-Canales, Jesús Lozano, and Daniel Martín-Vertedor. 2023. "Burn Defect and Phenol Prediction for Flavoured Californian-Style Black Olives Using Digital Sensors" Foods 12, no. 7: 1377. https://doi.org/10.3390/foods12071377






