Cefotaxime-, Ciprofloxacin-, and Extensively Drug-Resistant Escherichia coli O157:H7 and O55:H7 in Camel Meat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Samples
2.2. Isolation and Identification of E. coli Strains
2.3. Serological Identification of E. coli Isolates
2.4. Molecular Characterization of E. coli Isolates
2.4.1. Isolation of Genomic DNA
2.4.2. PCR for Detection of rfbO157 Gene Specific for E. coli O157
2.4.3. Detection of stx1, stx2, eaeA, and hylA Virulence Genes with PCR
2.5. Antimicrobial Resistance Profile and Multiple Antibiotic Resistance (MAR) index of E. coli O157:H7 and E. coli O55:H7 Isolated Strains
3. Results and Discussion
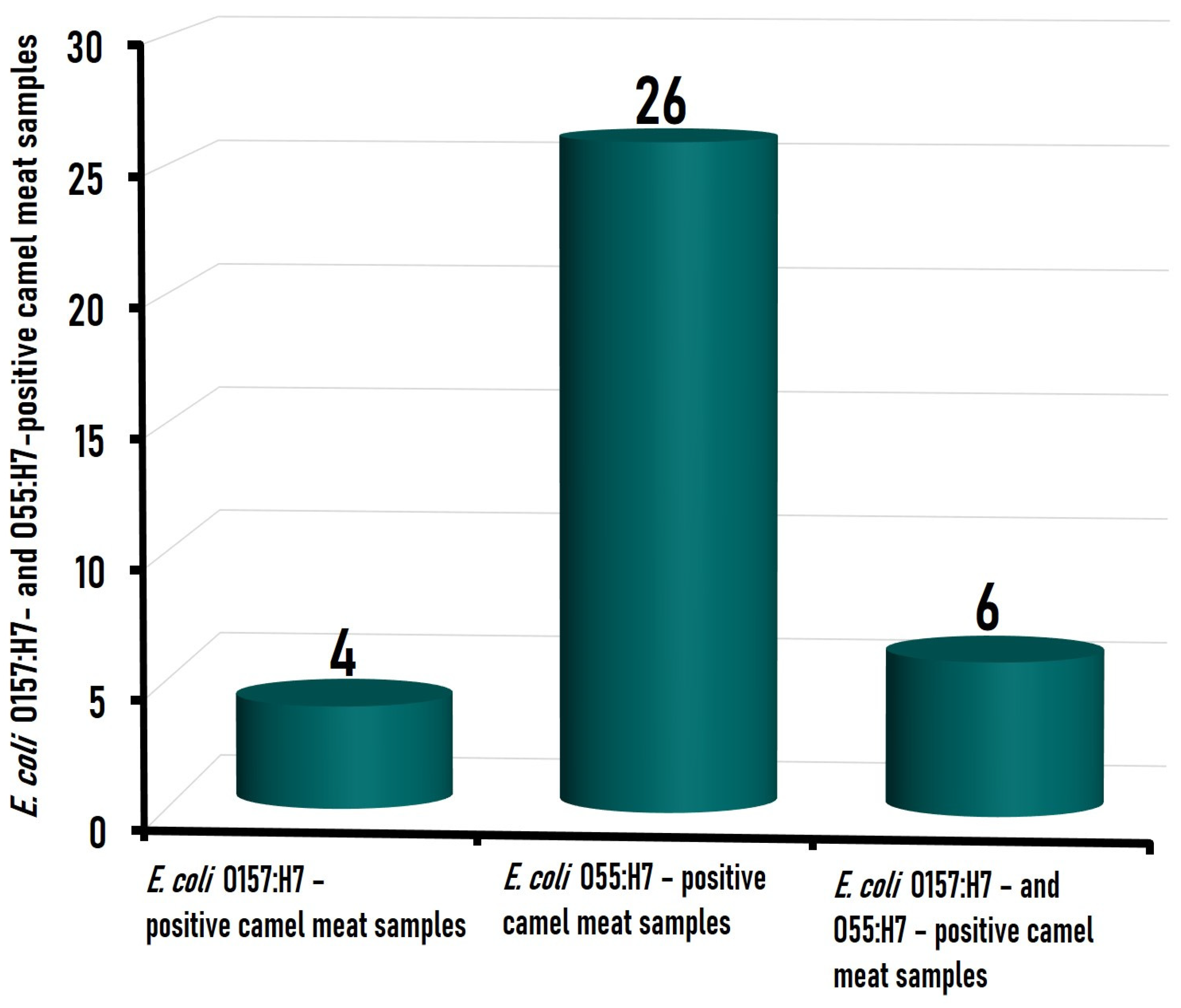
3.1. Prevalence of the Isolated E. coli Strains in Camel Meat Samples
3.2. Molecular Characterization and Virulence Genes Distribution among E. coli O157:H7 and E. coli O55:H7 Isolated from Camel Meat
3.2.1. Shiga Toxin Genes (stx1 and stx2)
3.2.2. Intimin (eaeA) and Enterohemolysin (hlyA) Genes
3.3. Antimicrobial Resistance Profile and MAR Index of E. coli O157:H7 and E. coli O55:H7 Strains
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ameer, M.A.; Wasey, A.; Salen, P. Escherichia coli (E. coli O157:H7). In Stat Pearls; Stat-Pearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK507845/ (accessed on 18 November 2022).
- Karch, H.; Bielaszewska, M. Sorbitol-fermenting Shiga toxin producing Escherichia coli O157:H-strains: Epidemiology, phenotypic and molecular characteristics, and microbiological diagnosis. J. Clin. Microbiol. 2001, 39, 2043–2049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawyer, C.; Vishram, B.; Jenkins, C.; Jorgensen, F.; Byrne, L.; Mikhail, A.F.W.; Dallman, T.J.; Carroll, K.; Ahyow, L.; Vahora, Q.; et al. Epidemiological investigation of recurrent outbreaks of haemolytic uraemic syndrome caused by Shiga toxin-producing Escherichia coli serotype O55:H7 in England, 2014–2018. Epidemiol. Infect. 2021, 149, E108. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.M.; Wilson, M.E.; Johnson, K.E.; Thorpe, C.M.; Sears, C.L. The emerging clinical importance of non-O157 Shiga toxin-producing Escherichia coli. Clin. Infect. Dis. 2006, 43, 1587–1595. [Google Scholar] [CrossRef] [PubMed]
- McFarland, N.; Bundle, N.; Jenkins, C.; Godbole, G.; Mikhail, A.; Dallman, T.; O’Connor, C.; McCarthy, N.; O’Connell, E.; Treacy, J.; et al. Recurrent seasonal outbreak of an emerging serotype of Shiga toxin-producing Escherichia coli (STEC O55:H7 Stx2a) in the south west of England, July 2014 to September 2015. Euro Surveill. 2017, 22, 30610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brock, A. Guildford woman critically ill in hospital after suffering organ failure from ‘confirmed case of E. coli’ infection. Get. Surrey 2017. Available online: https://www.getsurrey.co.uk (accessed on 29 November 2022).
- Available online: https://www.bbc.com/news/uk-england-leicestershire-45838401 (accessed on 11 January 2023).
- Iguchi, A.; Ooka, T.; Ogura, Y.; Nakayama, K.; Frankel, G.; Hayashi, T. Genomic comparison of the O-antigen biosynthesis gene clusters of Escherichia coli O55 strains belonging to three distinct lineages. Microbiology 2008, 154, 559–570. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Li, X.; Liu, B.; Beutin, L.; Xu, J.; Ren, Y.; Feng, L.; Lan, R.; Reeves, P.R.; Wang, L. Derivation of Escherichia coli O157:H7 from its O55:H7 precursor. PLoS ONE 2010, 5, e8700. [Google Scholar] [CrossRef] [Green Version]
- Wick, L.M.; Qi, W.; Lacher, D.W.; Whittam, T.S. Evolution of genomic content in the stepwise emergence of Escherichia coli O157:H7. J. Bacteriol. 2005, 187, 1783–1791. [Google Scholar] [CrossRef] [Green Version]
- Persad, A.K.; LeJeune, J.T. Animal Reservoirs of Shiga toxin-producing Escherichia coli. Microbiol. Spectr. 2014, 2, HEC-0027. [Google Scholar] [CrossRef]
- Gautam, R.; Kulow, M.; Park, D.; Gonzales, T.K.; Dahm, J.; Shiroda, M.; Stasic, A.J.; Döpfer, D.; Kaspar, C.W.; Ivanek, R. Transmission of Escherichia coli O157:H7 in cattle is influenced by the level of environmental contamination. Epidemiol. Infect. 2015, 143, 274–287. [Google Scholar] [CrossRef] [Green Version]
- Sallam, K.I.; Mohammed, M.; Ahdy, A.M.; Tamura, T. Prevalence, genetic characterization and virulence genes of sorbitol-fermenting Escherichia coli O157:H- and E. coli O157:H7 isolated from retail beef. Int. J. Food Microbiol. 2013, 165, 295–301. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Sallam, K.I.; Eldaly, E.A.Z.; Ahdy, A.M.; Tamura, T. Occurrence, serotypes and virulence genes of non-O157 Shiga toxin-producing Escherichia coli in fresh beef, ground beef, and beef burger. Food Control 2014, 37, 182–187. [Google Scholar] [CrossRef]
- Hessain, A.M.; Al-Arfaj, A.A.; Zakri, A.M.; El-Jakee, J.K.; Al-Zogibi, O.G.; Hemeg, H.A.; Ibrahim, I.M. Molecular characterization of Escherichia coli O157:H7 recovered from meat and meat products relevant to human health in Riyadh, Saudi Arabia. Saudi J. Biol. Sci. 2015, 22, 725–729. [Google Scholar] [CrossRef] [Green Version]
- Babolhavaeji, K.; Shokoohizadeh, L.; Yavari, M.; Moradi, A.; Alikhani, M.Y. Prevalence of Shiga toxin-producing Escherichia coli o157 and non-o157 serogroups isolated from fresh raw beef meat samples in an industrial slaughterhouse. Int. J. Microbiol. 2021, 2021, 1978952. [Google Scholar] [CrossRef]
- ISO 16649-2:2001; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Beta-Glucuronidase-Positive Escherichia coli—Part 2: Colony Count Technique at 44 Degrees C Using 5-Bromo-4-Chloro-3-Indolyl Beta-D-Glucuronide. International Organization for Standardization: Geneva, Switzerland, 2001.
- Dhanashree, B.; Mallya, P.S. Detection of Shiga-toxigenic Escherichia coli (STEC) in diarrhoeagenic stool & meat samples in Mangalore, India. Indian J. Med. Res. 2008, 128, 271–277. [Google Scholar]
- Mazaheri, S.; Ahrabi, S.; Aslani, M. Shiga toxin-producing Escherichia coli isolated from lettuce samples in Tehran, Iran. Jundishapur J. Microbiol. 2014, 7, e12346. [Google Scholar] [CrossRef] [Green Version]
- Fratamico, P.M.; Sackitey, S.K.; Wiedmann, M.; Deng, M.Y. Detection of Escherichia coli O157: H7 by multiplex PCR. J. Clin. Microbiol. 1995, 33, 2188–2191. [Google Scholar] [CrossRef] [Green Version]
- CLSI Standard M02; Performance Standards for Antimicrobial Disk Susceptibility Tests. 13th ed. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018.
- Singh, S.; Yadav, A.S.; Singh, S.M.; Bharti, P. Prevalence of Salmonella in chicken eggs collected from poultry farms and marketing channels and their antimicrobial resistance. Food Res. Int. 2010, 43, 2027–2030. [Google Scholar] [CrossRef]
- Rahimi, E.; Kazemeini, H.R.; Salajegheh, M. Escherichia coli O157: H7/NM prevalence in raw beef, camel, sheep, goat, and water buffalo meat in Fars and Khuzestan provinces, Iran. Vet. Res. Forum 2012, 3, 15–17. [Google Scholar]
- Hassan, M.A.; Heikal, G.I.; Barhoma, R.M. Traceability of enteropathogenic E. coli in cattle and camel carcasses. Benha Vet. Med. J. 2016, 31, 50–55. [Google Scholar] [CrossRef]
- Al-Gburi, N.M. Prevalence of Escherichia coli O157:H7 in camels’ fecal samples. J. Genet. Environ. Res. Conserv. 2016, 4, 46–50. [Google Scholar]
- Al Ajmi, D.S.; Banu, S.; Rahman, S. A Study on prevalence of Escherichia coli O157 in healthy camels, cattle, sheep and goat from slaughterhouse in Al Ain, the United Arab Emirates. J. Trauma Crit. Care 2019, 3, 41. [Google Scholar] [CrossRef]
- Elder, R.O.; Keen, J.E.; Siragusa, G.R.; Barkocy-Gallagher, G.A.; Koohmaraie, M.; Laegreid, W.W. Correlation of enterohemorrhagic Escherichia coli O157 prevalence in feces, hides, and carcasses of beef cattle during processing. Proc. Natl. Acad. Sci. USA 2000, 97, 2999–3003. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, K.; Sarkim, V.; Bitzan, M.; Karmali, M.A.; Bobrowski, C.; Ruder, H.; Laufs, R.; Sobottka, I.; Petric, M.; Karch, H.; et al. Shiga toxin-producing Escherichia coli infection and antibodies against Stx2 and Stx1 in household contacts of children with enteropathic hemolytic-uremic syndrome. J. Clin. Microbiol. 2002, 40, 1773–1782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thorpe, C.M.; Ritchie, J.M.; Acheson, D.W.K. Enterohemorrhagic and other Shiga toxin-producing Escherichia coli. In Escherichia coli: Virulence Mechanisms of a Versatile Pathogen; Donnenberg, M.S., Ed.; Academic Press: Boston, MA, USA, 2002; pp. 119–154. [Google Scholar]
- Brooks, J.T.; Sowers, E.G.; Wells, J.G.; Greene, K.D.; Griffin, P.M.; Hoekstra, R.M.; Strockbine, N.A. Non-O157 Shiga toxin–producing Escherichia coli infections in the United States, 1983–2002. J. Infect. Dis. 2005, 192, 1422–1429. [Google Scholar] [CrossRef] [Green Version]
- Pradel, N.; Bertin, Y.; Martin, C.; Livrelli, V. Molecular analysis of Shiga toxin-producing Escherichia coli strains isolated from hemolytic-uremic syndrome patients and dairy samples in France. Appl. Environ. Microbiol. 2008, 74, 2118–2128. [Google Scholar] [CrossRef] [Green Version]
- Louise, C.B.; Obrig, T.G. Specific interaction of Escherichia coli O157:H7-derived Shiga-like toxin II with human renal endothelial cells. J. Infect. Dis. 1995, 172, 1397–1401. [Google Scholar] [CrossRef]
- Lee, G.Y.; Jang, H.I.; Hwang, I.G.; Rhee, M.S. Prevalence and classification of pathogenic Escherichia coli isolated from fresh beef, poultry, and pork in Korea. Int. J. Food Microbiol. 2009, 134, 196–200. [Google Scholar] [CrossRef]
- Beutin, L.; Miko, A.; Krause, G.; Pries, K.; Haby, S.; Steege, K.; Albrecht, N. Identification of human-pathogenic strains of Shiga toxin-producing Escherichia coli from food by a combination of serotyping and molecular typing of Shiga toxin genes. Appl. Environ. Microbiol. 2007, 73, 4769–4775. [Google Scholar] [CrossRef] [Green Version]
- Mora, A.; Blanco, M.; Blanco, J.E.; Dahbi, G.; López, C.; Justel, P.; Alonso, M.P.; Echeita, A.; Bernárdez, M.I.; González, E.A.; et al. Serotypes, virulence genes and intimin types of Shiga toxin (verocytotoxin)-producing Escherichia coli isolates from minced beef in Lugo (Spain) from 1995 through 2003. BMC Microbiol. 2007, 7, 13. [Google Scholar] [CrossRef] [Green Version]
- Slanec, T.; Fruth, A.; Creuzburg, K.; Schmidt, H. Molecular analysis of virulence profiles and Shiga toxin genes in food-borne Shiga toxin-producing Escherichia coli. Appl. Environ. Microbiol. 2009, 75, 6187–6197. [Google Scholar] [CrossRef] [Green Version]
- Eid, H.I.; Algammal, A.M.; Nasef, S.A.; Elfeil, W.K.; Mansour, G.H. Genetic variation among avian pathogenic E. coli strains isolated from broiler chickens. Asian J. Anim. Vet. Adv. 2016, 11, 350–356. [Google Scholar] [CrossRef] [Green Version]
- Cagney, C.; Crowley, H.; Duffy, G.; Sheridan, J.J.; O’Brien, S.; Carney, E.; Anderson, W.; McDowell, D.A.; Blair, I.S.; Bishop, R.H. Prevalence and numbers of Escherichia coli O157:H7 in minced beef and beef burgers from butcher shops and supermarkets in the Republic of Ireland. Food Microbiol. 2004, 21, 203–212. [Google Scholar] [CrossRef]
- Chapman, P.A.; Siddons, C.A.; Cerdan Malo, A.T.; Harkin, M.A. A one year study of Escherichia coli O157 in raw beef and lamb products. Epidemiol. Infect. 2000, 124, 207–213. [Google Scholar] [CrossRef]
- Barlow, R.S.; Hirst, R.G.; Norton, R.E.; Ashhurst-Smith, C.; Bettelheim, K.A. A novel serotype of enteropathogenic Escherichia coli (EPEC) as a major pathogen in an outbreak of infantile diarrhoea. J. Med. Microbiol. 1999, 48, 1123–1125. [Google Scholar] [CrossRef] [Green Version]
- Beutin, L.; Krause, G.; Zimmermann, S.; Kaulfuss, S.; Gleier, K. Characterization of Shiga toxin-producing Escherichia coli strains isolated from human patients in Germany over a 3-year period. J. Clin. Microbiol. 2004, 42, 1099–1108. [Google Scholar] [CrossRef] [Green Version]
- Blanco, M.; Blanco, J.E.; Mora, A.; Dahbi, G.; Alonso, M.P.; González, E.A.; Bernárdez, M.I.; Blanco, J. Serotypes, virulence genes, and intimin types of Shiga toxin (verotoxin)-producing Escherichia coli isolates from cattle in Spain and identification of a new intimin variant gene (eae-ξ). J. Clin. Microbiol. 2004, 42, 645–651. [Google Scholar] [CrossRef] [Green Version]
- Binandeh, F.; Pajohi-Alamoti, M.; Mahmoodi, P.; Ahangari, A. Evaluation of stx1, stx2, hlyA, and eaeA virulence genes in Escherichia coli O157:H7 isolated from meat (beef and mutton) in Hamedan, Iran, during 2015–2016. Int. J. Enteric Pathog. 2020, 8, 55–59. [Google Scholar] [CrossRef]
- Jomehzadeh, N.; Saki, M.; Ahmadi, K.; Zandi, G. The prevalence of plasmid-mediated quinolone resistance genes among Escherichia coli strains isolated from urinary tract infections in southwest Iran. Mol. Biol. Rep. 2022, 49, 3757–3763. [Google Scholar] [CrossRef]
- Da Costa, P.M.; Loureiro, L.; Matos, A.J. Transfer of multidrug-resistant bacteria between intermingled ecological niches: The interface between humans, animals and the environment. Int. J. Environ. Res. Public Health 2013, 10, 278–294. [Google Scholar] [CrossRef] [Green Version]
- Sabala, R.F.; Usui, M.; Tamura, Y.; Abd-Elghany, S.M.; Sallam, K.I.; Elgazzar, M.M. Prevalence of colistin-resistant Escherichia coli harbouring mcr-1 in raw beef and ready-to-eat beef products in Egypt. Food Control 2021, 119, 107436. [Google Scholar] [CrossRef]
- Ahmed, A.M.; Shimamoto, T. Molecular analysis of multidrug resistance in Shiga toxin-producing Escherichia coli O157:H7 isolated from meat and dairy products. Int. J. Food Microbiol. 2015, 193, 6873. [Google Scholar] [CrossRef] [PubMed]
- Moawad, A.A.; Hotzel, H.; Awad, O.; Tomaso, H.; Neubauer, H.; Hafez, H.M.; El-Adawy, H. Occurrence of Salmonella enterica and Escherichia coli in raw chicken and beef meat in northern Egypt and dissemination of their antibiotic resistance markers. Gut Pathog. 2017, 9, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiko, A.; Asrat, D.; Zewde, G. Occurrence of Escherichia coli O157:H7 in retail raw meat products in Ethiopia. J. Infect. Dev. Ctries. 2008, 2, 389–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angulo, F.J.; Collinon, P.; Powers, J.H.; Chiller, T.M.; Aidara-Kane, A.; Aarestrup, F.M. World Health Organization ranking of antimicrobials according to their importance in human medicine: A critical step for developing risk management strategies for the use of antimicrobials in food production animals. Clin. Inf. Dis. 2009, 49, 132–141. [Google Scholar] [CrossRef] [Green Version]
- Tayh, G.; Boubaker, S.M.; Khedher, R.B.; Jbeli, M.; Chehida, F.B.; Mamlouk, A.; Dâaloul-Jedidi, M.; Messadi, L. Prevalence, virulence genes, and antimicrobial profiles of Escherichia coli O157: H7 isolated from healthy cattle in Tunisia. J. Infect. Dev. Ctries. 2022, 16, 1308–1316. [Google Scholar] [CrossRef]
- Grispoldi, L.; Karama, M.; Hadjicharalambous, C.; De Stefani, F.; Ventura, G.; Ceccarelli, M.; Revoltella, M.; Sechi, P.; Crotti, C.; D’Innocenzo, A.; et al. Bovine lymph nodes as a source of Escherichia coli contamination of the meat. Int. J. Food Microbiol. 2020, 331, 108715. [Google Scholar] [CrossRef]
- El-Ashmony, A.L.; Mostafa, A.E.; Tarabees, R. The antimicrobial pattern of E. coli isolated from various animal sources at El Beheira Governorate. Alex. J. Vet. Sci. 2022, 73, 7–19. [Google Scholar] [CrossRef]
- Nath, S.K.; Foster, G.A.; Mandell, L.A.; Rotstein, C. Antimicrobial activity of ceftriaxone compared with cefotaxime in the presence of serum albumin. Can. J. Infect. Dis. 1995, 6, 21–27. [Google Scholar] [CrossRef] [Green Version]
- Smith, C.R.; Petty, B.G.; Hendrix, C.W.; Kernan, W.N.; Garver, P.L.; Fox, K.; Beamer, A.; Carbone, K.; Threlkeld, M.; Lietman, P.S. Ceftriaxone compared with cefotaxime for serious bacterial infections. J. Infect. Dis. 1989, 160, 442–447. [Google Scholar] [CrossRef]
- Gums, J.G.; Boatwright, D.W.; Camblin, M.; Halstead, D.C.; Jones, M.E.; Sanderson, R. Differences between ceftriaxone and cefotaxime: Microbiological inconsistencies. Ann. Pharmacother. 2008, 42, 71–79. [Google Scholar] [CrossRef]
- El-Ghareeb, W.R.; Abdel-Raheem, S.M.; Al-Marri, T.M.; Alaql, F.A.; Fayez, M. Isolation and identification of extended spectrum β-lactamases (ESBLs) Escherichia coli from minced camel meat in Eastern Province, Saudi Arabia. Thai J. Vet. Med. 2020, 50, 155–161. [Google Scholar]
- Gessew, G.T.; Desta, A.F.; Adamu, E. High burden of multidrug resistant bacteria detected in Little Akaki River. Comp. Immunol. Microbiol. Infect. Dis. 2022, 80, 101723. [Google Scholar] [CrossRef]

| Serotype | Group | Number of E. coli Isolates | Virulence Genes | |||
|---|---|---|---|---|---|---|
| stx1 | stx2 | eaeA | hlyA | |||
| O157:H7 | A | 13 | + | + | + | + |
| B | 6 | − | + | + | + | |
| C | 3 | − | + | + | − | |
| D | 2 | + | + | + | − | |
| O55:H7 | I | 46 | − | + | + | − |
| II | 21 | − | + | + | + | |
| III | 12 | + | + | + | − | |
| IV | 10 | − | + | − | + | |
| V | 5 | + | + | + | + | |
| VI | 5 | − | + | − | − | |
| VII | 3 | + | − | − | − | |
| Total | 126 | 35 | 123 | 108 | 55 | |
| No | Antimicrobial Agent (Acronym) | Sensitive | Intermediate | Resistant | |||
|---|---|---|---|---|---|---|---|
| NO | % | NO | % | NO | % | ||
| 1 | Clindamycin (CL) | – | – | – | – | 126 | 100 |
| 2 | Penicillin (P) | – | – | – | – | 126 | 100 |
| 3 | Erythromycin (E) | – | – | – | – | 126 | 100 |
| 4 | Tetracycline (T) | 7 | 5.56 | 2 | 1.59 | 117 | 92.9 |
| 5 | Cephalothin (CN) | 15 | 11.9 | 4 | 3.17 | 107 | 84.9 |
| 6 | Ampicillin (AM) | 29 | 23.0 | 7 | 5.56 | 90 | 71.4 |
| 7 | Sulfamethoxazole (SXT) | 60 | 47.6 | 5 | 3.97 | 61 | 48.4 |
| 8 | Amikacin (AK) | 68 | 53.97 | 8 | 6.35 | 50 | 39.7 |
| 9 | Cefotaxime (CF) | 79 | 62.7 | 10 | 7.94 | 37 | 29.4 |
| 10 | Ciprofloxacin (CP) | 98 | 77.8 | – | – | 28 | 22.2 |
| 11 | Gentamicin (G) | 100 | 79.4 | 5 | 3.97 | 21 | 16.7 |
| 12 | Levofloxacin (L) | 109 | 86.5 | 6 | 4.76 | 11 | 8.7 |
| 13 | Imipenem (IPM) | 115 | 91.3 | 4 | 3.17 | 7 | 5.56 |
| 14 | Ceftriaxone (C) | 121 | 96.03 | – | – | 5 | 3.97 |
| E. coli Strains | Number of Isolates | Antimicrobial Resistance Profile | Classes with Resistance | Mar Index | Classification of Strains | |
|---|---|---|---|---|---|---|
| No. and (%) | ||||||
| O157:H7 (n = 24) | 2 | CL, P, E, T, CN, AM, SXT, AK, CF, CP, G, L, IPM | Macrolides, lincomycins, penicillins, tetracyclines, cephalosporins, aminoglycosides, sulfonamides, fluoroquinolones, carbapenemes | 0.929 | Extensively drug-resistant | 4 (16.7%) |
| 2 | CL, P, E, T, CN, AM, SXT, AK, CF, CP, G | Macrolides, lincomycins, penicillins, tetracyclines, cephalosporins, aminoglycosides, sulfonamides, fluoroquinolones | 0.786 | |||
| 2 | CL, P, E, T, CN, AM, SXT, AK, CF | Macrolides, lincomycins, penicillins, tetracyclines, cephalosporins, aminoglycosides, sulfonamides | 0.643 | Multidrug-resistant | 20 (83.3.0%) | |
| 4 | CL, P, E, T, CN, AM, SXT, AK | Macrolides, lincomycins, penicillins, tetracyclines, cephalosporins, aminoglycosides, sulfonamides | 0.571 | |||
| 5 | CL, P, E, T, CN, AM | Macrolides, lincomycins, penicillins, tetracyclines, cephalosporins, aminoglycosides | 0.429 | |||
| 6 | CL, P, E, T | Macrolides, lincomycins, penicillins, tetracyclines | 0.357 | |||
| 3 | CL, P, E | Macrolides, lincomycins, penicillins | 0.214 | |||
| Sum 24 | Average MAR Index for O157:H7 | 0.497 | ||||
| O55:H7 (n = 102) | 5 | CL, P, E, T, CN, AM, SXT, AK, CF, CP, G, L, IPM, C | Aminoglycosides, carbapenemes, cephalosporins, fluoroquinolones, macrolides, lincomycins, penicillins, sulfonamides, tetracyclines | 1.000 | Pan-drug resistant | 5 (4.9%) |
| 4 | CL, P, E, T, CN, AM, SXT, AK, CF, CP, G, L | Macrolides, lincomycins, penicillins, tetracyclines, cephalosporins, aminoglycosides, sulfonamides, fluoroquinolones | 0.857 | Extensively drug-resistant | 19 (18.6%) | |
| 8 | CL, P, E, T, CN, AM, SXT, AK, CF, CP, G | Macrolides, lincomycins, penicillins, tetracyclines, cephalosporins, aminoglycosides, sulfonamides, fluoroquinolones | 0.786 | |||
| 7 | CL, P, E, T, CN, AM, SXT, AK, CF, CP | Macrolides, lincomycins, penicillins, tetracyclines, cephalosporins, aminoglycosides, sulfonamides, fluoroquinolones | 0.714 | |||
| 7 | CL, P, E, T, CN, AM, SXT, AK, CF | Macrolides, lincomycins, penicillins, tetracyclines, cephalosporins, aminoglycosides, sulfonamides, fluoroquinolones | 0.643 | Multidrug-resistant | 78 (76.5%) | |
| 9 | CL, P, E, T, CN, AM, SXT, AK | Macrolides, lincomycins, penicillins, tetracyclines, cephalosporins, aminoglycosides, sulfonamides | 0.571 | |||
| 11 | CL, P, E, T, CN, AM, SXT | Macrolides, lincomycins, penicillins, tetracyclines, cephalosporins, aminoglycosides, sulfonamides | 0.500 | |||
| 24 | CL, P, E, T, CN, AM | Macrolides, lincomycins, penicillins, tetracyclines, cephalosporins, aminoglycosides | 0.429 | |||
| 17 | CL, P, E, T, CN | Macrolides, lincomycins, penicillins, tetracyclines, cephalosporins | 0.357 | |||
| 4 | CL, P, E, T | Macrolides, lincomycins, penicillins, tetracyclines | 0.286 | |||
| 6 | CL, P, E | Macrolides, lincomycins, penicillins, | 0.214 | |||
| Sum 102 | Average MAR Index for O55:H7 isolates | 0.527 | ||||
| Total | 126 | Average MAR index for O157:H7 and O55:H7 isolates | Type of resistance | 0.514 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sallam, K.I.; Abd-Elrazik, Y.; Raslan, M.T.; Imre, K.; Morar, A.; Herman, V.; Zaher, H.A. Cefotaxime-, Ciprofloxacin-, and Extensively Drug-Resistant Escherichia coli O157:H7 and O55:H7 in Camel Meat. Foods 2023, 12, 1443. https://doi.org/10.3390/foods12071443
Sallam KI, Abd-Elrazik Y, Raslan MT, Imre K, Morar A, Herman V, Zaher HA. Cefotaxime-, Ciprofloxacin-, and Extensively Drug-Resistant Escherichia coli O157:H7 and O55:H7 in Camel Meat. Foods. 2023; 12(7):1443. https://doi.org/10.3390/foods12071443
Chicago/Turabian StyleSallam, Khalid Ibrahim, Yasmine Abd-Elrazik, Mona Talaat Raslan, Kálmán Imre, Adriana Morar, Viorel Herman, and Hanan Ahmed Zaher. 2023. "Cefotaxime-, Ciprofloxacin-, and Extensively Drug-Resistant Escherichia coli O157:H7 and O55:H7 in Camel Meat" Foods 12, no. 7: 1443. https://doi.org/10.3390/foods12071443
APA StyleSallam, K. I., Abd-Elrazik, Y., Raslan, M. T., Imre, K., Morar, A., Herman, V., & Zaher, H. A. (2023). Cefotaxime-, Ciprofloxacin-, and Extensively Drug-Resistant Escherichia coli O157:H7 and O55:H7 in Camel Meat. Foods, 12(7), 1443. https://doi.org/10.3390/foods12071443











