Abstract
Opuntia spp. are crops well adapted to adverse environments and have great economic potential. Their constituents, including fruits, cladodes, and flowers, have a high nutritional value and are rich in value-added compounds. Cladodes have an appreciable content in dietary fiber, as well as bioactive compounds such as kaempferol, quercetin, and isorhamnetin. Fruits are a major source of bioactive compounds such as phenolic acids and vitamin C. The seeds are mainly composed of unsaturated fatty acids and vitamin E. The flowers are also rich in phenolic compounds. Therefore, in addition to their traditional uses, the different plant fractions can be processed to meet multiple applications in the food industry. Several bakery products have been developed with the incorporation of cladode flour. Pectin and mucilage obtained from cladodes can act as edible films and coatings. Fruits, fruit extracts, and fruit by-products have been mixed into food products, increasing their antioxidant capacity and extending their shelf life. Betalains, obtained from fruits, can be used as food colorants and demonstrate promising applications as a sensor in food packaging. This work reviews the most valuable components of the different fractions of this plant and emphasizes its most recent food applications, demonstrating its outstanding value.
1. Introduction
Prickly pear is a xerophytic plant belonging to the Cactaceae family [1]. This family comprises about 2000 species belonging to 130 genera [2]. Opuntia is among the most important and widely distributed genera of the family Cactaceae [3]. It is a native plant of arid and semi-arid lands owing to its particular adaptation mechanism to hostile climatic conditions [4]. It is originally from tropical and subtropical regions of America [5]. The species were recognized in Europe as early as the 15th century [6,7]. Afterwards, the cactus was introduced in North Africa, essentially in Morocco, Tunisia, and Algeria, around the 16th century [8]. It has been cultivated later in South Africa, Madagascar, India, Australia, Canada, Brazil, and Argentina [6]. Yet, the cultivation of the cactus is not limited to those areas, being currently present in more than 30 countries due to its importance and multiple applications [6], which are reflected in its environmental, nutritional, and economic benefits [9,10,11].
From an environmental point of view, cactus cladodes provide effective means to fight against desertification, erosion, and soil-related problems [12,13]. They have an exceptional capacity to adapt to drought, thanks to their ability to preserve water in the parenchyma through their specialized photosystem called Crassulacean Acid Metabolism (CAM) [5,14,15,16].
In addition to their environmental importance, this valuable plant is also of great nutritional interest. The cactus fruit is considered a nutritionally complete food, which can also be described as a nutraceutical food [17,18]. It is known for its very high nutritional value in vitamins, mainly ascorbic acid, and minerals, such as magnesium (Mg), calcium (Ca), and potassium (K), as well as being rich in antioxidants like phenolic compounds and flavonoids [19,20,21]. Typically, the cactus fruit is consumed fresh, yet it can be variously formulated into jams, drinks, tea, tinctures, and dietary supplements [22,23,24]. The cladodes have multiple applications as food and feed, most commonly consumed as nutritious fresh vegetables, processed into juice, or bread from their flour [19,25].
Several studies showed that the cactus could be used as a good source of bioactive compounds. due to their richness in secondary metabolites, especially phenolic compounds [25,26]. Among the reported compounds, eucomic acid, kaempferol 3-O-robinobioside-7-O-arabinofuranoside, isorhamnetin 3-O-galactoside, and isorhamnetin 3-O-rhamnoside-7-O-(rhamnosyl-hexoside) are those with high antioxidant activities [27]. In addition, different health benefits have been associated with cactus consumption and the richness of bioactive compounds, e.g., diabetes prevention [23,28], hypercholesterolemia [29,30], obesity [31], and hypertension [28].
Opuntia spp. plants can be used in full, as all vegetative parts are edible (cladodes, fruits, seeds, and flowers). Therefore, this paper represents a bibliographic review of the bioactive composition of prickly pear plants (cladodes, fruits/seeds, flowers, and roots). As knowledge on the extraction procedures of the bioactive compounds of the Opuntia plant and its fractions is not commonly reviewed, providing highlights and main findings from multiple studies was also a goal of this work. As this plant is gaining more attention due to its adaptability to marginal soils and arid conditions, which is increasing the area of cultivation and the yearly production, postharvest treatments and different processing options are also being implemented to increase its shelf life and maintain its quality. Hence, recent and innovative food applications of this valuable crop are also addressed in this analysis, with a focus on the full use of this crop, that is, by including examples of application of by-products, following a circular bioeconomy approach.
2. Prickly Pear Plant as a Source of Bioactive Macromolecules
2.1. Cladodes
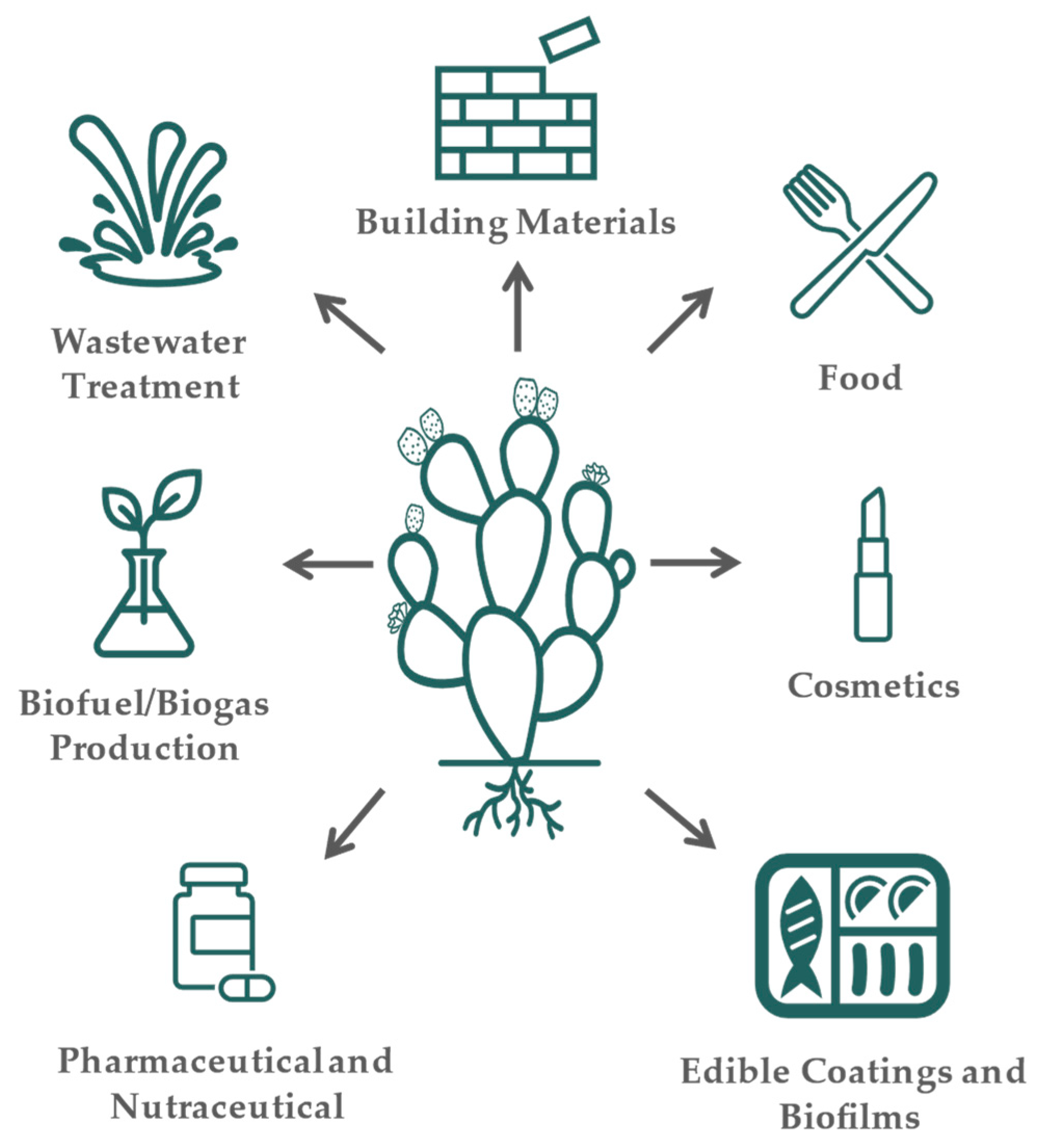
Cladodes from Opuntia spp. are mainly used as food; i.e., in Mexico, the younger cladodes, called nopalitos, are consumed as fresh vegetables in several dishes or transformed into several food products, foraged, and used as ornaments [9]. Moreover, they find applications in building construction, cosmetics, medicine, and wastewater treatment [32,33].
Cladodes have a high nutritional value due to their minerals, dietary fiber, and phytochemical content [34]. The chemical composition of cladodes varies depending on the type of cultivar, species, maturity stage, environmental conditions, harvesting and post-harvesting conditions, and treatments [27]. Cladodes are mainly composed of water (80–95%), carbohydrates (3–7%), and fiber (1–2%), with low contents of protein and lipids [27,34,35,36]. Furthermore, the pads are a great source of minerals, with a major presence of calcium and potassium and minor quantities of magnesium, manganese, iron, zinc, and copper [37]. The dietary fiber content in cladodes is composed of cellulose, hemicellulose, pectin, lignin, and mucilage [38]. The ingestion of cladodes due to their dietary fiber content may help in reducing body weight, playing an important role in the excretion of lipids [39,40]. Vitamins are also present in cladodes, mainly in the form of ascorbic acid and carotenoids [41,42].
Cladodes are an important source of bioactive compounds such as phenolic compounds (phenolic acids and flavonoids) and carotenoids [43,44]. The most common phenolic compounds found are kaempferol, quercetin, isorhamnetin, and isorhamnetin glucosides [34].
Several studies have been performed using different extraction procedures to access the phytochemical profile of cladodes; the most recent research is summarized in Table 1.

Table 1.
Bioactive composition and extraction procedures of Opuntia spp. cladodes.
In Opuntia ficus-indica, it was possible to identify several bioactive compounds, such as kaempferol, quercetin, and isorhamnetin glucosides [47,48,49,50]. Ben Lataief and collaborators performed both aqueous and ethanolic extractions on Opuntia dilenii cladodes to evaluate the differences in the composition of the compounds [46]. Ethanolic extracts allowed the detection of more phenolic and volatile compounds than the aqueous extract. The solvent choice displays an influence on the extraction efficiency and has an impact on the properties and composition of the resulting extracts [51]. Another important factor for bioactive compound extraction from cladodes is the high content of dietary fiber, which can retain those compounds and is where most of them are linked, so an appropriate solvent choice and extraction procedure are recommended [38].
Along with the solvent extraction process for bioactive compounds, new techniques are emerging, as in the case of enzyme-assisted extraction combined with supercritical carbon dioxide extraction. The use of supercritical carbon dioxide and enzymatic hydrolysis proved effective in the isolation of isorhamnetin conjugates [49]. The use of enzymes allowed the accessibility of the phenolic compounds through the breakage of the dietary fiber compounds that are eliminated by the action of CO2 and co-solvent during the extraction [52].
The high content of phenols, flavonoids, and also ascorbic acid in cladodes is highly related to several biological effects, such as antioxidant, antibacterial, antifungal, and cytotoxic activities [46,53].
As previously mentioned, cladodes have in their composition an hydrocolloid substance constituted by a complex polysaccharide of high molecular weight, named mucilage, and that substance is produced in specialized plant cells [32,54]. Mucilage represents about 14% of the cladode dry weight, and its physiological function is to regulate the cellular water content during prolonged drought and the calcium fluxes of the plant [35,55]. It is reported that cladodes in an older maturity stage have a decrease in mucilage content because, as a part of the soluble fiber, its content decreases along with the cladode maturation [32]. The mucilage is a complex polysaccharide resulting from the polymerization of monosaccharides like arabinose, galactose, rhamnose, xylose, and uronic acids (e.g., galacturonic acid) and is present in the internal layer of cladodes [56]. The polysaccharide allows the plant to retain a large amount of water and has several functional properties like gelling, thickening, and emulsifying [57]. The mucilage structure is composed of two different water-soluble fractions: pectin with gelling properties with Ca2+ and mucilage without gelling properties [58].
Despite the nutritional value of these bio-macromolecules, they are of good interest to the food industry due to their versatility, gelling, and film-forming abilities. Thus, the extraction of mucilage and pectin is comprised of these general steps: removing the outer layers of cladodes to eliminate the spines and the peel; washing and cutting; mixing with a solvent; pressing/centrifugation; precipitation; and drying [59]. Table 2 summarizes the most recent research on the extraction of mucilage and pectin from cladodes.

Table 2.
Mucilage and pectin extraction procedures, yields, and compositions from cladodes of Opuntia spp.
Conventional methods are used to extract mucilage and pectin from Opuntia cladodes, based on the extraction with solvents to isolate those compounds that are all mixed and compose dietary fiber [54,60,61]. The solvents to be used in the extraction and precipitation can be ethanol, methanol, isopropyl alcohol, acetone, or a combination of solvents and can influence the extraction yield, so it is important to determine the best option [70].
Other techniques employed to improve the extraction are the use of ultrasounds, microwave irradiation, and enzyme-assisted extraction [62,63,65]. It is also reported by several authors that acidic, neutral, or basic environments are possible to use in the extraction [66].
The use of ultrasounds allows the reduction of the particle size by the enhancement of the surface area and mass transfer [71]. In microwave-assisted extraction, the irradiation power helps with the diffusion of the solvent into the plant matrix by dissolving the compounds aimed to be extracted [72]. These techniques are helpful in the extraction process by enhancing the extraction efficiency and reducing the use of solvents and the time of extraction [63]. The use of chelating agents such as EDTA that interact with Ca2+ also helps with the extraction and improves the process [69]. Since pectins present in cladodes are categorized as low methoxyl pectins, the use of chelating agents for calcium sequestration allows the decrease of the degree of methoxylation by improving the gelling capacity of this pectin [73].
Low-methoxyl pectins have a large application in the food industry due to their gelling and stabilizing properties [34,39,60]. Mucilage can be used as a functional additive and has applications in different industries: as a water purifying agent, as an organic adhesive to lime in construction, as an inhibitor of the corrosion of aluminum, and in the food industry as an edible coating in fruits, while also performing as a stabilizer of emulsions, foams, and suspensions [32,74]. One of the novel potential applications of mucilage is to be used as a material for alternative food packaging once it can replace fossil-based plastics or reinforce polymeric matrices [75].
2.2. Fruits
Prickly pears are the succulent fruits from Opuntia spp., characterized by their high content in water (almost 92% wt), followed by carbohydrates (4–6% wt), proteins (1–2% wt), and minerals (around 1% wt), of which calcium, phosphorus, and sodium are highlighted [76].
Opuntia spp. fruits are also a good source of bioactive compounds, especially phenolic compounds, and vitamins (A and C) (Table 3). Their composition depends on several factors, such as soil, place of planting, environmental conditions, age, and species [74], which explains the differences observed in the data found in the literature.

Table 3.
Bioactive composition and extraction procedures of prickly pears and seed oil.
Regarding ascorbic acid (vitamin C), Opuntia spp. is rich in this compound due to the light intensity of the planting site. It may also be related to less irrigation and lower temperatures [81]. Regarding the mineral content of Opuntia ficus-indica, the values obtained were, considering mg/100 g, 63.4 Mg; 18.7 Na; 108.8 K; 316.5 Ca; 37.8 Mn; 25.9 Fe; 12.6 Zn; 0.01 Cu; and 0.05 P [81]. Thus, it is noticeable that fruits have a significant amount of minerals, in addition to vitamin C, making them advantageous for use as a food supplement [81]. Considering the pulp of the prickly pears, they are rich in biologically active compounds, such as vitamins, polyphenols, carotenoids, and betalains, among others, that can be extracted and used by the pharmaceutical and food industries [78]. It was found that the red-skinned fruit had a total content of phenolic compounds between 164.6 and 218.8 mg per 100 g. There is a large amount of quercetin, isorhamnetin, luteolin, and kaempferol, and it has a relevant content of flavonoids with higher concentrations than edible parts of papaya, banana, and watermelon, for example. Numerous polyphenolic acids such as ferulic acid, p-coumaric acid, 4-hydroxybenzoic acid, caffeic acid, salicylic acid, and gallic acid have also been identified [82]. Peels are also rich in phytochemicals and have a high potential to serve as functional compounds, e.g., in active food packaging films, as was observed with cranberry extracts [83], seaweed extracts [84], different essential oils [85], and plant extracts [86]. The main compounds in the peels are cellulose, hemicellulose, pectin, proteins, antioxidants, flavonoids, minerals, and other polysaccharides [82].
Moreover, their pH, taste, and color are other interesting characteristics that arouse interest for this fruit to be used as food, in addition to the absence of lead and cadmium, which brings greater safety for their consumption [81].
Yet, the amount of by-products reaches around 30% of the total weight after processing the fruits, making it feasible to look for ways to use these by-products in a circular bio-economy approach [77].
According to the study by Elsy De Santiago et al. (2018) [87], cacti have a significant amount of fiber, such as pectin, lignin, mucilage, cellulose, and hemicellulose, which help in the metabolism of glucose and lipids [87].
In addition to the antioxidant properties, other actions are also attributed to the phenolic compounds, namely, anti-inflammatory, anti-diabetes, and anti-cancer [77]. In the composition of these fruits, betalain pigments are also present, which present a red-violet (betacyanins) and yellow-orange (betaxanthins) color. This pigmentation is stable at a pH between 3 and 7, making it possible to use it as a natural color in food and as nutraceuticals, for example [77]. In the study by Tomás García-Cayuela et al. [79], the composition of betalains and phenolic compounds of the peels, pulps, and whole fruit was analyzed and quantified through the evaluation of three varieties from Spain and three from Mexico. The study made a complete comparison of the amounts of these compounds in Opuntia ficus-indica. In addition, betalains are natural pigments with active properties (antioxidant, antimicrobial), sensitivity to pH, and other interesting features that could be applied as a bio-based sensor for smart packaging systems. As these compounds are more pH-stable than anthocyanins, their use in smart packaging constitutes a promising alternative [88]. Tests made with the incorporation of amaranth leaf extracts, rich in betalains, in bio-based polymers support this application. In the mentioned study, following the degradation of poultry meat and fish, total volatile basic nitrogen content increased and pH was altered, modifying the betalain chemical structure, which changed the film’s color from red to yellow [89].
The seeds have a high number of compounds beneficial to health, such as unsaturated fatty acids, phytosterols, fat-soluble vitamins (vitamin E), and β-carotene, among others with antioxidant values. They can be used in the food and cosmetics industries and also for the prevention of chronic diseases [82].
The extraction of oil from seeds is traditionally performed using the solid-liquid extraction method with organic solvents such as hexane, chloroform, and petroleum ethers. With this procedure, about 13% of the seed oil can be extracted, depending on the species and the extraction conditions used. This extracted oil is rich in unsaturated acids such as oleic, vaccenic, and linolenic acids. They also have a significant number of tocopherols and phenolic compounds that have antioxidant activity. The total phenolic acid contents in the seed ethanol extract range from 48 to 89 mg in 100 g; and the contents of total flavonoids vary from 1.55 to 2.64 mg in 100 g; the total tannin contents vary between 4.1 and 6.6 mg in 100 g [82].
2.3. Opuntia Flowers and Roots
The flowers of Opuntia spp. are considered a vegetable, and the fruits and the young cladodes (or nopalitos) can be eaten as such [90]. Moreover, flowers from different plants are known to have wide medicinal properties and are being recognized for their antioxidant properties; however, few data are reported in the literature regarding the phytochemicals and antioxidant properties of Opuntia spp. flowers [51]. Traditionally, the flowers from these Cactaceae are used for medical purposes; in Tunisian markets, dried flowers of prickly pear are sold and used as an infusion to treat kidney stones [91]. The data found in recent literature on the phytochemical composition and the extraction procedures from Opuntia spp. flowers and roots are summarized in Table 4.

Table 4.
Bioactive composition and extraction procedures of Opuntia spp. flowers and roots.
It is noticeable that the flowers and roots of Opuntia spp. are rich in phenolic compounds with proven antioxidant and antimicrobial activities [92,93,95]. Once those parts of this perennial crop are generally neglected (i.e., lost in the cultivation process), there are opportunities to prepare such extracts for use either as food additives, with further purification in the pharmaceutical industry, or even as food supplements [94,95].
3. Applications in Food Products
3.1. Food Applications of Cladodes
Cladodes and their by-products can be used in a variety of industries (Figure 1). The most common uses of cladodes are food and feed consumption [44]. As food, it is consumed fresh or processed into several products such as soups, salads, juices, or bakery products to produce cookies, bread, and biscuits [45,96]. Due to the presence of several compounds with bioactive properties and a high content of dietary fiber, cladodes are also widely applied in cosmetics, pharmaceuticals, and nutraceutical products. As an example, the incorporation of cladodes (up to 10% w/w) into durum wheat bread revealed an improvement in the antioxidant activity of the bread without affecting the rheological properties [97]. Fortification of pasta with cladodes extracts in substitution of water demonstrated to be useful by increasing the fiber content with antioxidant features and with satisfactory acceptance in sensory analysis [98]. Furthermore, the use of cladodes powder (Opuntia ficus-indica f. inermis) as a substitute for wheat flour in cookies demonstrated an increase in dietary fiber and mineral content. The cookies produced contained a high level of fat, which makes the cookies highly susceptible to oxidation, but the use of cladodes powder showed a positive effect on reducing oxidation when compared with the control cookies, which were only produced with wheat flour [99]. Cladodes were also added to maize flour to improve the nutritional and physicochemical properties of tortillas in Mexico. Once again, the substitution of maize flour with cladodes powder at 6% improved the dietary fiber and mineral content (e.g., calcium), becoming a source for the intake of these nutrients [100]. In semi-arid regions, cladodes are commonly used for animal feed due to their richness in water and carbohydrates, necessary for their survival [101]. Cladodes were used as whole or supplementary foods, e.g., sheep and goats [102,103].
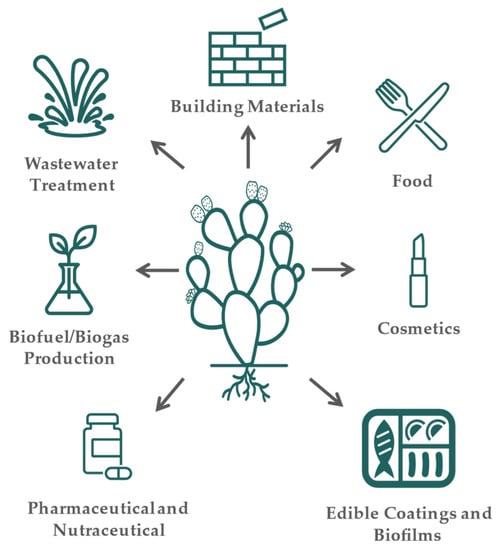
Figure 1.
Cladode applications.
Mucilage and pectins are also used in food packaging applications as edible coatings and biobased films [104,105]. Some studies showed that Opuntia ficus-indica mucilage is effective as a coating material to extend the shelf life of fresh strawberries [106], kiwifruit slices [107], and fig fruit [108]. Edible films produced from mucilage and pectin showed poor mechanical and physical properties, suggesting the need to incorporate other compounds to enhance those characteristics [75,109]. The incorporation of reinforcements, such as nanocellulose, nanoclays, and nanometal oxides, is an option, as it was observed with other biobased polymers (e.g., chitosan) [110,111]. Furthermore, the presence of bioactive compounds on cladodes makes them useful to enhance bioactive characteristics when incorporated in films, e.g., starch-based films, when compared to standalone films [112]. Several additives to improve cactus mucilage film’s mechanical resistance have been studied, e.g., calcium and gelatin [109,113] or beeswax to reduce water vapor permeability [113]. The use of different plasticizers (glycerol, sorbitol, PEG-200, and PEG-400) was also tested, showing that their structural features improved distinct interactions with mucilage polysaccharides [61]. Incorporating essential oils or extracts rich in phenolic compounds can add to the biobased films’ antimicrobial and antioxidant activities, as was observed in other works with biobased polymers [114].
Although more studies are needed to improve the characteristics of the materials, cladodes mucilage and pectins demonstrate promising applications as an alternative to fossil-based plastics currently used in the food industry.
3.2. Food Applications of Prickly Pear Fruits
The prickly pear and its by-products can be used in a variety of industries. Various products have been developed with cactus pear residues, such as yoghurts, snacks, and margarine.
In the food industry, one of the uses is the production of juices. To maintain stability and extend their shelf life, heat treatment is commonly used. However, exposure to high temperatures can cause the degradation of thermolabile compounds and modification of the organoleptic temperatures, so alternative technologies have been attempted such as the use of high pressure, pulsed electric fields (PEF), and ultrasound. The presence of phenolic compounds, vitamins, and other bioactive compounds in the juices can be a complement for the consumer [82]. In this sense, the use of PEF technology was applied to help in the inhibition of S. cerevisiae, along with pH reduction and the use of preservatives (sodium benzoate and potassium sorbate) in prickly pear juice [115]. The combination of these factors leads to microbial reduction and preservation for 21 days at 25 °C.
Prickly pear fruit is rich in several bioactive compounds, so the addition of this fruit to other food products can provide or enhance several properties, namely, antioxidant activity. That is the case with the incorporation of prickly pear into a gluten-free pasta made from rice-field bean flour, which allowed the increment of phenolic compounds and antioxidant properties at a percentage of 15% (w/w) [116]. This food product emerges as an alternative for celiac patients. Studies made on consumer preferences in Italy related to using Opuntia ficus-indica as an ingredient in new functional pasta showed a significant respondent interest regarding the health benefits and the nutritional and environmental aspects of this type of functional pasta [117]. However, the studies also reveal that the functional pasta should retain the organoleptic and physical properties of durum wheat-based pasta [118]. It was also demonstrated that the introduction of prickly pear peel powder (5% w/w) in cracker formulation could be a source of dietary fiber and bioactive compounds without affecting the quality of the product. The crackers presented an increment of around 8% in terms of antioxidant activity, and total dietary fiber went from 5.89 g/100 g to 8.11 g/100 g, when compared with the control [119].
Food supplements have been developed by using food by-products from Opuntia. Tablets were developed from Opuntia ficus-indica L. Mill fruits (green and red varieties) [120]. In this work, the formulation of tablets included the conjugation of microcrystalline cellulose with lactose and the addition of talcum powder and magnesium stearate. Each tablet presented a total weight of between 0.7 and 1 g. In terms of dietary fiber, the tablets presented a content of 0.24 g using green fruit and 0.15 g using red fruit. Furthermore, the tablets showed good antioxidant activity, with DPPH radical inhibition of 24% for the tablets made from red fruits and 20% for green fruits. According to this study, by-products of O. ficus-indica have a high potential for use in foods due to their high dietary fiber content and antioxidant activity, which can prevent free radical damage [120].
The use of hydro-ethanolic extracts from prickly pear peels was tested as an alternative to vitamin E in the prevention of margarine oxidation [121]. The extracts produced were rich in phenolic compounds, with a content of 1512.58 mg GAE/100 g of dry matter. Three different concentrations of the extracts were tested (50, 100, and 150 ppm). The use of prickly pear extract in margarine showed a positive effect on the reduction of oxidation when compared with the same product with vitamin E, even at the lowest concentration used (50 ppm). The margarine developed with 150 ppm of extract demonstrated a higher tendency to oxidation, which can be caused by the pro-oxidant effect of the phenolic compounds at high concentrations [121].
Prickly pear extract was added to cooked beef burger patties, and its effect on quality parameters was evaluated [122]. The extract (5% v/w) was added directly or encapsulated in alginate beads. The encapsulation of bioactive compounds from prickly pear could be a vehicle for their preservation for a long time. The use of prickly pear extracts showed no adverse effects on the cooked burgers. In fact, the intrinsic antioxidant activity present in the extracts, especially the ones encapsulated in alginate, not only enhances that property in the burgers but also avoids lipid oxidation when compared to other burgers in the study.
The seeds present in fruits also have the potential to be used in the food industry due to their richness in fatty acids, in particular, linoleic, palmitic, and stearic acids [123].
3.3. Food Applications of Opuntia spp. Flowers
The flowers from Opuntia spp. can also be used in food applications, but the most known uses are in decoctions and infusions made from dried flowers, which are widely used in traditional medicine [124]. It is reported that decoctions and infusions from the flowers of O. ficus-indica are a source of minerals, namely K and Ca, and also a source of polyphenols, flavonoids, and tannins [125]. The maceration of flowers from Opuntia ficus-indica was studied as a heat stabilizer of olive oil as well as its effect on the quality of the final product. The addition of 5% (w/w) of flowers to the olive oil leads to an increase in the phenolic compound content, improving the stability in terms of oxidation [126].
The knowledge of their characteristics, such as the hydration properties and oil holding capacity, is important as they may interfere with the functionality and nutritional quality of the food [91]. Flowers from Opuntia ficus-indica and Opuntia stricta harvested from a wild population located in Tunisia in the post-flowering stage were analyzed in terms of their oil holding capacity (OHC) and hydration properties through the determination of the swelling capacity (SWC), water solubility index (WSI), and water holding capacity (WHC). The hydration properties are related to the presence of soluble molecules (such as sugar; more sugar reflects superior WSI), to the polysaccharide content (correlated with SWC), and to the hydrophilic constituents (WHC). The OHC is an important parameter from an industrial point of view as it is correlated to the product’s emulsifying capacity. The results varied depending on the specimen, and overall, O. ficus-indica presented superior WSI, WHC, and OHC, while the SWC was higher for O. stricta. The values found for SWC were similar to those reported for wheat and carrots but smaller than in cauliflower. Regarding the WHC, Opuntia spp. flowers presented values of the same magnitude as other dietary fiber concentrates (from by-products) and some commercial dietary fiber-rich supplements. OHC found were similar to those reported in the cladodes and of the same magnitude as other fruits, vegetables, and seaweeds (around 2 g/g), but lower than for cereal fibers (2–4 g/g) [91].
Thus, Opuntia spp. flowers can be highlighted as an excellent alternative source of dietary fiber for human consumption but also as functional ingredients in the food industry as jellying agents to retard syneresis, modify the viscosity and texture of formulated foods, or stabilize food emulsions [91]. Together with the nutraceutical and pharmacological approaches, the use of Opuntia spp. flowers can always be explored, adding some economic value to these valuable by-products.
4. Conclusions
Opuntia spp. is a crop that has been gaining attention throughout the years and has been increasingly studied. Due to its adaptability to adverse environments, it has a high potential to generate value-added products from its fruits and cladodes. Cladodes are a good source of dietary fiber and rich in water, so their consumption should be more considered in the human diet due to their health benefits. Fruits, due to their richness in sugars, can easily be used in the production of juices, jams, and marmalades. Recent studies have used innovative technology to produce food products with greater stability and safety. Moreover, cladodes and fruits are rich in several bioactive compounds and have a high potential to be used in several nutraceutical products. Betalains, which are present in fruits, can also be used as food colorants as an alternative to the ones currently on the market and have a great potential to be used as a sensor in food packaging.
Nevertheless, Opuntia spp. by-products and their use in the food industry can be further investigated to better understand the potential uses of this crop in order to enhance its consumption globally.
Author Contributions
The review paper was planned and written with contributions of all the authors. Conceptualization, C.R., V.G.L.S., A.L.F. and I.C.; methodology, C.R. and V.G.L.S.; resources, C.R. and V.G.L.S.; writing—original draft preparation, C.R., C.D.d.P., S.L. and V.G.L.S.; writing—review and editing, C.R., V.G.L.S., A.M., A.O., M.R., L.P., I.C. and A.L.F.; supervision, V.G.L.S., A.M., A.O., I.C. and A.L.F.; funding acquisition, V.G.L.S., A.O., M.R., L.P., I.C. and A.L.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by national funding by the FCT, Foundation for Science and Technology, through the individual research grant (2020.04441.BD) of C.R. This work was supported by the Associate Laboratory for Green Chemistry—LAQV, which is financed by national funds from FCT/MCTES (UIDB/50006/2020 and UIDP/50006/2020), and by the Mechanical Engineering and Resource Sustainability Center—MEtRICs, which is financed by national funds from FCT/MCTES (UIDB/04077/2020 and UIDP/04077/2020). This work also received funds from FCT/MCTES through project ERANETMED/0001/2017—MediOpuntia (Portugal). The project MediOpuntia also received support through ERANETMED-MediOpuntia from Science and Technology Development Funding authority (STDF—Egypt), Ministry of Education, Universities and Research (MIUR—Italy), and Ministry of National Education, Vocational Training, Higher Education and Scientific Research (MENFPESRS—Morocco).
Data Availability Statement
Data sharing is not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ahmed, S.N.; Ahmad, M.; Zafar, M.; Rashid, S.; Sultana, S. Classification, Distribution and Morphological Characterization of Opuntia Species. In Opuntia spp.: Chemistry, Bioactivity and Industrial Applications; Springer: Berlin/Heidelberg, Germany, 2021; pp. 109–119. [Google Scholar]
- Abouseadaa, H.H.; Atia, M.A.M.; Younis, I.Y.; Issa, M.Y.; Ashour, H.A.; Saleh, I.; Osman, G.H.; Arif, I.A.; Mohsen, E. Gene-targeted molecular phylogeny, phytochemical profiling, and antioxidant activity of nine species belonging to family Cactaceae. Saudi J. Biol. Sci. 2020, 27, 1649–1658. [Google Scholar] [CrossRef] [PubMed]
- Agüero, J.I.; Galati, B.G.; Torretta, J.P. Structure and ultrastructure of floral nectaries of two Opuntia species (Cactaceae) in relation to their floral visitors. Plant Syst. Evol. 2018, 304, 1057–1067. [Google Scholar] [CrossRef]
- Edvan, R.L.; Mota, R.R.M.; Dias-Silva, T.P.; do Nascimento, R.R.; de Sousa, S.V.; da Silva, A.L.; de Araújo, M.J.; Araújo, J.S. Resilience of cactus pear genotypes in a tropical semi-arid region subject to climatic cultivation restriction. Sci. Rep. 2020, 10, 10040. [Google Scholar] [CrossRef] [PubMed]
- Khodaeiaminjan, M.; Nassrallah, A.A.; Kamal, K.Y. Potential Attribute of Crassulacean Acid Metabolism of Opuntia spp. Production in Water-Limited Conditions. In Opuntia spp.: Chemistry, Bioactivity and Industrial Applications; Springer: Berlin/Heidelberg, Germany, 2021; pp. 201–218. [Google Scholar]
- Arba, M.; Falisse, A.; Choukr-Allah, R.; Sindic, M. Biology, flowering and fruiting of the cactus Opuntia spp.: A review and some observations on three varieties in Morocco. Braz. Arch. Biol. Technol. 2017, 60, e17160568. [Google Scholar] [CrossRef]
- Anderson, E.F. The Cactus Family. Timber Press: Portland, OR, USA, 2001; ISBN 0881924989. [Google Scholar]
- Khatabi, O.; Hanine, H.; Elothmani, D.; Hasib, A. Extraction and determination of polyphenols and betalain pigments in the Moroccan Prickly pear fruits (Opuntia ficus-indica). Arab. J. Chem. 2016, 9, S278–S281. [Google Scholar] [CrossRef]
- Dubeux, J.C.B.; dos Santos, M.V.F.; da Cunha, M.V.; dos Santos, D.C.; de Alemeida Souza, R.T.; de Mello, A.C.L.; de Souza, T.C. Cactus (Opuntia and Nopalea) nutritive value: A review. Anim. Feed Sci. Technol. 2021, 275, 114890. [Google Scholar] [CrossRef]
- Sá Souza, M.; Júnior, G.N.A.; Souza, L.S.B.; Ferraz Jardim, A.M.R.; da Silva, G.I.N.; Araújo, G.G.L.; Campos, F.S.; Leite, M.L.M.V.; Tabosa, J.N.; Silva, T.G.F. Forage yield, competition and economic benefit of intercropping cactus and millet with mulch in a semi-arid environment. Afr. J. Range Forage Sci. 2022, 1–12. [Google Scholar] [CrossRef]
- Stavi, I. Ecosystem services related with Opuntia ficus-indica (prickly pear cactus): A review of challenges and opportunities. Agroecol. Sustain. Food Syst. 2022, 46, 815–841. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Hamid, A.; Imtiaz, H.; Rizwan, M.; Imran, M.; Sheikh, U.A.A.; Saira, I. Cactus pear: A weed of dry-lands for supplementing food security under changing climate. Planta Daninha 2020, 38, e020191761. [Google Scholar] [CrossRef]
- Rocha Filho, R.R.; Santos, D.C.; Véras, A.S.C.; Siqueira, M.C.B.; Novaes, L.P.; Mora-Luna, R.; Monteiro, C.C.F.; Ferreira, M.A. Can spineless forage cactus be the queen of forage crops in dryland areas? J. Arid Environ. 2021, 186, 104426. [Google Scholar] [CrossRef]
- Lahbouki, S.; Ben-Laouane, R.; Anli, M.; Boutasknit, A.; Ait-Rahou, Y.; Ait-El-Mokhtar, M.; El Gabardi, S.; Douira, A.; Wahbi, S.; Outzourhit, A. Arbuscular mycorrhizal fungi and/or organic amendment enhance the tolerance of prickly pear (Opuntia ficus-indica) under drought stress. J. Arid Environ. 2022, 199, 104703. [Google Scholar] [CrossRef]
- Scalisi, A.; Morandi, B.; Inglese, P.; Lo Bianco, R. Cladode growth dynamics in Opuntia ficus-indica under drought. Environ. Exp. Bot. 2016, 122, 158–167. [Google Scholar] [CrossRef]
- Elbana, M.; El-Gamal, E.; Mohamed, A.; Fernando, A.L.; Pari, L.; Outzourhit, A.; Elwakeel, M.; El-Sheikh, W.; Rashad, M. Effect of irrigation scheduling on canopy cover development and crop-water management related parameters of O. ficus-indica under prolonged drought conditions. Sci. J. Agric. Sci. 2020, 2, 113–122. [Google Scholar] [CrossRef]
- Yahia, E.M.; Mondragon-Jacobo, C. Nutritional components and anti-oxidant capacity of ten cultivars and lines of cactus pear fruit (Opuntia spp.). Food Res. Int. 2011, 44, 2311–2318. [Google Scholar] [CrossRef]
- Piga, A. Cactus Pear: A Fruit of Nutraceutical and Functional Importance. J. Prof. Assoc. Cactus Dev. 2004, 6, 9–22. [Google Scholar]
- Barba, F.J.; Garcia, C.; Fessard, A.; Munekata, P.E.S.; Lorenzo, J.M.; Aboudia, A.; Ouadia, A.; Remize, F. Opuntia ficus-indica Edible Parts: A Food and Nutritional Security Perspective. Food Rev. Int. 2020, 38, 930–952. [Google Scholar] [CrossRef]
- Aruwa, C.E.; Amoo, S.; Kudanga, T. Phenolic compound profile and biological activities of Southern African Opuntia ficus-indica fruit pulp and peels. LWT 2019, 111, 337–344. [Google Scholar] [CrossRef]
- Bakar, B.; Çakmak, M.; Ibrahim, M.S.; Özer, D.; Saydam, S.; Karatas, F. Investigation of amounts of vitamins, lycopene, and elements in the fruits of Opuntia ficus-indica subjected to different pretreatments. Biol. Trace Elem. Res. 2020, 198, 315–323. [Google Scholar] [CrossRef]
- Gouws, C.A.; Georgousopoulou, E.N.; Mellor, D.D.; McKune, A.; Naumovski, N. Effects of the consumption of prickly pear cacti (Opuntia spp.) and its products on blood glucose levels and insulin: A systematic review. Medicina B. Aires 2019, 55, 138. [Google Scholar] [CrossRef]
- Gouws, C.A.; McKune, A.; Tee, N.; Somerset, S.; Mortazavi, R. Prickly pear juice consumption after fat intake affects postprandial heart rate variability but not traditional risk factors of cardiovascular disease in healthy men. Nutrition 2022, 96, 111555. [Google Scholar] [CrossRef]
- Lamia, I.; Zouhir, C.; Youcef, A. Characterization and transformation of the Opuntia ficus-indica fruits. J. Food Meas. Charact. 2018, 12, 2349–2357. [Google Scholar] [CrossRef]
- Hernández-Becerra, E.; de los Angeles Aguilera-Barreiro, M.; Contreras-Padilla, M.; Pérez-Torrero, E.; Rodriguez-Garcia, M.E. Nopal cladodes (Opuntia ficus-indica): Nutritional properties and functional potential. J. Funct. Foods 2022, 95, 105183. [Google Scholar] [CrossRef]
- Lahbouki, S.; Anli, M.; El Gabardi, S.; Ait-El-Mokhtar, M.; Ben-Laouane, R.; Boutasknit, A.; Ait-Rahou, Y.; Outzourhit, A.; Wahbi, S.; Douira, A. Evaluation of arbuscular mycorrhizal fungi and vermicompost supplementation on growth, phenolic content and antioxidant activity of prickly pear cactus (Opuntia ficus-indica). Plant Biosyst. Int. J. Deal. all Asp. Plant Biol. 2021, 156, 882–892. [Google Scholar] [CrossRef]
- Astello-García, M.G.; Cervantes, I.; Nair, V.; Santos-Díaz, M.d.S.; Reyes-Agüero, A.; Guéraud, F.; Negre-Salvayre, A.; Rossignol, M.; Cisneros-Zevallos, L.; Barba de la Rosa, A.P. Chemical composition and phenolic compounds profile of cladodes from Opuntia spp. cultivars with different domestication gradient. J. Food Compos. Anal. 2015, 43, 119–130. [Google Scholar] [CrossRef]
- Pandit, V.; Kashive, D.; Sharma, T.K. Formulation and Evaluation of Novel Formulation for Diabetes Induced Hypertension using Modified Innate Superdisintegrant. J. Drug Deliv. Ther. 2020, 10, 240–250. [Google Scholar] [CrossRef]
- Gouws, C.; Mortazavi, R.; Mellor, D.; McKune, A.; Naumovski, N. The effects of Prickly Pear fruit and cladode (Opuntia spp.) consumption on blood lipids: A systematic review. Complement. Ther. Med. 2020, 50, 102384. [Google Scholar] [CrossRef]
- Ennouri, M.; Fetoui, H.; Bourret, E.; Zeghal, N.; Attia, H. Evaluation of some biological parameters of Opuntia ficus-indica. 1. Influence of a seed oil supplemented diet on rats. Bioresour. Technol. 2006, 97, 1382–1386. [Google Scholar] [CrossRef]
- Sirotkin, A.V. Can nopal cactus (Opuntia ficus-indica L. Miller) treat obesity? Obes. Med. 2022, 30, 100390. [Google Scholar] [CrossRef]
- Contreras-Padilla, M.; Rodríguez-García, M.E.; Gutiérrez-Cortez, E.; Valderrama-Bravo, M.d.C.; Rojas-Molina, J.I.; Rivera-Muñoz, E.M. Physicochemical and rheological characterization of Opuntia ficus-indica mucilage at three different maturity stages of cladode. Eur. Polym. J. 2016, 78, 226–234. [Google Scholar] [CrossRef]
- Nharingo, T.; Moyo, M. Application of Opuntia ficus-indica in bioremediation of wastewaters. A critical review. J. Environ. Manag. 2016, 166, 55–72. [Google Scholar] [CrossRef]
- del Socorro Santos-Díaz, M.; Barba de la Rosa, A.P.; Héliès-Toussaint, C.; Guéraud, F.; Nègre-Salvayre, A. Opuntia spp.: Characterization and Benefits in Chronic Diseases. Oxid. Med. Cell. Longev. 2017, 2017, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Ginestra, G.; Parker, M.L.; Bennett, R.N.; Robertson, J.; Mandalari, G.; Narbad, A.; Lo Curto, R.B.; Bisignano, G.; Faulds, C.B.; Waldron, K.W. Anatomical, chemical, and biochemical characterization of cladodes from prickly pear [Opuntia ficus-indica (L.) Mill.]. J. Agric. Food Chem. 2009, 57, 10323–10330. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Carle, R. Cactus stems (Opuntia spp.): A review on their chemistry, technology, and uses. Mol. Nutr. Food Res. 2005, 49, 175–194. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.A.; Albuquerque, T.G.; Pereira, P.; Ramalho, R.; Vicente, F.; Oliveira, M.B.P.P.; Costa, H.S. Opuntia ficus-indica (L.) Mill.: A Multi-Benefit Potential to Be Exploited. Molecules 2021, 26, 951. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Urbiola, M.I.; Pérez-Torrero, E.; Rodríguez-García, M.E. Chemical Analysis of Nutritional Content of Prickly Pads (Opuntia ficus-indica) at Varied Ages in an Organic Harvest. Int. J. Environ. Res. Public Health 2011, 8, 1287–1295. [Google Scholar] [CrossRef]
- Aragona, M.; Lauriano, E.R.; Pergolizzi, S.; Faggio, C. Opuntia ficus-indica (L.) Miller as a source of bioactivity compounds for health and nutrition. Nat. Prod. Res. 2018, 32, 2037–2049. [Google Scholar] [CrossRef]
- Uebelhack, R.; Busch, R.; Alt, F.; Beah, Z.M.; Chong, P.W. Effects of Cactus Fiber on the Excretion of Dietary Fat in Healthy Subjects: A Double Blind, Randomized, Placebo-Controlled, Crossover Clinical Investigation. Curr. Ther. Res. Clin. Exp. 2014, 76, 39–44. [Google Scholar] [CrossRef]
- Méndez, L.P.; Flores, F.T.; Martín, J.D.; Rodríguez Rodríguez, E.M.; Díaz Romero, C. Physicochemical characterization of cactus pads from Opuntia dillenii and Opuntia ficus-indica. Food Chem. 2015, 188, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Feugang, J.M.; Konarski, P.; Zou, D.; Stintzing, F.C.; Zou, C. Nutritional and medicinal use of Cactus pear (Opuntia spp.) cladodes and fruits. Front. Biosci. 2006, 11, 2574–2589. [Google Scholar] [CrossRef]
- El-Mostafa, K.; El Kharrassi, Y.; Badreddine, A.; Andreoletti, P.; Vamecq, J.; Saïd, M.H.; Kebbaj, E.; Latruffe, N.; Lizard, G.; Nasser, B.; et al. Nopal Cactus (Opuntia ficus-indica) as a Source of Bioactive Compounds for Nutrition, Health and Disease. Molecules 2014, 19, 14879–14901. [Google Scholar] [CrossRef]
- Ciriminna, R.; Chavarría-Hernández, N.; Rodríguez-Hernández, A.I.; Pagliaro, M. Toward unfolding the bioeconomy of nopal (Opuntia spp.). Biofuels Bioprod. Biorefining 2019, 13, 1417–1427. [Google Scholar] [CrossRef]
- Msaddak, L.; Abdelhedi, O.; Kridene, A.; Rateb, M.; Belbahri, L.; Ammar, E.; Nasri, M.; Zouari, N. Opuntia ficus-indica cladodes as a functional ingredient: Bioactive compounds profile and their effect on antioxidant quality of bread. Lipids Health Dis. 2017, 16, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ben Lataief, S.; Zourgui, M.N.; Rahmani, R.; Najjaa, H.; Gharsallah, N.; Zourgui, L. Chemical composition, antioxidant, antimicrobial and cytotoxic activities of bioactive compounds extracted from Opuntia dillenii cladodes. J. Food Meas. Charact. 2020, 15, 782–794. [Google Scholar] [CrossRef]
- Missaoui, M.; D′Antuono, I.; D′Imperio, M.; Linsalata, V.; Boukhchina, S.; Logrieco, A.F.; Cardinali, A. Characterization of micronutrients, bioaccessibility and antioxidant activity of prickly pear cladodes as functional ingredient. Molecules 2020, 25, 2176. [Google Scholar] [CrossRef] [PubMed]
- Rocchetti, G.; Pellizzoni, M.; Montesano, D.; Lucini, L. Italian opuntia ficus-indica cladodes as rich source of bioactive compounds with health-promoting properties. Foods 2018, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Antunes-Ricardo, M.; Mendiola, J.A.; García-Cayuela, T.; Ibañez, E.; Gutiérrez-Uribe, J.A.; Pilar Cano, M.; Guajardo-Flores, D. Enzyme-assisted supercritical fluid extraction of antioxidant isorhamnetin conjugates from Opuntia ficus-indica (L.) Mill. J. Supercrit. Fluids 2020, 158, 104713. [Google Scholar] [CrossRef]
- De Santiago, E.; Juániz, I.; Cid, C.; De Peña, M.P. Extraction of (Poly)phenolic Compounds of Cactus (Opuntia ficus-indica (L.) Mill.) Cladodes. Food Anal. Methods 2021, 14, 1167–1175. [Google Scholar] [CrossRef]
- Ammar, I.; Ennouri, M.; Attia, H. Phenolic content and antioxidant activity of cactus (Opuntia ficus-indica L.) flowers are modified according to the extraction method. Ind. Crops Prod. 2015, 64, 97–104. [Google Scholar] [CrossRef]
- Antunes-Ricardo, M.; García-Cayuela, T.; Mendiola, J.A.; Ibañez, E.; Gutiérrez-Uribe, J.A.; Cano, M.P.; Guajardo-Flores, D. Supercritical CO2 enzyme hydrolysis as a pretreatment for the release of isorhamnetin conjugates from Opuntia ficus-indica (L.) Mill. J. Supercrit. Fluids 2018, 141, 21–28. [Google Scholar] [CrossRef]
- Aruwa, C.E.; Amoo, S.O.; Kudanga, T. Opuntia (Cactaceae) plant compounds, biological activities and prospects—A comprehensive review. Food Res. Int. 2018, 112, 328–344. [Google Scholar] [CrossRef]
- Dick, M.; Dal Magro, L.; Rodrigues, R.C.; Rios, A.d.O.; Flôres, S.H. Valorization of Opuntia monacantha (Willd.) Haw. cladodes to obtain a mucilage with hydrocolloid features: Physicochemical and functional performance. Int. J. Biol. Macromol. 2019, 123, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Sáenz, C.; Sepúlveda, E.; Matsuhiro, B. Opuntia spp. mucilage’s: A functional component with industrial perspectives. J. Arid Environ. 2004, 57, 275–290. [Google Scholar] [CrossRef]
- Rodríguez-González, S.; Martínez-Flores, H.E.; Chávez-Moreno, C.K.; Macías-Rodríguez, L.I.; Zavala-Mendoza, E.; Garnica-Romo, M.G.; Chacõn-García, L. Extraction and characterization of mucilage from wild species of Opuntia. J. Food Process Eng. 2014, 37, 285–292. [Google Scholar] [CrossRef]
- Bayar, N.; Kriaa, M.; Kammoun, R. Extraction and characterization of three polysaccharides extracted from Opuntia ficus-indica cladodes. Int. J. Biol. Macromol. 2016, 92, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda, E.; Sáenz, C.; Aliaga, E.; Aceituno, C. Extraction and characterization of mucilage in Opuntia spp. J. Arid Environ. 2007, 68, 534–545. [Google Scholar] [CrossRef]
- Rodrigues, C.; Souza, V.G.L.; Rashad, M.; Pari, L.; Outzourhit, A.; Fernando, A.L. Mucilage extraction from Opuntia spp. for production of biofilms. In Proceedings of the 27th European Biomass Conference and Exhibition, Lisbon, Portugal, 27–30 May 2019; pp. 1456–1459. [Google Scholar]
- Lefsih, K.; Delattre, C.; Pierre, G.; Michaud, P.; Aminabhavi, T.M.; Dahmoune, F.; Madani, K. Extraction, characterization and gelling behavior enhancement of pectins from the cladodes of Opuntia ficus-indica. Int. J. Biol. Macromol. 2016, 82, 645–652. [Google Scholar] [CrossRef]
- Gheribi, R.; Puchot, L.; Verge, P.; Jaoued-Grayaa, N.; Mezni, M.; Habibi, Y.; Khwaldia, K. Development of plasticized edible films from Opuntia ficus-indica mucilage: A comparative study of various polyol plasticizers. Carbohydr. Polym. 2018, 190, 204–211. [Google Scholar] [CrossRef]
- Bayar, N.; Bouallegue, T.; Achour, M.; Kriaa, M.; Bougatef, A.; Kammoun, R. Ultrasonic extraction of pectin from Opuntia ficus-indica cladodes after mucilage removal: Optimization of experimental conditions and evaluation of chemical and functional properties. Food Chem. 2017, 235, 275–282. [Google Scholar] [CrossRef]
- Felkai-Haddache, L.; Remini, H.; Dulong, V.; Mamou-Belhabib, K.; Picton, L.; Madani, K.; Rihouey, C. Conventional and Microwave-Assisted Extraction of Mucilage from Opuntia ficus-indica Cladodes: Physico-Chemical and Rheological Properties. Food Bioprocess Technol. 2016, 9, 481–492. [Google Scholar] [CrossRef]
- Kalegowda, P.; Chauhan, A.S.; Nanjaraj Urs, S.M. Opuntia dillenii (Ker-Gawl) Haw cladode mucilage: Physico-chemical, rheological and functional behavior. Carbohydr. Polym. 2017, 157, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Bayar, N.; Friji, M.; Kammoun, R. Optimization of enzymatic extraction of pectin from Opuntia ficus-indica cladodes after mucilage removal. Food Chem. 2018, 241, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Chaouch, M.A.; Hafsa, J.; Rihouey, C.; Le Cerf, D.; Majdoub, H. Effect of pH during Extraction on the Antioxidant and Antiglycated Activities of Polysaccharides from Opuntia ficus-indica. J. Food Biochem. 2016, 40, 316–325. [Google Scholar] [CrossRef]
- Quinzio, C.; Ayunta, C.; Alancay, M.; de Mishima, B.L.; Iturriaga, L. Physicochemical and rheological properties of mucilage extracted from Opuntia ficus-indica (L. Miller). Comparative study with guar gum and xanthan gum. J. Food Meas. Charact. 2017, 12, 459–470. [Google Scholar] [CrossRef]
- Madera-Santana, T.J.; Vargas-Rodríguez, L.; Núñez-Colín, C.A.; González-García, G.; Peña-Caballero, V.; Núñez-Gastélum, J.A.; Gallegos-Vázquez, C.; Rodríguez-Núñez, J.R. Mucilage from cladodes of Opuntia spinulifera Salm-Dyck: Chemical, morphological, structural and thermal characterization. CyTA-J. Food 2018, 16, 650–657. [Google Scholar] [CrossRef]
- Pérez-Martínez, J.D.; Sánchez-Becerril, M.; Ornelas-Paz, J.J.; González-Chávez, M.M.; Ibarra-Junquera, V.; Escalante-Minakata, P. The Effect of Extraction Conditions on the Chemical Characteristics of Pectin from Opuntia ficus-indica Cladode Flour. J. Polym. Environ. 2013, 21, 1040–1051. [Google Scholar] [CrossRef]
- Inglese, P.; Saenz, C.; Mondragon, C.; Nefzaoui, A.; Louhaichi, M. Crop Ecology, Cultivation and Uses of Cactus Pear; FAO: Rome, Italy, 2017; ISBN 978-92-5-109860-8. [Google Scholar]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Thirugnanasambandham, K.; Sivakumar, V.; Maran, J.P. Microwave-assisted extraction of polysaccharides from mulberry leaves. Int. J. Biol. Macromol. 2015, 72, 1–5. [Google Scholar] [CrossRef]
- Gawkowska, D.; Cybulska, J.; Zdunek, A. Structure-related gelling of pectins and linking with other natural compounds: A review. Polymers 2018, 10, 762. [Google Scholar] [CrossRef]
- Monrroy, M.; García, E.; Ríos, K.; García, J.R. Extraction and Physicochemical Characterization of Mucilage from Opuntia cochenillifera (L.) Miller. J. Chem. 2017, 2017, 4301901. [Google Scholar] [CrossRef]
- Gheribi, R.; Khwaldia, K. Cactus Mucilage for Food Packaging Applications. Coatings 2019, 9, 655. [Google Scholar] [CrossRef]
- Tegegne, F. Nutritional value of Opuntia ficus-indica as a ruminant feed in Ethiopia. In Cactus (Opuntia spp.) as Forage; Mondragon, C., Gonzalez, S.P., Eds.; Food and Agriculture Organization: Rome, Italy, 2001; pp. 91–100. [Google Scholar]
- Melgar, B.; Inês, M.; Ciric, A.; Sokovic, M.; Garcia-castello, E.M.; Rodriguez-lopez, A.D.; Barros, L.; Ferreira, I. By-product recovery of Opuntia spp. peels: Betalainic and phenolic profiles and bioactive properties. Ind. Crops Prod. 2017, 107, 353–359. [Google Scholar] [CrossRef]
- Kuti, J.O. Antioxidant compounds from four Opuntia cactus pear fruit varieties. Food Chem. 2004, 85, 527–533. [Google Scholar] [CrossRef]
- García-Cayuela, T.; Gómez-Maqueo, A.; Guajardo-Flores, D.; Welti-Chanes, J.; Cano, M.P. Characterization and quantification of individual betalain and phenolic compounds in Mexican and Spanish prickly pear (Opuntia ficus-indica L. Mill) tissues: A comparative study. J. Food Compos. Anal. 2019, 76, 1–13. [Google Scholar] [CrossRef]
- Chougui, N.; Tamendjari, A.; Hamidj, W.; Hallal, S.; Barras, A.; Richard, T.; Larbat, R. Oil composition and characterisation of phenolic compounds of Opuntia ficus-indica seeds. Food Chem. 2013, 139, 796–803. [Google Scholar] [CrossRef]
- Chiteva, R.; Wairagu, N. Chemical and nutritional content of Opuntia ficus-indica (L.). Afr. J. Biotechnol. 2013, 12, 3309–3312. [Google Scholar]
- Barba, F.J.; Putnik, P.; Bursać Kovačević, D.; Poojary, M.M.; Roohinejad, S.; Lorenzo, J.M.; Koubaa, M. Impact of conventional and non-conventional processing on prickly pear (Opuntia spp.) and their derived products: From preservation of beverages to valorization of by-products. Trends Food Sci. Technol. 2017, 67, 260–270. [Google Scholar] [CrossRef]
- Severo, C.; Anjos, I.; Souza, V.G.L.; Canejo, J.P.; Bronze, M.R.; Fernando, A.L.; Coelhoso, I.; Bettencourt, A.F.; Ribeiro, I.A.C. Development of cranberry extract films for the enhancement of food packaging antimicrobial properties. Food Packag. Shelf Life 2021, 28, 100646. [Google Scholar] [CrossRef]
- Andrade, M.A.; Barbosa, C.H.; Souza, V.G.L.; Coelhoso, I.M.; Reboleira, J.; Bernardino, S.; Ganhão, R.; Mendes, S.; Fernando, A.L.; Vilarinho, F.; et al. Novel Active Food Packaging Films Based on Whey Protein Incorporated with Seaweed Extract: Development, Characterization, and Application in Fresh Poultry Meat. Coatings 2021, 11, 229. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Rodrigues, C.; Ferreira, L.; Pires, J.R.A.; Duarte, M.P.; Coelhoso, I.; Fernando, A.L. In vitro bioactivity of novel chitosan bionanocomposites incorporated with different essential oils. Ind. Crops Prod. Prod. 2019, 140, 111563. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Rodrigues, P.F.; Duarte, M.P.; Fernando, A.L. Antioxidant Migration Studies in Chitosan Films Incorporated with Plant Extracts. J. Renew. Mater. 2018, 6, 548–558. [Google Scholar] [CrossRef]
- De Santiago, E.; Domínguez-Fernández, M.; Cid, C.; De Peña, M.P. Impact of cooking process on nutritional composition and antioxidants of cactus cladodes (Opuntia ficus-indica). Food Chem. 2018, 240, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.; Souza, V.G.L.; Coelhoso, I.; Fernando, A.L. Bio-Based Sensors for Smart Food Packaging—Current Applications and Future Trends. Sensors 2021, 21, 2148. [Google Scholar] [CrossRef] [PubMed]
- Kanatt, S.R. Development of active/intelligent food packaging film containing Amaranthus leaf extract for shelf life extension of chicken/fish during chilled storage. Food Packag. Shelf Life 2020, 24, 100506. [Google Scholar] [CrossRef]
- Sáenz, C.; Berger, H.; Rodríguez-Félix, A.; Galletti, L.; García, J.C.; Sepúlveda, E.; Teresa, M.; Víctor, V.; De Cortázar, G.; Cuevas García, R.; et al. Agro-Industrial Utilization of Cactus Pear; Food and Agriculture Organization: Rome, Italy, 2013; ISBN 978-92-5-107987-4. [Google Scholar]
- Ammar, I.; Ennouri, M.; Bali, O.; Attia, H. Characterization of two prickly pear species flowers growing in Tunisia at four flowering stages. LWT-Food Sci. Technol. 2014, 59, 448–454. [Google Scholar] [CrossRef]
- Ammar, I.; Ennouri, M.; Khemakhem, B.; Yangui, T.; Attia, H. Variation in chemical composition and biological activities of two species of Opuntia flowers at four stages of flowering. Ind. Crops Prod. 2012, 37, 34–40. [Google Scholar] [CrossRef]
- Ammar, I.; Bardaa, S.; Mzid, M.; Sahnoun, Z.; Rebaii, T.; Attia, H.; Ennouri, M. Antioxidant, antibacterial and in vivo dermal wound healing effects of Opuntia flower extracts. Int. J. Biol. Macromol. 2015, 81, 483–490. [Google Scholar] [CrossRef]
- De Leo, M.; De Abreu, M.B.; Pawlowska, A.M.; Cioni, P.L.; Braca, A. Profiling the chemical content of Opuntia ficus-indica flowers by HPLC–PDA-ESI-MS and GC/EIMS analyses. Phytochem. Lett. 2010, 3, 48–52. [Google Scholar] [CrossRef]
- Alimi, H.; Hfaiedh, N.; Bouoni, Z.; Hfaiedh, M.; Sakly, M.; Zourgui, L.; Rhouma, K. Ben Antioxidant and antiulcerogenic activities of Opuntia ficus-indica f. inermis root extract in rats. Phytomedicine 2010, 17, 1120–1126. [Google Scholar] [CrossRef]
- Ventura-Aguilar, R.I.; Bosquez-Molina, E.; Bautista-Baños, S.; Rivera-Cabrera, F. Cactus stem (Opuntia ficus-indica Mill): Anatomy, physiology and chemical composition with emphasis on its biofunctional properties. J. Sci. Food Agric. 2017, 97, 5065–5073. [Google Scholar] [CrossRef]
- Sciacca, F.; Palumbo, M.; Pagliaro, A.; Di Stefano, V.; Scandurra, S.; Virzì, N.; Melilli, M.G. Opuntia cladodes as functional ingredient in durum wheat bread: Rheological, sensory, and chemical characterization. CYTA-J. Food 2021, 19, 96–104. [Google Scholar] [CrossRef]
- Attanzio, A.; Diana, P.; Barraja, P.; Carbone, A.; Spanò, V.; Parrino, B.; Cascioferro, S.M.; Allegra, M.; Cirrincione, G.; Tesoriere, L.; et al. Quality, functional and sensory evaluation of pasta fortified with extracts from Opuntia ficus-indica cladodes. J. Sci. Food Agric. 2019, 99, 4242–4247. [Google Scholar] [CrossRef] [PubMed]
- Msaddak, L.; Siala, R.; Fakhfakh, N.; Ayadi, M.A.; Nasri, M.; Zouari, N. Cladodes from prickly pear as a functional ingredient: Effect on fat retention, oxidative stability, nutritional and sensory properties of cookies. Int. J. Food Sci. Nutr. 2015, 66, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Moreno, E.; Cordoba-Díaz, M.; de Cortes Sánchez-Mata, M.; Marqués, C.D.; Goñi, I. The addition of cladodes (Opuntia ficus-indica L. Miller) to instant maize flour improves physicochemical and nutritional properties of maize tortillas. LWT 2015, 62, 675–681. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; Pitacas, F.I.; Reis, C.M.G.; Blasco, M. Nutritional value of Opuntia ficus-indica cladodes from Portuguese ecotypes. Bulg. J. Agric. Sci. 2016, 22, 40–45. [Google Scholar]
- Rezende, F.M.; Véras, A.S.C.; Siqueira, M.C.B.; Conceição, M.G.; Lima, C.L.; Almeida, M.P.; Mora-Luna, R.E.; Neves, M.L.M.W.; Monteiro, C.C.F.; Ferreira, M.A. Nutritional effects of using cactus cladodes (Opuntia stricta Haw Haw) to replace sorghum silage in sheep diet. Trop. Anim. Health Prod. 2020, 52, 1875–1880. [Google Scholar] [CrossRef] [PubMed]
- Mokoboki, K.; Sebola, N. Chemical composition and feed intake of Opuntia cladodes varieties offered to goats. J. Anim. Plant Sci. 2017, 32, 5096–5103. [Google Scholar]
- Souza, V.G.L.; Mello, I.P.; Khalid, O.; Pires, J.R.A.; Rodrigues, C.; Alves, M.M.; Santos, C.; Fernando, A.L.; Coelhoso, I. Strategies to Improve the Barrier and Mechanical Properties of Pectin Films for Food Packaging: Comparing Nanocomposites with Bilayers. Coatings 2022, 12, 108. [Google Scholar] [CrossRef]
- Freitas, C.M.P.; Coimbra, J.S.R.; Souza, V.G.L.; Sousa, R.C.S. Structure and Applications of Pectin in Food, Biomedical, and Pharmaceutical Industry: A Review. Coatings 2021, 11, 922. [Google Scholar] [CrossRef]
- Del-Valle, V.; Hernández-Muñoz, P.; Guarda, A.; Galotto, M.J. Development of a cactus-mucilage edible coating (Opuntia ficus-indica) and its application to extend strawberry (Fragaria ananassa) shelf-life. Food Chem. 2005, 91, 751–756. [Google Scholar] [CrossRef]
- Allegra, A.; Inglese, P.; Sortino, G.; Settanni, L.; Todaro, A.; Liguori, G. The influence of Opuntia ficus-indica mucilage edible coating on the quality of “Hayward” kiwifruit slices. Postharvest Biol. Technol. 2016, 120, 45–51. [Google Scholar] [CrossRef]
- Allegra, A.; Sortino, G.; Inglese, P.; Settanni, L.; Todaro, A.; Gallotta, A. The effectiveness of Opuntia ficus-indica mucilage edible coating on post-harvest maintenance of ‘Dottato’ fig (Ficus carica L.) fruit. Food Packag. Shelf Life 2017, 12, 135–141. [Google Scholar] [CrossRef]
- Espino-Díaz, M.; De Jesús Ornelas-Paz, J.; Martínez-Téllez, M.A.; Santillán, C.; Barbosa-Cánovas, G.V.; Zamudio-Flores, P.B.; Olivas, G.I. Development and characterization of edible films based on mucilage of Opuntia ficus-indica (L.). J. Food Sci. 2010, 75, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Pires, J.R.A.; de Souza, V.G.L.; Fernando, A.L. Production of Nanocellulose from Lignocellulosic Biomass Wastes: Prospects and Limitations; Springer: Cham, Switzerland, 2019; pp. 719–725. [Google Scholar]
- Pires, J.; Paula, C.D.; Souza, V.G.L.; Fernando, A.L.; Coelhoso, I. Understanding the barrier and mechanical behavior of different nanofillers in chitosan films for food packaging. Polymers 2021, 13, 721. [Google Scholar] [CrossRef] [PubMed]
- De Farias, P.M.; de Vasconcelos, L.B.; Ferreira, M.E.S.; Pascall, M.; Tapia-Blácido, D.R. Nopal cladode (Opuntia ficus-indica) flour: Production, characterization, and evaluation for producing bioactive film. Food Packag. Shelf Life 2021, 29, 100703. [Google Scholar] [CrossRef]
- Lira-Vargas, A.A.; Corrales-Garcia, J.J.E.; Valle-Guadarrama, S.; Peña-Valdivia, C.B.; Trejo-Marquez, M.A. Biopolymeric films based on cactus (Opuntia ficus-indica) mucilage incorporated with gelatin and beeswax. J. Prof. Assoc. Cactus Dev. 2014, 16, 51–70. [Google Scholar]
- Souza, V.G.L.; Ferreira, L.S.; Rodrigues, C.P.; Pires, J.R.; Duarte, M.P.; Coelhoso, I.M.; Fernando, A.L. Antimicrobial and antioxidant activity of novel biocomposites incorporated with essential oils. In Proceedings of the 27th European Biomass Conference and Exhibition Proceedings, Lisbon, Portugal, 27–30 May 2019; pp. 1083–1086. [Google Scholar]
- García-García, R.; Escobedo-Avellaneda, Z.; Tejada-Ortigoza, V.; Martín-Belloso, O.; Valdez-Fragoso, A.; Welti-Chanes, J. Hurdle technology applied to prickly pear beverages for inhibiting Saccharomyces cerevisiae and Escherichia coli. Lett. Appl. Microbiol. 2015, 60, 558–564. [Google Scholar] [CrossRef]
- Oniszczuk, A.; Wójtowicz, A.; Oniszczuk, T.; Matwijczuk, A.; Dib, A.; Markut-Miotła, E. Opuntia fruits as food enriching ingredient, the first step towards new functional food products. Molecules 2020, 25, 916. [Google Scholar] [CrossRef]
- Palmieri, N.; Stefanoni, W.; Latterini, F.; Pari, L. An Italian explorative study of willingness to pay for a new functional pasta featuring Opuntia ficus-indica. Agriculture 2021, 11, 701. [Google Scholar] [CrossRef]
- Palmieri, N.; Suardi, A.; Stefanoni, W.; Pari, L. Opuntia ficus-indica as an ingredient in new functional pasta: Consumer preferences in Italy. Foods 2021, 10, 803. [Google Scholar] [CrossRef]
- Elhassaneen, Y. Improvement of Bioactive Compounds Content and Antioxidant Properties in Crackers with the Incorporation of Prickly Pear and Potato Peels Powder. Int. J. Nutr. Food Sci. 2016, 5, 53. [Google Scholar] [CrossRef]
- Manzur-Valdespino, S.; Ramírez-Moreno, E.; Arias-Rico, J.; Jaramillo-Morales, O.A.; Calderón-Ramos, Z.G.; Delgado-Olivares, L.; Córdoba-Díaz, M.; Córdoba-Díaz, D.; Cruz-Cansino, N.d.S. Opuntia ficus-indica L. Mill residues-Properties and application possibilities in food supplements. Appl. Sci. 2020, 10, 3260. [Google Scholar] [CrossRef]
- Chougui, N.; Djerroud, N.; Naraoui, F.; Hadjal, S.; Aliane, K.; Zeroual, B.; Larbat, R. Physicochemical properties and storage stability of margarine containing Opuntia ficus-indica peel extract as antioxidant. Food Chem. 2015, 173, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Parafati, L.; Restuccia, C.; Palmeri, R.; Fallico, B.; Arena, E. Impact of prickly pear extract on the quality parameters of beef burger patties after cooking. Food Biosci. 2021, 42, 101146. [Google Scholar] [CrossRef]
- Ciriminna, R.; Delisi, R.; Albanese, L.; Meneguzzo, F.; Pagliaro, M. Opuntia ficus-indica seed oil: Biorefinery and bioeconomy aspects. Eur. J. Lipid Sci. Technol. 2017, 119, 1700013. [Google Scholar] [CrossRef]
- Berrabah, H.; Taïbi, K.; Ait Abderrahim, L.; Boussaid, M. Phytochemical composition and antioxidant properties of prickly pear (Opuntia ficus-indica L.) flowers from the Algerian germplasm. J. Food Meas. Charact. 2019, 13, 1166–1174. [Google Scholar] [CrossRef]
- Ammar, I.; Ennouri, M.; Bouaziz, M.; Amira, A. Ben Phenolic Profiles, Phytchemicals and Mineral Content of Decoction and Infusion of Opuntia ficus-indica Flowers. Plant Foods Hum. Nutr. 2015, 70, 388–394. [Google Scholar] [CrossRef]
- Ammar, I.; BenAmira, A.; Khemakem, I.; Attia, H.; Ennouri, M. Effect of Opuntia ficus-indica flowers maceration on quality and on heat stability of olive oil. J. Food Sci. Technol. 2017, 54, 1502–1510. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).