Effect of Adding Bovine Skin Gelatin Hydrolysates on Antioxidant Properties, Texture, and Color in Chicken Meat Processing
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Thermal Treatment
2.4. Texture Determination of Meat Samples
2.5. Color Determination of Meat Samples
2.6. Antioxidant Activity of BSGHs
2.7. Statistical Analysis
3. Results and Discussion
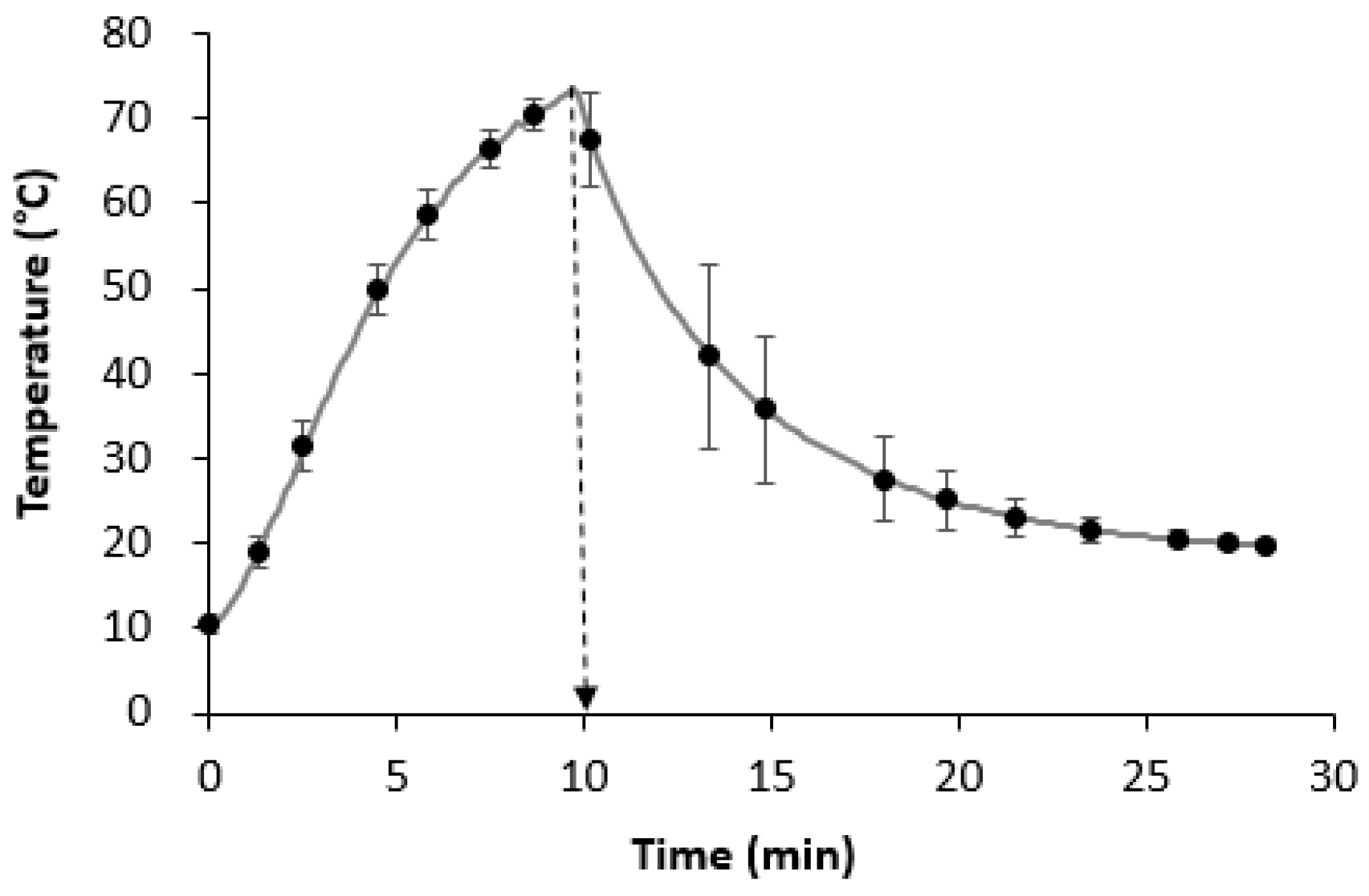
3.1. Thermal Treatment
3.2. Texture Determination of Meat Samples
3.3. Color Determination of Meat Samples
3.4. Antioxidant Activity of BSGHs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McCluskey, J.J.; Loureiro, M.L. Consumer Preferences and Willingness to Pay for Food Labeling: A Discussion of Empirical Studies. J. Food Distrib. Res. 2003, 34, 1–8. [Google Scholar]
- Higdon, J. Phosphorus. Available online: https://lpi.oregonstate.edu/mic/minerals/phosphorus (accessed on 13 May 2019).
- Ritz, E.; Hahn, K.; Ketteler, M.; Kuhlmann, M.K.; Mann, J. Phosphate Additives in Food. Dtsch. Aerzteblatt Online 2012, 109, 49–55. [Google Scholar] [CrossRef]
- Polizer Rocha, Y.J.; de Noronha, R.L.F.; Trindade, M.A. Understanding the consumer’s perception of traditional frankfurters and frankfurters with healthy attributes through sorting task and hard laddering techniques. Meat Sci. 2019, 149, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Chambers, E.; Chambers, E.; Castro, M. What Is “Natural”? Consumer Responses to Selected Ingredients. Foods 2018, 7, 65. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Food Additives and Flavourings (FAF); Younes, M.; Aquilina, G.; Castle, L.; Engel, K.; Fowler, P.; Frutos Fernandez, M.J.; Fürst, P.; Gürtler, R.; Husøy, T.; et al. Re-evaluation of phosphoric acid–phosphates—Di-, Tri- and polyphosphates (E 338–341, E 343, E 450–452) as food additives and the safety of proposed extension of use. EFSA J. 2019, 17, e05674. [Google Scholar] [CrossRef] [PubMed]
- Shroff, R. Phosphate is a vascular toxin. Pediatr. Nephrol. 2013, 28, 583–593. [Google Scholar] [CrossRef]
- Shuto, E.; Taketani, Y.; Tanaka, R.; Harada, N.; Isshiki, M.; Sato, M.; Nashiki, K.; Amo, K.; Yamamoto, H.; Higashi, Y.; et al. Dietary Phosphorus Acutely Impairs Endothelial Function. J. Am. Soc. Nephrol. 2009, 20, 1504–1512. [Google Scholar] [CrossRef]
- Kralik, G.; Kralik, Z.; Grčević, M.; Hanžek, D. Quality of chicken meat. Anim. Husb. Nutr. 2018, 63–92. [Google Scholar] [CrossRef]
- Devi, S.M.; Balachandar, V.; Lee, S.I.; Kim, I.H. An Outline of Meat Consumption in the Indian Population—A Pilot Review. Korean J. Food Sci. Anim. Resour. 2014, 34, 507–515. [Google Scholar] [CrossRef]
- Ahn, D.U. Use of Chicken Meat and Processing Technologies. Korean J. Poult. Sci. 2004, 31, 67–88. [Google Scholar]
- Arango, C.M.; Restrepo, D.A. Efectos del uso de diferentes fuentes de fosfatos sobre la capacidad de retención de agua (CRA) y las características de textura de una salchicha. Rev. Fac. Nal. Agron. Medellín 2002, 55, 1425–1440. [Google Scholar]
- Glorieux, S.; Goemaere, O.; Steen, L.; Fraeye, I. Phosphate Reduction in Emulsified Meat Products: Impact of Phosphate Type and Dosage on Quality Characteristics. Food Technol. Biotechnol. 2017, 55, 390–397. [Google Scholar] [CrossRef]
- Kristinsson, H.G.; Rasco, B.A. Fish protein hydrolysates: Production, biochemical, and functional properties. Crit. Rev. Food Sci. Nutr. 2000, 40, 43–81. [Google Scholar] [CrossRef]
- Avila Rodríguez, M.I.; Rodríguez Barroso, L.G.; Sánchez, M.L. Collagen: A review on its sources and potential cosmetic applications. J. Cosmet. Dermatol. 2018, 17, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Turkiewicz, M. Collagen hydrolysates as a new diet supplement. Food Chem. Biotechnol. 2009, 73, 83–92. [Google Scholar]
- Sibilla, S.; Godfrey, M.; Brewer, S.; Budh-Raja, A.; Genovese, L. An Overview of the Beneficial Effects of Hydrolysed Collagen as a Nutraceutical on Skin Properties: Scientific Background and Clinical Studies. Open Nutraceuticals J. 2015, 8, 29–42. [Google Scholar] [CrossRef]
- Yazaki, M.; Ito, Y.; Yamada, M.; Goulas, S.; Teramoto, S.; Nakaya, M.; Ohno, S.; Yamaguchi, K. Oral Ingestion of Collagen Hydrolysate Leads to the Transportation of Highly Concentrated Gly-Pro-Hyp and Its Hydrolyzed Form of Pro-Hyp into the Bloodstream and Skin. J. Agric. Food Chem. 2017, 65, 2315–2322. [Google Scholar] [CrossRef]
- Sionkowska, A.; Skrzyński, S.; Śmiechowski, K.; Kołodziejczak, A. The review of versatile application of collagen: Versatile Application of Collagen. Polym. Adv. Technol. 2017, 28, 4–9. [Google Scholar] [CrossRef]
- Sousa, S.C.; Fragoso, S.P.; Penna, C.R.A.; Arcanjo, N.M.O.; Silva, F.A.P.; Ferreira, V.C.S.; Barreto, M.D.S.; Araújo, Í.B.S. Quality parameters of frankfurter-type sausages with partial replacement of fat by hydrolyzed collagen. LWT Food Sci. Technol. 2017, 76, 320–325. [Google Scholar] [CrossRef]
- Hashim, P.; Ridzwan, M.; Bakar, J.; Hashim, D. Collagen in food and beverage industries. Int. Food Res. J. 2015, 22, 1–8. [Google Scholar]
- Landi, G.; Fedi, F.; Sorrentino, A.; Neitzert, H.C.; Iannace, S. Gelatin/graphene systems for low cost energy storage. AIP Conf. Proc. 2014, 1599, 202–205. [Google Scholar] [CrossRef]
- Huda, N.; Seow, E.K.; Normawati, M.N.; Nik Aisyah, N.M.; Fazilah, A.; Easa, A.M. Effect of duck feet collagen addition on physicochemical properties of surimi. Int. Food Res. J. 2013, 20, 537–544. [Google Scholar]
- Nuñez, S.M.; Cárdenas, C.; Pinto, M.; Valencia, P.; Cataldo, P.; Guzmán, F.; Almonacid, S. Bovine skin gelatin hydrolysates as potential substitutes for polyphosphates: The role of degree of hydrolysis and pH on water-holding capacity. J. Food Sci. 2020, 85, 1988–1996. [Google Scholar] [CrossRef] [PubMed]
- Bourne, M.C. Basic Principles of Food Texture Measurement; Springer: Boston, MA, USA, 1990. [Google Scholar]
- Ibarra, J. Addition of Fish Protein Hydrolysate for Enhanced Water Retention in Sous Vide Processing of Salmon. J. Food Process. Technol. 2013, 4, 1–7. [Google Scholar] [CrossRef]
- Hauck, C.A. Efecto del Procesamiento Térmico De zanahoria (D.carota) en la Biodisponibilidad de β-caroteno Obtenida Mediante Estudios In Vitro. (Effect of Thermal Processing of Carrot (D.Carota) on the Bioavailability of β-Carotene Obtained through In Vitro Studies). Chemical Engineering Degree Bachelor’s Thesis, Universidad Técnica Federico Santa María, Valaparíso, Chile, 2017. [Google Scholar]
- Alam, M.N.; Bristi, N.J.; Rafiquzzaman, M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef]
- Tounkara, F.; Sodio, B.; Amza, T.; Le, G.-W.; Shi, Y.-H. Antioxidant Effect and Water-Holding Capacity of Roselle (Hibiscus sabdariffa L.) Seed Protein Hydrolysates. Adv. J. Food Sci. Technol. 2013, 5, 752–757. [Google Scholar] [CrossRef]
- Pereira, A.G.T.; Ramos, E.M.; Teixeira, J.T.; Cardoso, G.P.; Ramos, A.d.L.S.; Fontes, P.R. Effects of the addition of mechanically deboned poultry meat and collagen fibers on quality characteristics of frankfurter-type sausages. Meat Sci. 2011, 89, 519–525. [Google Scholar] [CrossRef]
- Kristinsson, H.G.; Rasco, B.A. Biochemical and Functional Properties of Atlantic Salmon (Salmo salar) Muscle Proteins Hydrolyzed with Various Alkaline Proteases. J. Agric. Food Chem. 2000, 48, 657–666. [Google Scholar] [CrossRef]
- Fernández-López, J.; Sayas-Barberá, E.; Pérez-Alvarez, J.A.; Aranda-Catalá, V. Effect of Sodium Chloride, Sodium Tripolyphosphate and pH on Color Properties of Pork Meat. Color Res. Appl. 2004, 29, 67–74. [Google Scholar] [CrossRef]
- Fennema, O.R. Comparative Water Holding Properties of Various Muscle Foods: A Critical Review Relating to Definitions, Methods of Measurement, Governing Factors, Comparative Data and Mechanistic Matters. J. Muscle Foods 1990, 1, 363–381. [Google Scholar] [CrossRef]
- Paterson, B.C.; Parrish, F.C.; Stromer, M.H. Effects of Salt and Pyrophosphate on the Physical and Chemical Properties of Beef Muscle. J. Food Sci. 1988, 53, 1258–1265. [Google Scholar] [CrossRef]
- Fernández-López, J.; Pérez-Alvarez, J.A.; Aranda-Catalá, V. Dry-Cured Ham: Contributions on the Influence of pH and Temperature During the Salting Stage; Reproval SL: Valencia, Spain, 1994; Volume III. [Google Scholar]
- Perez-Alvarez, J.A.; Sanchez-Rodríguez, E.; Fernandez-Lopez, J.; Gago-Gago, M.A.; Ruíz-Peluffo, C.; Rosini, M.; Pagan-Moreno, M.J.; Lopez-Santovena, F.; Aranda-Catalá, V. Chemical and Color Characteristics of “Lomo Embuchado” During Salting Seasoning. J. Muscle Foods 1997, 8, 395–411. [Google Scholar] [CrossRef]
- Nurilmala, M.; Hizbullah, H.H.; Karnia, E.; Kusumaningtyas, E.; Ochiai, Y. Characterization and Antioxidant Activity of Collagen, Gelatin, and the Derived Peptides from Yellowfin Tuna (Thunnus albacares) Skin. Mar. Drugs 2020, 18, 98. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xia, Y.; Hua, M.; Li, Z.; Zhang, L.; Li, S.; Gong, R.; Liu, S.; Wang, Z.; Sun, Y. Functional properties and antioxidant activity of gelatine and hydrolysate from deer antler base. Food Sci. Nutr. 2020, 8, 3402–3412. [Google Scholar] [CrossRef] [PubMed]
- Pakbin, B.; Allahyari, S.; Dibazar, S.P.; Brück, W.M.; Vahidi, R.; Mahmoudi, R.; Khanjari, A. Production of Bovine Collagen Hydrolysate with Antioxidant Activity; Optimized by Response Surface Methodology. Sci. Pharm. 2022, 90, 62. [Google Scholar] [CrossRef]
- Irshad, I.; Kanekanian, A.; Peters, A.; Masud, T. Antioxidant activity of bioactive peptides derived from bovine casein hydrolysate fractions. J. Food Sci. Technol. 2015, 52, 231–239. [Google Scholar] [CrossRef]
- Gómez, L.J.; Figueroa, O.A.; Zapata, J.E. Actividad Antioxidante de Hidrolizados Enzimáticos de Plasma Bovino Obtenidos por Efecto de Alcalasa® 2.4 L. Inf. Tecnológica 2013, 24, 33–42. [Google Scholar] [CrossRef]
- Kong, B.; Xiong, Y.L. Antioxidant Activity of Zein Hydrolysates in a Liposome System and the Possible Mode of Action. J. Agric. Food Chem. 2006, 54, 6059–6068. [Google Scholar] [CrossRef]
- Salgado, P.R.; Fernández, G.B.; Drago, S.R.; Mauri, A.N. Addition of bovine plasma hydrolysates improves the antioxidant properties of soybean and sunflower protein-based films. Food Hydrocoll. 2011, 25, 1433–1440. [Google Scholar] [CrossRef]
- Elias, R.J.; Kellerby, S.S.; Decker, E.A. Antioxidant Activity of Proteins and Peptides. Crit. Rev. Food Sci. Nutr. 2008, 48, 430–441. [Google Scholar] [CrossRef]
- Ohashi, Y.; Onuma, R.; Naganuma, T.; Ogawa, T.; Naude, R.; Nokihara, K.; Muramoto, K. Antioxidant Properties of Tripeptides Revealed by a Comparison of Six Different Assays. Food Sci. Technol. Res. 2015, 21, 695–704. [Google Scholar] [CrossRef]


| Parameter | Value |
|---|---|
| Type of sample | Cylinder |
| Sample length | 40 mm |
| Sample diameter | 26.2 mm |
| Charge | 25,000 g |
| Test speed | 1 mm/s |
| Activation charge | 0.5 N |
| % deformation | 30 |
| Test | Compression |
| Probe | TA 18 |
| Concentration (% w/w Meat) | Parameter L* | ||
|---|---|---|---|
| STPP | BSGH1 | BSGH2 | |
| Control | 86.31 ± 0.99 a | 86.31 ± 0.99 a | 86.31 ± 0.99 a |
| 0.5 | 84.96 ± 0.16 b | 85.42 ± 0.31 ab | 85.81 ± 0.09 ab |
| 1 | 83.87 ± 0.30 c | 85.18 ± 0.15 b | 85.20 ± 0.19 b |
| 2 | 79.89 ± 1.12 h | 83.81 ± 0.08 c | 83.07 ± 0.50 cd |
| 3 | 76.63 ± 0.55 i | 82.37 ± 0.12 de | 81.35 ± 0.21 fg |
| 4 | 76.17 ± 0.64 i | 81.81 ± 0.78 ef | 80.60 ± 0.74 g |
| 5 | 75.23 ± 0.67 j | 80.74 ± 0.57 g | 78.02 ± 0.58 h |
| Concentration (% w/w Meat) | Parameter a* | ||
|---|---|---|---|
| STPP | BSGH1 | BSGH2 | |
| Control | 1.49 ± 0.86 de | 1.49 ± 0.86 de | 1.49 ± 0.86 de |
| 0.5 | 1.16 ± 0.24 cd | 2.14 ± 0.11 hi | 1.63 ± 0.20 ef |
| 1 | 0.88 ± 0.28 bc | 2.10 ± 0.27 hi | 1.67 ± 0.24 efg |
| 2 | 0.59 ± 0.36 ab | 2.07 ± 0.10 ghi | 1.81 ± 0.27 efgh |
| 3 | 0.30 ± 0.15 a | 2.24 ± 0.05 i | 2.18 ± 0.13 hi |
| 4 | 0.43 ± 0.09 a | 1.99 ± 0.34 fghi | 2.10 ± 0.29 hi |
| 5 | 0.19 ± 0.08 a | 2.16 ± 0.17 hi | 2.09 ± 0.17 ghi |
| Concentration (% w/w Meat) | Parameter b* | ||
|---|---|---|---|
| STPP | BSGH1 | BSGH2 | |
| Control | 12.80 ± 0.90 abcd | 12.80 ± 0.90 abcd | 12.80 ± 0.90 abcd |
| 0.5 | 13.38 ± 0.15 a | 12.04 ± 0.37 d | 12.02 ± 0.31 d |
| 1 | 12.81 ± 0.63 abcd | 12.50 ± 0.14 bcd | 12.33 ± 0.11 cd |
| 2 | 10.20 ± 1.51 e | 13.05 ± 0.11 abc | 13.09 ± 0.09 abc |
| 3 | 9.49 ± 0.27 e | 13.34 ± 0.18 ab | 13.46 ± 0.31 a |
| 4 | 9.81 ± 1.07 e | 13.36 ± 0.16 ab | 13.54 ± 0.21 a |
| 5 | 10.05 ± 0.19 e | 13.39 ± 0.45 a | 13.03 ± 0.09 abc |
| Concentration (% w/w Meat) | ΔE* | ||
|---|---|---|---|
| STPP | BSGH1 | BSGH2 | |
| 0.5 | 2.08 ± 0.19 b | 0.45 ± 0.15 a | 0.61 ± 0.06 a |
| 1 | 3.24 ± 0.39 cd | 0.60 ± 0.14 a | 0.65 ± 0.18 a |
| 2 | 8.78 ± 1.60 h | 2.01 ± 0.07 b | 2.73 ± 0.49 bc |
| 3 | 11.13 ± 0.50 i | 3.45 ± 0.14 cd | 4.46 ± 0.29 ef |
| 4 | 11.47 ± 0.65 Ij | 4.01 ± 0.71 de | 5.21 ± 0.65 f |
| 5 | 12.27 ± 0.67 j | 5.03 ± 0.61 f | 7.64 ± 0.57 g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nuñez, S.M.; Cárdenas, C.; Valencia, P.; Pinto, M.; Silva, J.; Pino-Cortés, E.; Almonacid, S. Effect of Adding Bovine Skin Gelatin Hydrolysates on Antioxidant Properties, Texture, and Color in Chicken Meat Processing. Foods 2023, 12, 1496. https://doi.org/10.3390/foods12071496
Nuñez SM, Cárdenas C, Valencia P, Pinto M, Silva J, Pino-Cortés E, Almonacid S. Effect of Adding Bovine Skin Gelatin Hydrolysates on Antioxidant Properties, Texture, and Color in Chicken Meat Processing. Foods. 2023; 12(7):1496. https://doi.org/10.3390/foods12071496
Chicago/Turabian StyleNuñez, Suleivys M., Constanza Cárdenas, Pedro Valencia, Marlene Pinto, Javier Silva, Ernesto Pino-Cortés, and Sergio Almonacid. 2023. "Effect of Adding Bovine Skin Gelatin Hydrolysates on Antioxidant Properties, Texture, and Color in Chicken Meat Processing" Foods 12, no. 7: 1496. https://doi.org/10.3390/foods12071496
APA StyleNuñez, S. M., Cárdenas, C., Valencia, P., Pinto, M., Silva, J., Pino-Cortés, E., & Almonacid, S. (2023). Effect of Adding Bovine Skin Gelatin Hydrolysates on Antioxidant Properties, Texture, and Color in Chicken Meat Processing. Foods, 12(7), 1496. https://doi.org/10.3390/foods12071496








