Effects of Non-Enzymatic Browning and Lipid Oxidation on Color of Ready-to-Eat Abalone during Accelerated Storage and Its Control
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Sample Treatment
2.3. Color Measurement
2.4. Determination of Lipid Oxidation
2.4.1. Determination of Oil-Extraction Rate
2.4.2. Determination of Fatty Acid Composition
2.4.3. Determination of Lipid Hydroperoxides
2.4.4. Determination of Aldehydes Formation
2.5. Determination of Total Polyphenol Content
2.6. Determination of Maillard Reaction Products (MRPs)
2.6.1. Determination of Advanced Glycation End Products (AGEs) Fluorescence Intensity
2.6.2. Determination of 5-Hydroxymethylfurfural (5-HMF) Content
2.6.3. Determination of Maillard Intermediate Product and Browning Index
2.7. Fatty Acid Composition
2.8. Statistical Analysis
3. Results and Discussion
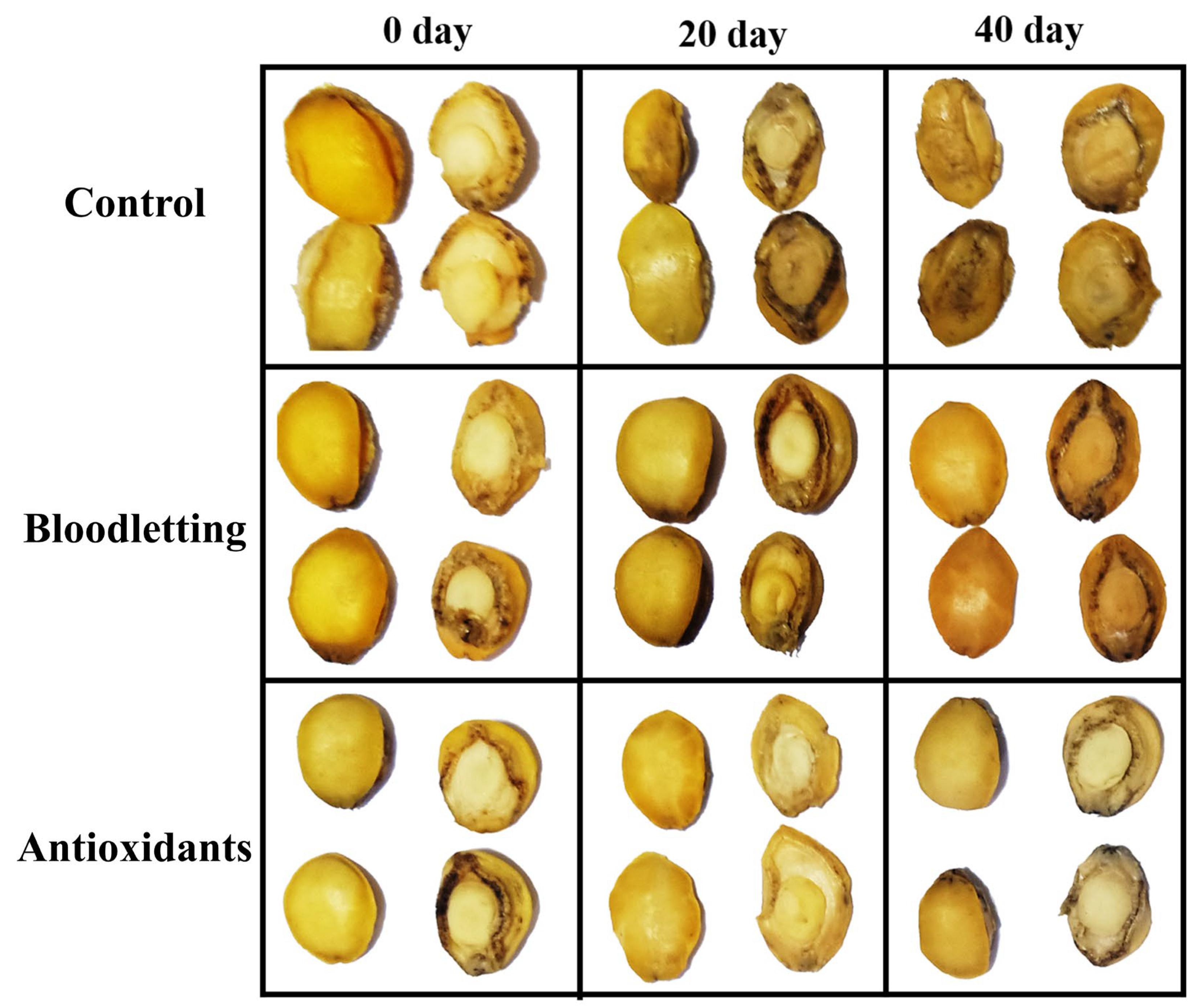
3.1. Appearance and Color of RTE Abalone Muscle during Storage
3.1.1. Appearance
3.1.2. Color
3.2. Lipid Oxidation in RTE Abalone Muscle during Storage
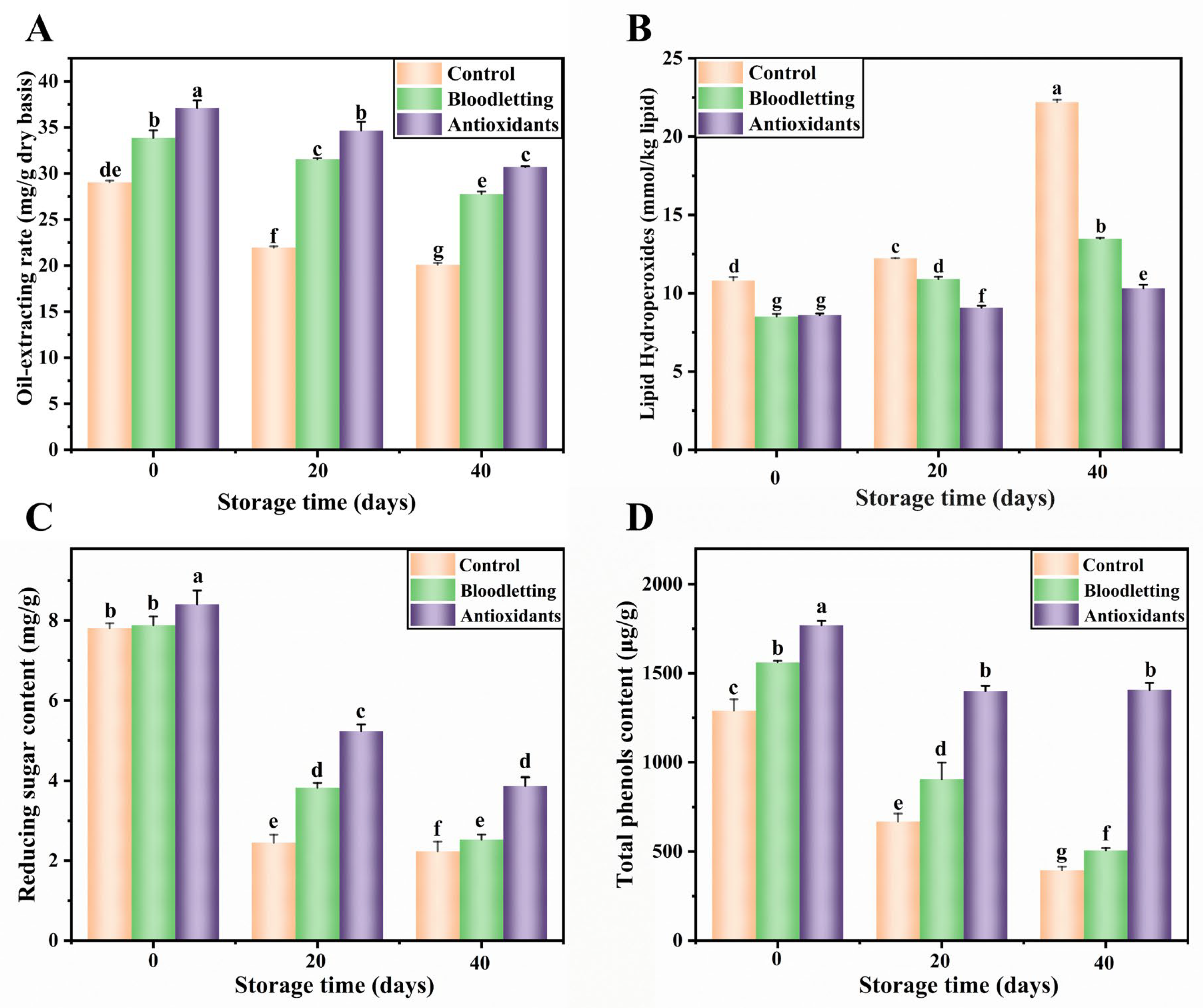
3.2.1. Lipid Extraction Rate
3.2.2. Fatty Acid Composition
3.2.3. Lipid Hydroperoxide Value
3.2.4. Aldehydes Content
3.3. Total Phenols Content in RTE Abalone Muscle during Storage
3.4. The Maillard Reaction in RTE Abalone Muscle during Storage
3.4.1. Reducing Sugar Content
3.4.2. The Maillard Reaction Products
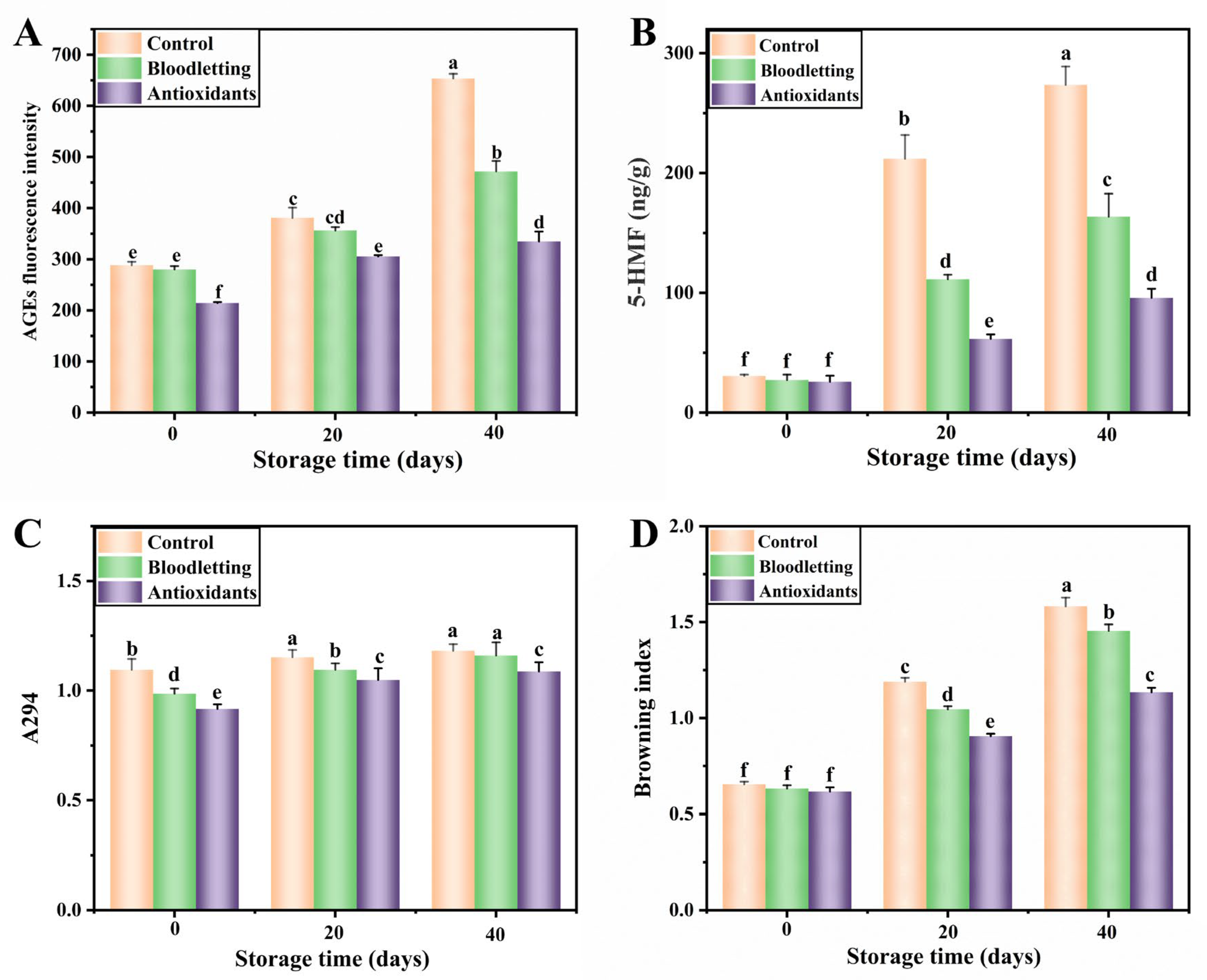
AGEs Fluorescence Intensity
5-Hydroxymethylfurfural Content
Maillard Intermediate Product (A294)
Browning Index (BI)
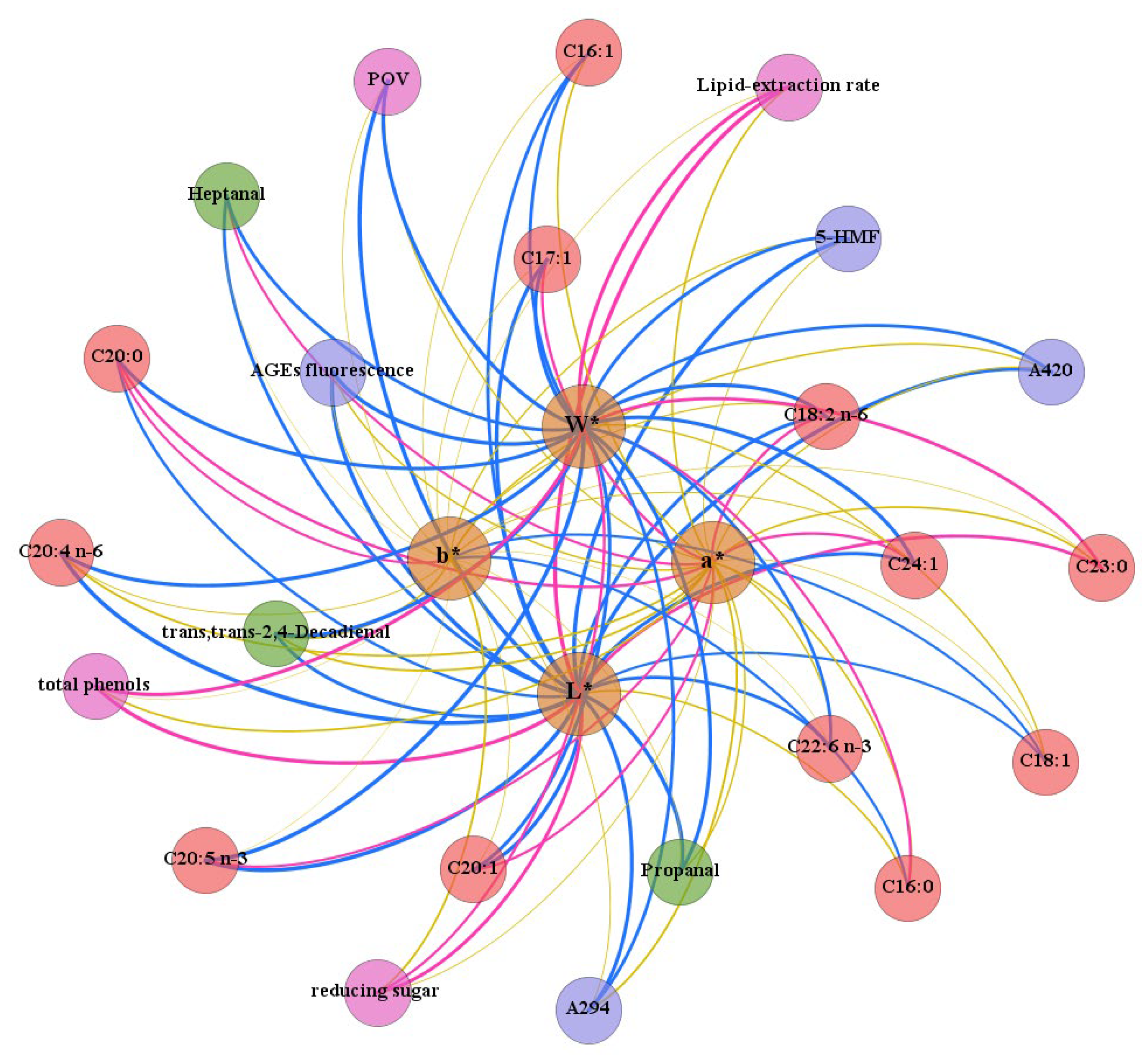
3.5. Correlation Analysis of Various Factors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. FIGIS List of Species for Fishery Global Production Statistics. Available online: http://www.fao.org/fishery/statistics/en (accessed on 14 January 2022).
- Chiou, T.; Tsai, C.; Lan, H. Chemical, physical and sensory changes of small abalone meat during cooking. Fish. Sci. 2004, 70, 867–874. [Google Scholar] [CrossRef]
- Singh, A.; Benjakul, S. Effect of serine protease inhibitor from squid ovary on gel properties of surimi from Indian mackerel. Texture Stud. 2017, 48, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Jiao, S.; Zhu, D.; Deng, Y.; Zhao, Y.Y. Effects of hot air-assisted radio frequency heating on quality and shelf-life of roasted peanuts. Food Bioproc. Technol. 2016, 9, 308–319. [Google Scholar] [CrossRef]
- Wang, X.F.; Cong, H.; Wang, H.B. Research review of snack and cooked fish food. Wuhan Polytech. Univ. 2011, 30, 37–41. [Google Scholar]
- Nunak, N.; Schleining, G. Instrumental textural changes in raw white shrimp during iced storage. Aquat. Food Prod. Technol. 2011, 20, 350–360. [Google Scholar] [CrossRef]
- Dong, X.P.; Hou, Y.W.; Wang, Y.; Xu, X.B.; Wang, K.X.; Zhao, M.Y.; Prakash, S.; Yu, C.X. Effect of temperature-time pretreatments on the texture and microstructure of abalone (Haliotis discus hanai). Texture Stud. 2018, 49, 503–511. [Google Scholar] [CrossRef]
- Friedman, M. Food browning and its prevention: An overview. J. Agric. Food Chem. 1996, 44, 631–653. [Google Scholar] [CrossRef]
- Li, D.Y.; Liu, Z.Q.; Liu, B.; Qi, Y.; Liu, Y.X.; Liu, X.Y.; Qin, L.; Zhou, D.Y.; Shahidi, F. Effect of protein oxidation and degradation on texture deterioration of ready-to-eat shrimps during storage. J. Food Sci. 2020, 85, 2673–2680. [Google Scholar] [CrossRef]
- Mozuraityte, R.; Rustad, T.; Storrø, I. Pro-oxidant activity of Fe2+ in oxidation of cod phospholipids in liposomes. Eur. J. Lipid Sci. Technol. 2010, 108, 218–226. [Google Scholar] [CrossRef]
- Wang, Y.; Hui, T.; Zhang, Y.W.; Liu, B.; Wang, F.L.; Li, J.K.; Cui, B.W.; Guo, X.Y.; Peng, Z.Q. Effects of frying conditions on the formation of heterocyclic amines and trans fatty acids in grass carp (Ctenopharyngodon idellus). Food Chem. 2015, 167, 251–257. [Google Scholar] [CrossRef]
- Burmester, T. Origin and evolutiong of arthropod hemocyanin and related proteins. Comp Physiol. 2002, 172, 95–107. [Google Scholar]
- Jaenicke, E.; Decker, H. Conversion of crustacean hemocyanin to catecholoxidase. Micron 2004, 35, 89–90. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Zhang, Y.L.; Wang, F.; Pang, M.X.; Qi, J.H. Relationship in between Chemical Oxidation and Browning of Flavanols. IOP Conf. Ser. Earth Environ. Sci. 2016, 41, 012012. [Google Scholar] [CrossRef] [Green Version]
- Lian, F.; Måge, I.; Lorentzen, G.; Siikavuopio, S.I.; Øverbø, K.; Vang, B.; Lindberg, D. Exploring the effect of inhibitors, cooking and freezing on melanosis in snow crab (Chionoecetes opilio) clusters. Food Control 2018, 92, 255–266. [Google Scholar] [CrossRef]
- Martínez-Alvarez, O.; López-Caballero, M.E.; Montero, P.; Gómez-Guillén, M.D.C. The effect of different melanosis-inhibiting blends on the quality of frozen deep-water rose shrimp (Parapenaeus longirostris). Food Control 2020, 109, 106889. [Google Scholar] [CrossRef]
- Skipnes, D.; Johnsen, S.O.; Skåra, T.; Sivertsvik, M.; Lekang, O. Optimization of heat processing of farmed Atlantic cod (Gadus morhua) muscle with respect to cook loss, water holding capacity, colour, and texture. J. Aquat. Food Prod. Technol. 2011, 20, 331–340. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Xie, H.K.; Zhou, D.Y.; Hu, X.P.; Liu, Z.Y.; Song, L.; Zhu, B.W. Changes in lipid profiles of dried clams (Mactra chinensis Philippi and Ruditapes philippinarum) during accelerated storage and prediction of shelf life. J. Agric. Food Chem. 2018, 66, 7764–7774. [Google Scholar] [CrossRef]
- Xie, H.K.; Zhou, D.Y.; Liu, Z.Y. Effects of natural phenolics on shelf life and lipid stability of freeze-dried scallop adductor muscle. Food Chem. 2019, 295, 423–431. [Google Scholar] [CrossRef]
- Zhou, F.Z.; Zeng, T.; Yin, S.W.; Tang, C.H.; Yuan, D.B.; Yang, X.Q. Development of antioxidant gliadin particle stabilized Pickering high internal phase emulsions (HIPEs) as oral delivery systems and the in vitro digestion fate. Food Funct. 2018, 9, 959–970. [Google Scholar] [CrossRef]
- Zhao, G.H.; Hu, Y.Y.; Liu, Z.Y. Simultaneous quantification of 24 aldehydes and ketones in oysters (Crassostrea gigas) with different thermal processing procedures by HPLC-electrospray tandem mass spectrometry. Food Res. Int. 2021, 147, 110559. [Google Scholar] [CrossRef]
- Zhao, M.T.; Liu, Z.Y.; Li, A.; Zhao, G.H.; Xie, H.K.; Zhou, D.Y.; Wang, T. Gallic acid and its alkyl esters emerge as effective antioxidants against lipid oxidation during hot air drying process of Ostrea talienwhanensis. LWT Food Sci. Technol. 2021, 139, 110551. [Google Scholar] [CrossRef]
- Matiacevich, S.B.; Pilar Buera, M. A critical evaluation of fluorescence as a potential marker for the Maillard reaction. Food Chem. 2006, 95, 423–430. [Google Scholar] [CrossRef]
- Serra-Cayuela, A.; Jourdes, M.; Riu-Aumatell, M.; Buxaderas, S.; Teissedre, P.L.; López-Tamames, E. Kinetics of browning, phenolics, and 5-hydroxymethylfurfural in commercial sparkling wines. J. Agric. Food Chem. 2014, 62, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Ajandouz, E.H.; Tchiakpe, L.S.; Ore, F.D.; Benajiba, A.; Puigserver, A. Effects of pH on caramelization and Maillard reaction kinetics in fructose-lysine model systems. J. Food Sci. 2001, 66, 926–931. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, X.; Gao, Y. Path analysis of non-enzymatic browning in Dongbei Suancai during storage caused by different fermentation conditions. Food Chem. 2020, 335, 127620. [Google Scholar] [CrossRef] [PubMed]
- Li, D.Y.; Li, N.; Dong, X.H. Effect of phytic acid combined with lactic acid on color and texture deterioration of ready-to-eat shrimps during storage. Food Chem. 2022, 396, 133702. [Google Scholar] [CrossRef] [PubMed]
- Uematsu, T.; Parkányiová, L.; Endo, T.; Matsuyama, C.; Pokorný, J. Effect of the unsaturation degree on browning reactions of peanut oil and other edible oils with proteins under storage and frying conditions. Int. Congr. 2002, 1245, 445–446. [Google Scholar] [CrossRef]
- Wang, Z.M.; He, Z.F.; Gan, X. Interrelationship among ferrous myoglobin, lipid and protein oxidations in rabbit meat during refrigerated and superchilled storage. Meat Sci. 2018, 146, 131–139. [Google Scholar] [CrossRef]
- Cyprian, O.O.; Nguyen, M.V.; Sveinsdottir, K. Influence of blanching treatment and drying methods on the drying characteristics and quality changes of dried sardine (Sardinella gibbosa) during storage. Dry. Technol. 2017, 35, 478–489. [Google Scholar] [CrossRef]
- Li, D.Y.; Xie, H.K.; Liu, Z.; Li, A.; Li, J.; Liu, B. Shelf life prediction and changes in lipid profiles of dried shrimp (Penaeus vannamei) during accelerated storage. Food Chem. 2019, 297, 124951. [Google Scholar] [CrossRef]
- Lertittikul, W.; Benjakul, S.; Tanaka, M. Characteristics and antioxidative activity of Maillard reaction products from a porcine plasma protein-glucose model system as influenced by pH. Food Chem. 2007, 100, 669–677. [Google Scholar] [CrossRef]
- Rannou, C.; Laroque, D.; Renault, E. Mitigation strategies of acrylamide, furans, heterocyclic amines and browning during the Maillard reaction in foods. Food Res. Int. 2016, 90, 154–176. [Google Scholar] [CrossRef] [PubMed]
- Hayase, F.; Shibuya, T.; Sato, J.; Yamamoto, M. Effect of oxygen and transition metals on the advanced Maillard reaction of proteins with glucose. Biosci. Biotechnol. Biochem. 1996, 60, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Demirok, E.; Kolsarici, N. Effect of green tea extract and microwave pre-cooking on the formation of acrylamide in fried chicken drumsticks and chicken wings. Food Res. Int. 2014, 63, 290–298. [Google Scholar] [CrossRef]
- Liu, S.C.; Huang, M.C.; James, S.W. A study on the mechanism of browning in mei liqueur using model solutions. Food Res. Int. 2003, 36, 579–585. [Google Scholar] [CrossRef]
- Kowalski, S.; Lukasiewicz, M.; Duda-Chodak, A.; Zięć, G. 5-Hydroxymethyl-2-furfural (HMF)–heat-induced formation, occurrence in food and biotransformation—A review. Pol. J. Food Nutr. Sci. 2013, 63, 207–225. [Google Scholar] [CrossRef] [Green Version]
- Burdurlu, H.S.; Karadeniz, F. Effect of storage on nonenzymatic browning of apple juice concentrates. Food Chem. 2003, 80, 91–97. [Google Scholar] [CrossRef]
- Yu, P.; Xu, X.B.; Yu, S.J. The effect of pH and amino acids on the formation of methylglyoxal in a glucose-amino acid model system. J. Sci. Food Agric. 2017, 97, 3159–3165. [Google Scholar] [CrossRef]
- Zamora, R.; Hidalgo, F.J. The Maillard reaction and lipid oxidation. Lipid Technol. 2011, 23, 59–62. [Google Scholar] [CrossRef]
| Sample | L* | a* | b* | W* | ΔE | |
|---|---|---|---|---|---|---|
| Control | 0D | 59.47 ± 1.96 ab | 6.91 ± 1.24 b | 37.80 ± 3.78 a | 44.15 ± 4.51 bc | -- |
| 20D | 54.48 ± 5.32 c | 4.39 ± 1.51 d | 32.17 ± 1.84 a | 44.08 ± 2.98 bc | 62.95 ± 6.75 d | |
| 40D | 44.80 ± 7.50 d | 4.88 ± 0.30 cd | 32.55 ± 4.95 a | 35.73 ± 3.02 d | 246.77 ± 14.18 a | |
| Bloodletting | 0D | 64.14 ± 3.79 a | 5.03 ± 0.88 c | 38.89 ± 4.39 a | 46.86 ± 3.88 b | -- |
| 20D | 61.02 ± 3.21 ab | 2.70 ± 1.45 e | 34.46 ± 5.05 a | 47.90 ± 5.89 b | 34.78 ± 5.69 e | |
| 40D | 52.58 ± 1.43 c | 8.17 ± 0.96 a | 36.30 ± 2.25 a | 39.72 ± 3.69 c | 150.21 ± 9.84 b | |
| Antioxidants | 0D | 65.16 ± 3.30 a | 2.83 ± 0.34 e | 33.14 ± 2.56 a | 51.84 ± 5.69 a | -- |
| 20D | 63.25 ± 2.30 a | 3.33 ± 1.34 e | 29.87 ± 4.67 a | 52.52 ± 6.24 a | 14.62 ± 2.14 f | |
| 40D | 56.95 ± 2.62 b | 4.35 ± 0.75 d | 29.40 ± 2.50 a | 47.69 ± 2.78 b | 83.86 ± 9.58 c | |
| Time (Days) | 0 | 20 | 40 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | Control | Bloodletting | Antioxidants | Control | Bloodletting | Antioxidants | Control | Bloodletting | Antioxidants |
| C16:0 | 0.19 ± 0.02 a | 0.19 ± 0.02 a | 0.19 ± 0.02 a | 0.20 ± 0.02 a | 0.19 ± 0.02 a | 0.19 ± 0.01 a | 0.20 ± 0.02 a | 0.21 ± 0.01 a | 0.22 ± 0.02 a |
| C16:1 | 2.60 ± 0.07 a | 2.63 ± 0.10 a | 2.70 ± 0.08 a | 2.27 ± 0.07 c | 2.49 ± 0.03 b | 2.45 ± 0.09 b | 2.11 ± 0.04 d | 2.32 ± 0.09 c | 2.31 ± 0.06 c |
| C17:1 | 0.75 ± 0.01 ab | 0.78 ± 0.01 a | 0.81 ± 0.01 a | 0.65 ± 0.03 b | 0.73 ± 0.01 ab | 0.81 ± 0.01 a | 0.58 ± 0.02 c | 0.63 ± 0.02 b | 0.73 ± 0.02 ab |
| C18:1 | 0.48 ± 0.01 b | 0.53 ± 0.02 ab | 0.58 ± 0.01 a | 0.48 ± 0.03 b | 0.50 ± 0.02 ab | 0.52 ± 0.04 ab | 0.41 ± 0.02 c | 0.52 ± 0.03 ab | 0.68 ± 0.01 ab |
| C18:2 n-6 | 3.94 ± 0.13 ab | 3.98 ± 0.09 ab | 4.10 ± 0.09 a | 3.36 ± 0.06 c | 3.59 ± 0.15 b | 3.89 ± 0.08 ab | 2.71 ± 0.09 d | 2.81 ± 0.11 d | 3.59 ± 0.11 b |
| C20:0 | 1.13 ± 0.04 a | 1.11 ± 0.08 a | 1.13 ± 0.06 a | 1.09 ± 0.03 a | 1.11 ± 0.04 a | 1.13 ± 0.01 a | 1.09 ± 0.04 a | 1.06 ± 0.09 a | 1.08 ± 0.03 a |
| C20:1 | 0.55 ± 0.01 a | 0.56 ± 0.01 a | 0.59 ± 0.02 a | 0.48 ± 0.01 b | 0.54 ± 0.01 a | 0.59 ± 0.03 a | 0.46 ± 0.02 b | 0.52 ± 0.02 a | 0.54 ± 0.02 a |
| C20:4 n-6 | 1.06 ± 0.03 b | 1.11 ± 0.03 ab | 1.16 ± 0.02 a | 1.01 ± 0.02 bc | 1.05 ± 0.02 b | 1.11 ± 0.04 ab | 0.99 ± 0.03 bc | 1.04 ± 0.04 b | 1.06 ± 0.01 b |
| C20:5 n-3 | 0.88 ± 0.03 b | 0.91 ± 0.01 b | 1.00 ± 0.02 a | 0.83 ± 0.02 bc | 0.86 ± 0.02 b | 0.94 ± 0.03 ab | 0.77 ± 0.02 c | 0.79 ± 0.04 c | 0.90 ± 0.03 b |
| C22:6 n-3 | 1.05 ± 0.03 b | 1.14 ± 0.02 a | 1.11 ± 0.03 a | 1.01 ± 0.03 b | 1.03 ± 0.04 b | 1.03 ± 0.03 b | 0.91 ± 0.02 c | 0.98 ± 0.02 bc | 1.01 ± 0.02 b |
| C23:0 | 1.52 ± 0.01 a | 1.53 ± 0.02 a | 1.49 ± 0.02 a | 1.54 ± 0.02 a | 1.51 ± 0.04 a | 1.52 ± 0.06 a | 1.60 ± 0.02 a | 1.55 ± 0.04 a | 1.52 ± 0.06 a |
| C24:1 | 0.37 ± 0.01 a | 0.38 ± 0.01 a | 0.38 ± 0.02 a | 0.34 ± 0.01 ab | 0.36 ± 0.01 a | 0.38 ± 0.02 a | 0.31 ± 0.04 b | 0.33 ± 0.02 ab | 0.35 ± 0.02 a |
| SFAs | 2.85 ± 0.15 a | 2.83 ± 0.13 a | 2.81 ± 0.16 a | 2.82 ± 0.06 a | 2.81 ± 0.21 a | 2.84 ± 0.15 a | 2.89 ± 0.14 a | 2.81 ± 0.19 a | 2.82 ± 0.15 a |
| MUFAs | 4.74 ± 0.21 b | 4.88 ± 0.19 ab | 5.05 ± 0.23 a | 4.21 ± 0.25 c | 4.62 ± 0.23 bc | 4.74 ± 0.14 b | 3.88 ± 0.26 d | 4.32 ± 0.21 c | 4.60 ± 0.31 bc |
| PUFAs | 6.92 ± 0.19 b | 7.14 ± 0.23 ab | 7.37 ± 0.21 a | 6.21 ± 0.18 d | 6.53 ± 0.16 c | 6.97 ± 0.19 b | 5.38 ± 0.16 f | 5.62 ± 0.23 e | 6.55 ± 0.21 c |
| Time (Days) | 0 | 20 | 40 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | Control | Bloodletting | Antioxidants | Control | Bloodletting | Antioxidants | Control | Bloodletting | Antioxidants |
| Propanal | 32.50 ± 1.52 c | 13.31 ± 1.90 f | 11.77 ± 2.56 f | 37.67 ± 0.07 b | 23.19 ± 1.99 d | 15.42 ± 1.49 f | 55.47 ± 2.34 a | 26.15 ± 0.79 d | 21.40 ± 2.06 e |
| Heptanal | 16.12 ± 0.01 a | 6.70 ± 0.36 c | 6.02 ± 0.03 d | 11.96 ± 0.63 b | 8.90 ± 0.71 c | 9.52 ± 0.24 c | 16.64 ± 2.15 a | 11.61 ± 4.23 b | 10.61 ± 1.54 b |
| trans,trans-2,4-Decadienal | 200.21 ± 2.09 e | 232.58 ± 5.62 d | 125.02 ± 10.94 g | 261.96 ± 4.35 c | 260.23 ± 1.00 c | 124.36 ± 5.62 g | 337.08 ± 8.65 a | 296.5 ± 19.13 b | 161.67 ± 31.11 f |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Y.; Yu, M.; Li, D.; Zhao, G.; Zhang, M.; Wang, Z.; Liu, Y.; Zhou, D. Effects of Non-Enzymatic Browning and Lipid Oxidation on Color of Ready-to-Eat Abalone during Accelerated Storage and Its Control. Foods 2023, 12, 1514. https://doi.org/10.3390/foods12071514
Fan Y, Yu M, Li D, Zhao G, Zhang M, Wang Z, Liu Y, Zhou D. Effects of Non-Enzymatic Browning and Lipid Oxidation on Color of Ready-to-Eat Abalone during Accelerated Storage and Its Control. Foods. 2023; 12(7):1514. https://doi.org/10.3390/foods12071514
Chicago/Turabian StyleFan, Yingchen, Manman Yu, Deyang Li, Guanhua Zhao, Min Zhang, Zonghan Wang, Yuxin Liu, and Dayong Zhou. 2023. "Effects of Non-Enzymatic Browning and Lipid Oxidation on Color of Ready-to-Eat Abalone during Accelerated Storage and Its Control" Foods 12, no. 7: 1514. https://doi.org/10.3390/foods12071514
APA StyleFan, Y., Yu, M., Li, D., Zhao, G., Zhang, M., Wang, Z., Liu, Y., & Zhou, D. (2023). Effects of Non-Enzymatic Browning and Lipid Oxidation on Color of Ready-to-Eat Abalone during Accelerated Storage and Its Control. Foods, 12(7), 1514. https://doi.org/10.3390/foods12071514





