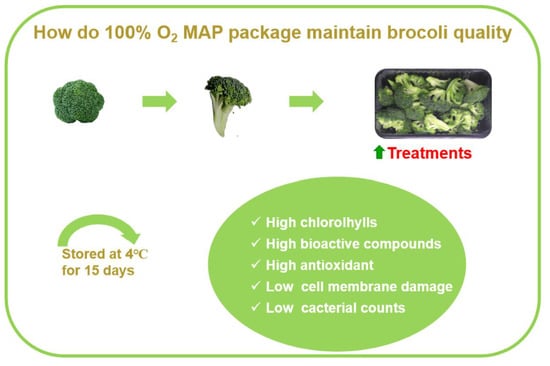
Effect of 100% Oxygen-Modified Atmosphere Packaging on Maintaining the Quality of Fresh-Cut Broccoli during Refrigerated Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
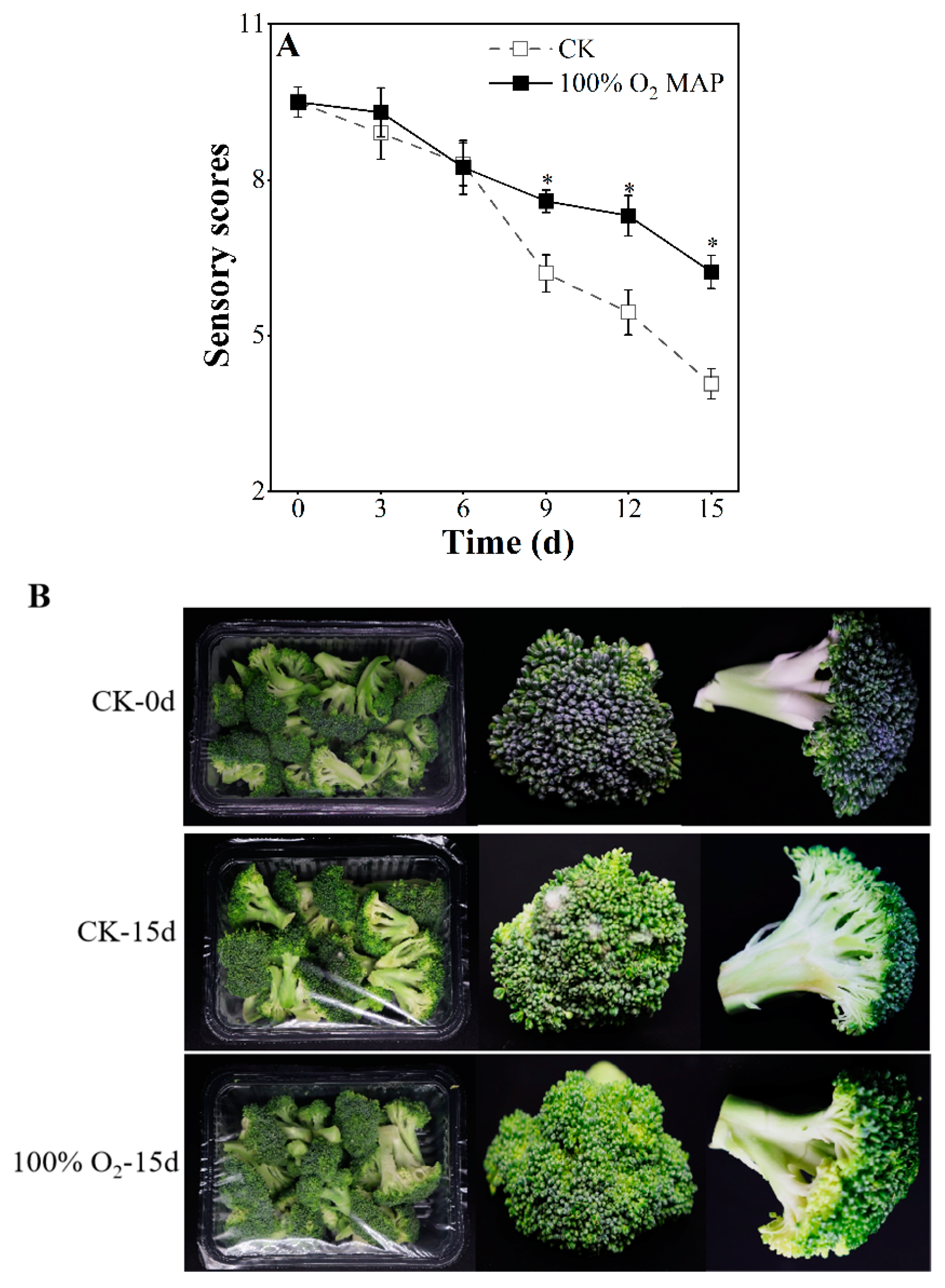
2.3. Visual Appearance, Color, Sensory Quality
2.4. Gas Composition
2.5. Texture Analysis
2.6. Respiration Rate
2.7. Electrolyte Leakage (EL) Rate and Malondialdehyde (MDA) Content
2.8. Total Bacterial Count (TBC)
2.9. Total Chlorophyll and Total Carotenoid Content
2.10. Total Glucosinolates and Sulforaphane
2.11. Vitamin C (VC) Content
2.12. Total Phenolic (TP) Content
2.13. Superoxide (O2−) and Hydrogen Peroxide (H2O2) Content
2.14. Measurement of Antioxidant Activity: DPPH, ABTS, SOD, and CAT
2.15. Statistical Analyses
3. Results and Discussion
3.1. Effect of 100% O2 MAP on Gas Composition and Respiration Rate of Fresh-Cut Broccoli
3.2. Effect of 100% O2 MAP on the Appearance, Color, Chlorophylls, and Carotenoids of Fresh-Cut Broccoli
3.3. Effect of 100% O2 MAP on the Microbiological Quality of Fresh-Cut Broccoli
3.4. Effect of 100% O2 MAP on Nutrients in Fresh-Cut Broccoli
3.5. Effect of 100% O2 MAP on Membrane Structure of Fresh-Cut Broccoli
3.6. Effect of 100% O2 MAP on Antioxidant Activity of Fresh-Cut Broccoli
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ansah, F.A.; Amodio, M.L.; Colelli, G. Quality of fresh-cut products as affected by harvest and postharvest operations. J. Sci. Food Agri. 2018, 98, 3614–3626. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xia, Y.; Liu, H.Y.; Guo, H.; He, X.Q.; Liu, Y.; Gan, R.Y. Nutritional values, beneficial effects, and food applications of broccoli (Brassica oleracea var. italica Plenck). Trends Food Sci. Technol. 2022, 119, 288–308. [Google Scholar] [CrossRef]
- Luo, F.; Fang, H.; Wei, B.; Cheng, S.; Zhou, Q.; Zhou, X.; Ji, S. Advance in yellowing mechanism and the regulation technology of post-harvested broccoli. Food Qua. Saf. 2020, 4, 107–113. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Chen, Y.; Liu, S.; Yun, L.; Guo, Y.; Wang, F. Investigating the relationship between volatile components and differentially expressed proteins in broccoli heads during storage in high CO2 atmospheres. Postharvest Biol. Technol. 2019, 153, 43–51. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Ali, M.R.; Ramadan, K.M.A.; Anwar, R.; Shalaby, T.A.; Rezk, A.A.; El-Mogy, M.M. Exogenous postharvest application of calcium chloride and salicylic acid to maintain the quality of broccoli florets. Plants 2022, 11, 1513. [Google Scholar] [CrossRef] [PubMed]
- Kao, N.Y.; Tu, Y.F.; Sridhar, K.; Tsai, P.J. Effect of a high voltage electrostatic field (HVEF) on the shelf-life of fresh-cut broccoli (Brassica oleracea var. italica). LWT–Food Sci.Technol. 2019, 116, 108532. [Google Scholar] [CrossRef]
- Collazo, C.; Charles, F.; Aguiló-Aguayo, I.; Marín-Sáez, J.; Lafarga, T.; Abadias, M.; Viñas, I. Decontamination of Listeria innocua from fresh-cut broccoli using UV-C applied in water or peroxyacetic acid, and dry-pulsed light. Innov. Food Sci. Emerg. Technol. 2019, 52, 438–449. [Google Scholar] [CrossRef] [Green Version]
- Park, J.J.; Lee, W.Y. Reduction in Listeria monocytogenes on fresh-cut broccoli by chlorine dioxide with ultrasonication. Food Preserv. 2018, 5, 755–762. [Google Scholar] [CrossRef]
- Ren, G.; Liu, Y.; Deng, B.; Wang, Y.; Lin, W.; Zhang, Y.; Yang, J. Gene expression analyses reveal mechanisms of inhibited yellowing by applying selenium chitosan on fresh cut broccoli. Foods 2022, 11, 3123. [Google Scholar] [CrossRef]
- Ali, J.; Sadegh, S.; Manfred, L.; Mahajan, P. A comprehensive simulation program for modified atmosphere and humidity packaging (MAHP) of fresh fruits and vegetables. Food Eng. 2017, 206, 88–97. [Google Scholar] [CrossRef]
- Gao, J.; Zhu, Y.; Luo, F. Effects of ethanol combined with ascorbic acid and packaging on the inhibition of browning and microbial growth in fresh-cut Chinese yam. Food Sci. Nut. 2018, 6, 998–1005. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhao, X.; Ma, Y.; Guan, H.; Liang, H.; Wang, D. Inhibitory effect of modified atmosphere packaging on Escherichia coli O157:H7 in fresh-cut cucumbers (Cucumis sativus L.) and effectively maintain quality during storage. Food Chem. 2022, 369, 130969. [Google Scholar] [CrossRef]
- Haque, M.A.; Asaduzzaman, M.; Mahomud, M.S.; Alam, M.R.; Khaliduzzaman, A.; Pattadar, S.N.; Ahmmed, R. High carbon di-oxide modified atmospheric packaging on quality of ready-to-eat minimally processed fresh-cut iceberg lettuce. Food Sci. Biotechnol. 2021, 30, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Zhang, M.; Devahastin, S.; Guo, Z. Effects of pressurized argon and nitrogen treatments in combination with modified atmosphere on quality characteristics of fresh-cut potatoes. Postharvest Biol. Technol. 2019, 149, 159–165. [Google Scholar] [CrossRef]
- Liu, E.C.; Niu, L.F.; Yi, Y.; Wang, L.M.; Ai, Y.W.; Zhao, Y.; Min, T. Expression analysis of ERFs during storage under modified atmosphere packaging (high-concentration of CO2) of fresh-cut Lotus Root. Hort. Sci. 2020, 55, 216–223. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Li, C.B.; Sun, J. Synergistic effects of ultraviolet light irradiation and high-oxygen modified atmosphere packaging on physiological quality, microbial growth and lignification metabolism of fresh-cut carrots. Postharvest Biol. Technol. 2021, 173, 111365. [Google Scholar] [CrossRef]
- Fan, M.; Li, W.; Hu, X.; Sun, Y.N.; Yu, G.; Zhang, X. Effect of micro-vacuum storage on active oxygen metabolism, internal browning and related enzyme activities in Laiyang pear (Pyrus bretschneideri Reld). LWT–Food Sci. Technol. 2016, 72, 467–474. [Google Scholar] [CrossRef]
- Kader, A.; Ben-Yehoshua, S. Effects of superatmospheric oxygen levels on postharvest, physiology and quality of fresh fruits and vegetables. Postharvest Biol. Technol. 2000, 20, 1–13. [Google Scholar] [CrossRef]
- Alvarez, M.V.; Ortega-Ramirez, L.A.; Silva-Espinoza, B.A.; Gonzalez-Aguilar, G.A.; Ayala-Zavala, J.F. Antimicrobial, antioxidant, and sensorial impacts of oregano and rosemary essential oils over broccoli florets. J. Food Process. Pres. 2019, 43, 8892. [Google Scholar] [CrossRef]
- He, Q.; Xiao, K. Quality of broccoli (Brassica oleracea L. var. italica) in modified atmosphere packaging made by gas barrier-gas promoter blending materials. Postharvest Biol. Technol. 2018, 144, 63–69. [Google Scholar] [CrossRef]
- Lv, J.; Wu, J.; Zuo, J.; Fan, L.; Shi, J.; Gao, L.; Wang, Q. Effect of Se treatment on the volatile compounds in broccoli. Food Chem. 2017, 216, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Tang, J.; Xiao, J.; Geng, X.; Guo, L. Purple light-emitting diode (Pereyra et al.) lights controls chlorophyll degradation and enhances nutraceutical quality of postharvest broccoli florets. Sci. Hort. 2022, 294, 110768. [Google Scholar] [CrossRef]
- Xu, D.; Zuo, J.; Li, P.; Yan, Z.; Gao, L.; Wang, Q.; Jiang, A. Effect of methyl jasmonate on the quality of harvested broccoli after simulated transport. Food Chem. 2020, 319, 126561. [Google Scholar] [CrossRef] [PubMed]
- Mawlong, I.; Sujith, K.; Gurung, B.; Singh, K.H.; Singh, D. A simple spectrophotometric method for estimating total glucosinolates in mustard de-oiled cake. Inter. J. Food Prop. 2017, 20, 3274–3281. [Google Scholar] [CrossRef] [Green Version]
- Huang, K.; Lin, J.C.; Wu, Q.Y.; Yan, J.Y.; Liu, M.Y.; Zhang, S.; Xiao, W.J. Changes in sulforaphane and selenocysteine methyltransferase transcript levels in broccoli treated with sodium selenite. Plant Mol. Biol. Rep. 2015, 34, 807–814. [Google Scholar] [CrossRef]
- Blainski, A.; Lopes, G.C.; Mello, J.C. Application and analysis of the folin ciocalteu method for the determination of the total phenolic content from Limonium brasiliense L. Molecules 2013, 18, 6852–6865. [Google Scholar] [CrossRef] [Green Version]
- Lanubile, A.; Maschietto, V.; De Leonardis, S.; Battilani, P.; Paciolla, C.; Marocco, A. Defense responses to mycotoxin-producing fungi Fusarium proliferatum, F. subglutinans, and Aspergillus flavus in kernels of susceptible and resistant maize genotypes. Mol. Plant Microbe Interact. 2015, 28, 546–557. [Google Scholar] [CrossRef] [Green Version]
- Ancos, B.D.; Sgroppo, S.; Plaza, L.; Cano, M.P. Possible nutritional and health-related value promotion in orange juice preserved by high-pressure treatment. J. Sci. Food Agri. 2002, 82, 790–796. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying and improved ABTS radical cation decolorization assay. Free Radical Bio. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Li, B.; Ding, Y.; Tang, X.; Wang, G.; Wu, S.; Li, X.; Tang, X. Effect of L-Arginine on Maintaining Storage Quality of the White Button Mushroom (Agaricus bisporus). Food and Bioprocess Tech. 2019, 12, 563–574. [Google Scholar] [CrossRef]
- Sun, Y.; Li, W. Effects the mechanism of micro-vacuum storage on broccoli chlorophyll degradation and builds prediction model of chlorophyll content based on the color parameter changes. Sci. Hort. 2017, 224, 206–214. [Google Scholar] [CrossRef]
- Barbosa, C.; Machado, T.B.; Alves, M.R.; Oliveira, M. Fresh-cut bell peppers in modified atmosphere packaging: Improving shelf life to answer food security concerns. Molecules 2020, 25, 2323. [Google Scholar] [CrossRef] [PubMed]
- Beaudry, R.M. Responses of horticultural commodities to low oxygen: Limits to the expanded use of modified atmosphere packaging. Hort. Technol. 2000, 10, 491–500. [Google Scholar] [CrossRef] [Green Version]
- Guo, Q.; Wu, B.; Peng, X.; Wang, J.; Li, Q.; Jin, J.; Ha, Y. Effects of chlorine dioxide treatment on respiration rate and ethylene synthesis of postharvest tomato fruit. Postharvest Biol. Technol. 2014, 93, 9–14. [Google Scholar] [CrossRef]
- Bal, E. Postharvest application of chitosan and low temperature storage affect respiration rate and quality of plum fruits. Agr. Sci. Technol. 2013, 15, 1219–1230. [Google Scholar] [CrossRef]
- Loredana, L.; Francesca, M.; Florinda, F.; Filomena, N.; Paola, O.; Donatella, A. Effect of argon-enriched modified atmosphere on the over quality and bioactive compounds of ready-to-use broccoli rabe (Brassica rapa sylvestris L. var. esculenta) during the storage. Food Sci. Technol. Int. 2023, 29, 84–94. [Google Scholar] [CrossRef]
- Wang, Z.; Li, C.; Zou, D.; Ji, S.; Cheng, S.; Zhou, Q.; Wei, B. Chlorine dioxide alleviates the yellowing process of broccoli by regulating chlorophyll degrading enzyme activity and gene expression. J. Food Proces. Pres. 2022, 46, e16985. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Guo, Y.; Ma, Y.; Yang, M.; Fu, R.; Sun, Y. Elevated CO2 delayed yellowing by maintaining chlorophyll biosynthesis and inhibiting chlorophyll degradation and carotenoid accumulation of postharvest broccoli. Postharvest Biol. Technol. 2022, 94, 112089. [Google Scholar] [CrossRef]
- Bico, S.L.S.; Raposo, M.F.J.; Morais, R.M.S.C.; Morais, A.M.M.B. Combined effects of chemical dip and/or carrageenan coating and/or controlled atmosphere on quality of fresh-cut banana. Food Control 2009, 20, 508–514. [Google Scholar] [CrossRef]
- Woods, F.M.; Dozier, W.A.; Ebel, R.C.; Thomas, R.H.; Nesbitt, M.; Wilkins, B.S.; Himelrick, D.G. Fruit quality and antioxidant properties in alabama-grown blackberries during fruit maturation. Inter. J. Fruit Sci. 2007, 6, 67–85. [Google Scholar] [CrossRef]
- Wei, L.; Liu, C.; Zheng, H.; Zheng, L. Melatonin treatment affects the glucoraphanin-sulforaphane system in postharvest fresh-cut broccoli (Brassica oleracea L.). Food Chem. 2020, 307, 125562. [Google Scholar] [CrossRef] [PubMed]
- Pe´rez, A.G.; Sanz, C. Effect of high oxygen and high carbon dioxide atmospheres on strawberry flavor and other quality traits. J. Agric. Food Chem. 2001, 49, 2370–2375. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Yang, Y.H.; Sridhar, K.; Tsai, P.J. Synergies of modified atmosphere packaging and high-voltage electrostatic field to extend the shelf-life of fresh-cut cabbage and baby corn. LWT–Food Sci. and Technol. 2021, 138, 110559. [Google Scholar] [CrossRef]
- Jiang, A.; Zuo, J.; Zheng, Q.; Guo, L.; Gao, L.; Zhao, S.; Hu, W. Red LED irradiation maintains the postharvest quality of broccoli by elevating antioxidant enzyme activity and reducing the expression of senescence-related genes. Hort. Sci. 2019, 251, 73–79. [Google Scholar] [CrossRef]
- Aiamla-or, S.; Shigyo, M.; Yamauchi, N. UV-B treatment controls chlorophyll degradation and related gene expression in broccoli (Brassica oleracea L. Italica Group) florets during storage. Sci. Hort. 2019, 243, 524–527. [Google Scholar] [CrossRef]
- Shi, J.; Zuo, J.; Zhou, F.; Gao, L.; Wang, Q.; Jiang, A. Low-temperature conditioning enhances chilling tolerance and reduces damage in cold-stored eggplant (Solanum melongena L.) fruit. Postharvest Biol. Technol. 2018, 141, 33–38. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, Y.; Zhao, X.; Zuo, J.; Zheng, Y. Effect of 100% Oxygen-Modified Atmosphere Packaging on Maintaining the Quality of Fresh-Cut Broccoli during Refrigerated Storage. Foods 2023, 12, 1524. https://doi.org/10.3390/foods12071524
Dai Y, Zhao X, Zuo J, Zheng Y. Effect of 100% Oxygen-Modified Atmosphere Packaging on Maintaining the Quality of Fresh-Cut Broccoli during Refrigerated Storage. Foods. 2023; 12(7):1524. https://doi.org/10.3390/foods12071524
Chicago/Turabian StyleDai, Yukexin, Xiaoyan Zhao, Jinhua Zuo, and Yanyan Zheng. 2023. "Effect of 100% Oxygen-Modified Atmosphere Packaging on Maintaining the Quality of Fresh-Cut Broccoli during Refrigerated Storage" Foods 12, no. 7: 1524. https://doi.org/10.3390/foods12071524
APA StyleDai, Y., Zhao, X., Zuo, J., & Zheng, Y. (2023). Effect of 100% Oxygen-Modified Atmosphere Packaging on Maintaining the Quality of Fresh-Cut Broccoli during Refrigerated Storage. Foods, 12(7), 1524. https://doi.org/10.3390/foods12071524