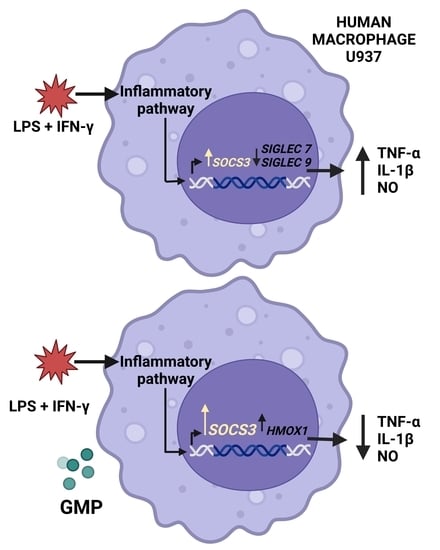
Protective Effect of Glycomacropeptide on the Inflammatory Response of U937 Macrophages
Abstract
1. Introduction
2. Materials and Methods
2.1. Production of Asialo GMP
2.2. Electrophoresis and Western Blot
2.3. Monocyte Culture, Differentiation, and Stimulation
2.4. Measurement of NO Production
2.5. Quantification of IL-1β and TNF-α
2.6. RNA Isolation, Reverse Transcription, and Real-Time Quantitative Polymerase Chain Reaction
2.7. Statistical Analysis
3. Results
3.1. GMP Effect on U937 Macrophage Activation by LPS and IFN-γ
3.2. Effect of GMP on the Gene Expression of Siglec Receptors
3.3. Regulatory Effect of GMP on SOCS3 and HMOX1 Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Status Report on Noncommunicable Diseases 2010; World Health Organization: Geneva, Switzerland, 2011; Available online: https://apps.who.int/iris/handle/10665/44579 (accessed on 13 May 2022).
- Myles, I.A. Fast food fever: Reviewing the impacts of the Western diet on immunity. Nutr. J. 2014, 13, 61. [Google Scholar] [CrossRef]
- Saitoh, S.; Van Wijk, K.; Nakajima, O. Crosstalk between Metabolic Disorders and Immune Cells. Int. J. Mol. Sci. 2021, 22, 10017. [Google Scholar] [CrossRef]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef]
- Lumeng, C.N.; Deyoung, S.M.; Bodzin, J.L.; Saltiel, A.R. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes 2007, 56, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, G.I.; Langley, K.G.; Berglund, N.A.; Kammoun, H.L.; Reibe, S.; Estevez, E.; Weir, J.; Mellett, N.A.; Perne, G.; Conway, J.R.; et al. Evidence that TLR4 Is Not a Receptor for Saturated Fatty Acids but Mediates Lipid-Induced Inflammation by Reprogramming Macrophage Metabolism. Cell Metab. 2018, 27, 1096–1110.e5. [Google Scholar] [CrossRef]
- De Taeye, B.M.; Novitskaya, T.; McGuinness, O.P.; Gleaves, L.; Medda, M.; Covington, J.W.; Vaughan, D.E. Macrophage TNF-alpha contributes to insulin resistance and hepatic steatosis in diet-induced obesity. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E713–E725. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Barnes, G.T.; Yang, Q.; Tan, G.; Yang, D.; Chou, C.J.; Sole, J.; Nichols, A.; Ross, J.S.; Tartaglia, L.A.; et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Investig. 2003, 112, 1821–1830. [Google Scholar] [CrossRef]
- Bode, J.G.; Ehlting, C.; Häussinger, D. The macrophage response towards LPS and its control through the p38(MAPK)-STAT3 axis. Cell. Signal. 2012, 24, 1185–1194. [Google Scholar] [CrossRef] [PubMed]
- Linossi, E.M.; Babon, J.J.; Hilton, D.J.; Nicholson, S.E. Suppression of cytokine signaling: The SOCS perspective. Cytokine Growth Factor. Rev. 2013, 24, 241–248. [Google Scholar] [CrossRef]
- Zaky, A.A.; Simal-Gandara, J.; Eun, J.B.; Shim, J.H.; El-Aty, A.M.A. Bioactivities, Applications, Safety, and Health Benefits of Bioactive Peptides From Food and By-Products: A Review. Front. Nutr. 2022, 8, 815640. [Google Scholar] [CrossRef]
- Muro Urista, C.; Álvarez Fernández, R.; Riera Rodriguez, F.; Cuenca, A.A.; Jurado, T.A. Review: Production and functionality of active peptides from milk. Food Sci. Technol. Int. 2011, 17, 293–317. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Wei, J.; Hao, L.; Shan, Q.; Li, H.; Gao, D.; Jin, Y.; Sun, P. Bioactive Proteins and their Physiological Functions in Milk. Curr. Protein Pept. Sci. 2019, 20, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Eigel, W.N.; Butler, J.E.; Emstrom, C.A.; Farrel, J.M., Jr.; Harwalkar, V.R.; Jenness, R.; Whitney, R.M. Nomenclature of proteins of cow’s milk fifth revision. J. Dairy. Sci. 1984, 67, 1599–1631. [Google Scholar] [CrossRef]
- Yvon, M.; Beucher, S.; Guilloteau, P.; Huerou-Luron, L.; Corring, T. Effects of caseinomacropeptide (CMP) on digestion regulation. Reprod. Nutr. Dev. 1994, 34, 527–537. [Google Scholar] [CrossRef]
- Huang, L.J.; Lin, J.H.; Tsai, J.H.; Chu, W.W.; Chen, W.Y.; Chen, S.L.; Chen, S.H. Identification of protein O-glycosylation site and corresponding glycans using liquid chromatography-tandem mass spectrometry via mapping accurate mass and retention time shift. J. Chromatogr. A 2014, 1371, 136–145. [Google Scholar] [CrossRef]
- Pisano, A.; Packe, N.H.; Redmond, J.W.; Williams, K.L.; Gooley, A.A. Characterization of O-linked glycosylation motifs in the glycopeptide domain of bovine kappa-casein. Glycobiology 1994, 4, 837–844. [Google Scholar] [CrossRef]
- Saito, T.; Itoh, T. Variations and distributions of O-glycosidically linked sugar chains in bovine kappa-casein. J. Dariy Sci. 1992, 75, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Li, E.W.; Mine, Y. Immunoenhancing effects of bovine glycomacropeptide and its derivatives on the proliferative response and phagocytic activities of human macrophagelike cells, U937. J. Agric. Food Chem. 2004, 52, 2704–2708. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, F.C.; Cervantes, M.M.; Cervantes-García, D.; Jimenz, M.; Ventura-Juarez, J.; Salinas, E. Glycomacropeptide Attenuates Inflammation, Pruritus, and Th2 Response Associated with Atopic Dermatitis Induced by 2,4-Dinitrochlorobenzene in Rat. J. Immunol. Res. 2017, 2017, 6935402. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Pavón, D.; Cervantes-García, D.; Bermúdez-Humarán, L.G.; Córdova-Dávalos, L.E.; Quintanar-Stephano, A.; Jimenez, M.; Salinas, E. Protective Effect of Glycomacropeptide on Food Allergy with Gastrointestinal Manifestations in a Rat Model through Down-Regulation of Type 2 Immune Response. Nutrients 2020, 12, 2942. [Google Scholar] [CrossRef] [PubMed]
- Roldán, N.R.; Jiménez, M.; Cervantes-García, D.; Marin, E.; Salinas, E. Glycomacropeptide administration attenuates airway inflammation and remodeling associated to allergic asthma in rat. Inflamm. Res. 2016, 65, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Cervantes-García, D.; Bahena-Delgado, A.I.; Jiménez, M.; Córdova-Dávalos, L.-E.; Ruiz-Esparza, V.; Sánchez-Aleman, E.; Martìnez-Saldaña, M.C.; Salinas, E. Glycomacropeptide Ameliorates Indomethacin-Induced Enteropathy in Rats by Modifying Intestinal Inflammation and Oxidative Stress. Molecules 2020, 25, 2351. [Google Scholar] [CrossRef]
- Daddaoua, A.; Puerta, V.; Zarzuelo, A.; Suárez, M.D.; Sánchez, M.F.; Martínez-Agustín, O. Bovine glycomacropeptide is anti-inflammatory in rats with hapten-induced colitis. J. Nutr. 2005, 135, 1164–1170. [Google Scholar] [CrossRef]
- López-Posadas, R.; Requena, P.; González, D.M.D.; Zarzuelo, A.; Sánchez, M.F.; Martínez-Agustín, O. Bovine glycomacropeptide has intestinal antiinflammatory effects in rats with dextran sulfate-induced colitis. J. Nutr. 2010, 140, 2014–2019. [Google Scholar] [CrossRef] [PubMed]
- Requena, P.; Daddaoua, A.; Martínez-Plata, E.; González, M.; Zarzuelo, A.; Suárez, M.D.; Sánchez de Medina, F.; Martínez-Augustin, O. Bovine glycomacropeptide ameliorates experimental rat ileitis by mechanisms involving downregulation of interleukin 17. Br. J. Pharm. 2008, 154, 825–832. [Google Scholar] [CrossRef]
- Sauvé, M.F.; Feldman, F.; Koudoufio, M.; Ould-Chikh, N.; Ahmarani, L.; Sane, A.; N’Timbane, T.; El-Jalbout, R.; Patey, N.; Spahis, S.; et al. Glycomacropeptide for Management of Insulin Resistance and Liver Metabolic Perturbations. Biomedicines 2021, 9, 1140. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhan, B.; Chang, D.M.; Mao, X. Antidiabetic Effect of Casein Glycomacropeptide Hydrolysates on High-Fat Diet and STZ-Induced Diabetic Mice via Regulating Insulin Signaling in Skeletal Muscle and Modulating Gut Microbiota. Nutrients 2020, 12, 220. [Google Scholar] [CrossRef]
- Xu, S.P.; Mao, X.Y.; Ren, F.Z.; Chen, H.L. Attenuating effect of casein glycomacropeptide on proliferation, differentiation, and lipid accumulation of in vitro Sprague-Dawley rat preadipocytes. J. Dairy. Sci. 2011, 94, 676–683. [Google Scholar] [CrossRef]
- Xu, S.P.; Mao, X.Y.; Cheng, X.; Chen, B. Ameliorating effects of casein glycomacropeptide on obesity induced by high-fat diet in male Sprague-Dawley rats. Food Chem. Toxicol. 2013, 56, 1–7. [Google Scholar] [CrossRef]
- Song, J.J.; Wang, Q.; Duc, M.; Chen, B.; Mao, X. Peptide IPPKKNQDKTE ameliorates insulin resistance in HepG2 cells via blocking ROS-mediated MAPK signaling. J. Funct. Foods 2017, 31, 287–294. [Google Scholar] [CrossRef]
- Cheng, X.; Gao, D.; Chen, B.; Mao, X. Endotoxin-Binding Peptides Derived from Casein Glycomacropeptide Inhibit Lipopolysaccharide-Stimulated Inflammatory Responses via Blockade of NF-κB activation in macrophages. Nutrients 2015, 7, 3119–3137. [Google Scholar] [CrossRef]
- Cheng, X.; Gao, D.X.; Song, J.J.; Ren, F.Z.; Mao, X. Casein glycomacropeptide hydrolysate exerts cytoprotection against H2O2-induced oxidative stress in RAW 264.7 macrophages via ROS dependent heme oxygenase-1 expression. RSC Adv. 2015, 5, 4511. [Google Scholar] [CrossRef]
- Naito, Y.; Takagi, T.; Higashimura, Y. Heme oxygenase-1 and anti-inflammatory M2 macrophages. Arch. Biochem. Biophys. 2014, 564, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 277, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Chávez, N.A.; Salinas, E.; Jauregui, J.; Macías, K. Detection of bovine milk adulterated with cheese whey by western blot immunoassay. Food Agric. Immunol. 2008, 19, 265–272. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Suchy, D.; Łabuzek, K.; Machnik, G.; Okopien, B. The influence of ezetimibe on classical and alternative activation pathways of monocytes/macrophages isolated from patients with hypercholesterolemia. Naunyn-Schmiedeberg’s Arch. Pharm. 2014, 387, 733–742. [Google Scholar] [CrossRef][Green Version]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Ross, E.A.; Devitt, A.; Johnson, J.R. Macrophages: The Good, the Bad, and the Gluttony. Front. Immunol. 2021, 12, 708186. [Google Scholar] [CrossRef]
- Gonzalez-Gil, A.; Schnaar, R.L. Siglec Ligands. Cells 2021, 10, 1260. [Google Scholar] [CrossRef]
- Nediani, C.; Giovannelli, L. Oxidative Stress and Inflammation as Targets for Novel Preventive and Therapeutic Approches in Non Communicable Diseases. Antioxidants 2020, 9, 290. [Google Scholar] [CrossRef]
- Chabance, B.; Jollès, P.; Izquierdo, C.; Francoual, C.; Drouet, F. Characterization of an antithrombotic peptide from kappa-casein in newborn plasma after milk ingestion. Br. J. Nutr. 1995, 73, 583–590. [Google Scholar] [CrossRef]
- Chabance, B.; Marteau, P.; Rambaud, J.C.; Migliore-Samour, D.; Boynard, M.; Perrotin, P.; Guillet, R.; Jollès, P.; Fiat, A.M. Casein peptide release and passage to the blood in humans during digestion of milk or yogurt. Biochimie 1998, 80, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Juge, N.; Tailford, L.; Owen, C.D. Sialidases from gut bacteria: A mini-review. Biochem. Soc. Trans. 2016, 44, 166–175. [Google Scholar] [CrossRef]
- Mitsuki, M.; Nara, K.; Yamaji, T.; Enomoto, A.; Kanno, M.; Yamaguchi, Y.; Yamada, A.; Waguri, S.; Hashimoto, Y. Siglec-7 mediates nonapoptotic cell death independently of its immunoreceptor tyrosine-based inhibitory motifs in monocytic cell line U937. Glycobiology 2010, 20, 395–402. [Google Scholar] [CrossRef]
- Nordström, T.; Movert, E.; Olin, A.I.; Ali, S.R.; Nizet, V.; Varki, A.; Areschoug, T. Human Siglec-5 inhibitory receptor and immunoglobulin A (IgA) have separate binding sites in streptococcal beta protein. J. Biol. Chem. 2011, 286, 33981–33991. [Google Scholar] [CrossRef] [PubMed]
- Ando, M.; Tu, W.; Nishijima, K.; Iijima, S. Siglec-9 enhances IL-10 production in macrophages via tyrosine-based motifs. Biochem. Biophys. Res. Commun. 2008, 369, 878–883. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, Y.; Isoda, H.; Shinmoto, H.; Tanimoto, M.; Dosako, S.; Idota, T.; Nakajima, I. Inhibition by kappa-casein glycomacropeptide and lactoferrin of influenza virus hemagglutination. Biosci. Biotechnol. Biochem. 1993, 57, 1214–1215. [Google Scholar] [CrossRef]
- Idota, T.; Kawakami, H.; Nakajima, I. Growth-promoting Effects of N-Acetylneuraminic Acid-containing Substances on Bifidobacteria. Biosci. Biotechnol. Biochem. 1994, 58, 1720–1722. [Google Scholar] [CrossRef]
- Otani, H.; Monnai, M.; Kawasaki, Y.; Kawakami, H.; Tanimoto, M. Inhibition of mitogen-induced proliferative responses of lymphocytes by bovine kappa-caseinoglycopeptides having different carbohydrate chains. J. Dairy. Res. 1995, 62, 349–357. [Google Scholar] [CrossRef]
- Robitaille, G.; Ng-Kwai-Hang, K.F.; Monardes, H.G. Association of kappa-casein glycosylation with milk production and composition in Holsteins. J. Dairy. Sci. 1991, 74, 3314–3317. [Google Scholar] [CrossRef] [PubMed]
- Carow, B.; Rottenberg, M.E. SOCS3, a Major Regulator of Infection and Inflammation. Front. Immunol. 2014, 5, 58. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Holdbrooks, A.T.; Liu, Y.; Reynolds, S.L.; Yanagisawa, L.L.; Benveniste, E.N. SOCS3 deficiency promotes M1 macrophage polarization and inflammation. J. Immunol. 2012, 189, 3439–3448. [Google Scholar] [CrossRef] [PubMed]
- Pae, H.O.; Chung, H.T. Heme oxygenase-1: Its therapeutic roles in inflammatory diseases. Immune Netw. 2009, 9, 12–19. [Google Scholar] [CrossRef]
- Li, T.; Chen, B.; Du, M.; Song, J.; Cheng, X.; Wang, X.; Mao, X. Casein Glycomacropeptide Hydrolysates Exert Cytoprotective Effect against Cellular Oxidative Stress by Up-Regulating HO-1 Expression in HepG2 Cells. Nutrients 2017, 9, 31. [Google Scholar] [CrossRef]






| Target | Oligonucleotide | Accession Number (NCBI) |
|---|---|---|
| SIGLEC5 | Fw: TGTGCCCTGCTCCTTCTCTTA | NM_003830.3 |
| Rv: TTCCCGTGTCCTCCATTCTG | ||
| SIGLEC7 | Fw: TGACCACGAACAGGACCATC | NM_014385.3 |
| Rv: TCAACAGCACAGACCAAGCG | ||
| SIGLEC9 | Fw: GGCGGAAGGACAGACAAGTA | NM_001198558.1 |
| Rv: GCATCCTGGTCTGTATTGGC | ||
| SOCS3 | Fw: CACAAGAAGCCAACCAGGAG | NM_003955.4 |
| Rv: CTTGTGGTTGCTATCGTCCC | ||
| HMOX1 | Fw: AAGACTGCGTTCCTGCTCAAC | NM_002133.3 |
| Rv: AAAGCCCTACAGCAACTGTCG | ||
| GAPDH | Fw: ATCCCATCACCATCTTCCAG | NM_002046.6 |
| Rv: GGCAGAGATGATGACCCTTT |
| LPS/IFN-γ | GMP | aGMP |
|---|---|---|
| 100 ± 0.03% | 88.03 ± 5.26% | 94.13 ± 3.65% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Córdova-Dávalos, L.E.; Cervantes-García, D.; Ballona-Alba, M.F.; Santos-López, A.; Esquivel-Basaldúa, A.S.; Gallegos-Alcalá, P.; Jiménez, M.; Salinas, E. Protective Effect of Glycomacropeptide on the Inflammatory Response of U937 Macrophages. Foods 2023, 12, 1528. https://doi.org/10.3390/foods12071528
Córdova-Dávalos LE, Cervantes-García D, Ballona-Alba MF, Santos-López A, Esquivel-Basaldúa AS, Gallegos-Alcalá P, Jiménez M, Salinas E. Protective Effect of Glycomacropeptide on the Inflammatory Response of U937 Macrophages. Foods. 2023; 12(7):1528. https://doi.org/10.3390/foods12071528
Chicago/Turabian StyleCórdova-Dávalos, Laura Elena, Daniel Cervantes-García, Maria Fernanda Ballona-Alba, Alejandra Santos-López, Alma Saraí Esquivel-Basaldúa, Pamela Gallegos-Alcalá, Mariela Jiménez, and Eva Salinas. 2023. "Protective Effect of Glycomacropeptide on the Inflammatory Response of U937 Macrophages" Foods 12, no. 7: 1528. https://doi.org/10.3390/foods12071528
APA StyleCórdova-Dávalos, L. E., Cervantes-García, D., Ballona-Alba, M. F., Santos-López, A., Esquivel-Basaldúa, A. S., Gallegos-Alcalá, P., Jiménez, M., & Salinas, E. (2023). Protective Effect of Glycomacropeptide on the Inflammatory Response of U937 Macrophages. Foods, 12(7), 1528. https://doi.org/10.3390/foods12071528