The Possibility of Using Sulphur Shelf Fungus (Laetiporus sulphureus) in the Food Industry and in Medicine—A Review
Abstract
:1. Introduction
2. Characteristics and Occurrence of Laetiporus sulphureus
3. Nutritional Value of Fruiting Bodies
4. Bioactivity of Laetiporus sulphureus
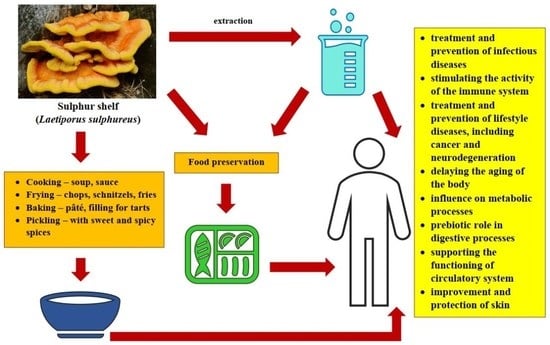
5. Fruiting bodies of Laetiporus sulphureus in Food Production
6. Other Applications
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kovács, D.; Vetter, J. Chemical composition of the mushroom Laetiporus sulphureus (Bull.) Murill. Acta Aliment. 2015, 44, 104–110. [Google Scholar] [CrossRef] [Green Version]
- Szymański, M.; Kolendowicz, M.; Szymański, A. Badania wyciągów z owocników grzyba Laetiporus sulphureus (Bull.). Post. Fitoter. 2021, 22, 14–22. [Google Scholar] [CrossRef]
- Elkhateeb, W.A.; El Ghwas, D.E.; Gundoju, N.R.; Somasekhar, T.; Akram, M.; Daba, G.M. Chicken of the Woods Laetiporus Sulphureus and Schizophyllum Commune Treasure of Medicinal Mushrooms. Open Access J. Microbiol. Biotechnol. 2021, 6, 000201. [Google Scholar] [CrossRef]
- van den Brandhof, J.G.; Wösten, H.A.B. Risk assessment of fungal materials. Fungal. Biol. Biotechnol. 2022, 9, 3. [Google Scholar] [CrossRef]
- Kuo, M. MushroomExpert.Com. Laetiporus sulphureus. 2017. Available online: http://www.mushroomexpert.com/laetiporus_sulphureus.html (accessed on 23 February 2023).
- Discover Life. Available online: https://www.discoverlife.org/mp/20m?kind=Laetiporus+sulphureus (accessed on 21 October 2022).
- Mortimer, P.E.; Xu, J.; Karunarathna, S.C.; Hyde, K.D. Mushrooms for Trees and People: A Field Guide to Useful Mushrooms of the Mekong Region; The World Agroforestry Centre, East Asia: Kunming, China, 2014; pp. 16–17. [Google Scholar]
- Bulam, S.; Üstün, N.S.; Pekşen, A. Nutraceutical and Food Preserving Importance of Laetiporus sulphureus. Turk. J. Agric.-Food Sci. Technol. 2019, 7, 94–100. [Google Scholar] [CrossRef] [Green Version]
- Siwulski, M.; Pleszczyńska, M.; Wiater, A.; Wong, J.-J.; Szczodrak, J. The influence of different media on the Laetiporus sulphureus (Bull.: Fr.) Murr. mycelium growth. Herba Pol. 2009, 55, 278–284. [Google Scholar]
- Luangharn, T.; Karunarathna, S.C.; Hyde, K.D.; Chukeatirote, E. Optimal conditions of mycelia growth of Laetiporus sulphureus sensu lato. Mycology 2014, 5, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Simões, R. Isolation, Cultivation and Antioxidant Capacity of Laetiporus sulphureus. Master’s Thesis, Faculdade de Ciências e Tecnologias da Universidade de Coimbra, Coimbra, Portugal, 2019. [Google Scholar]
- Bergmann, P.; Frank, C.; Reinhardt, O.; Takenberg, M.; Werner, A.; Berger, R.G.; Ersoy, F.; Zschätzsch, M. Pilot-Scale Production of the Natural Colorant Laetiporic Acid, Its Stability and Potential Applications. Fermentation 2022, 8, 684. [Google Scholar] [CrossRef]
- Maria Curie-Skłodowska University; Poznań University of Life Sciences; Biernacki Józef Krzysztof. A Method of Cultivating the Fruiting Bodies of the Sulfur Yellow (Laetiporus sulphureus). Application: 2 January 2012. Patent PL219753 B1, 31 July 2015. [Google Scholar]
- Pleszczyńska, M.; Wiater, A.; Siwulski, M.; Szczodrak, J. Successful large-scale production of fruiting bodies of Laetiporus sulphureus (Bull.: Fr.) Murrill on an artificial substrate. World J. Microbiol. Biotechnol. 2013, 29, 753–758. [Google Scholar] [CrossRef] [Green Version]
- Jasińska, A.; Dawidowicz, L.; Siwulski, M.; Kilinowski, P. Growth of Mycelium of Different Edible and Medicinal Mushrooms on Medium Supplemented with Digestate from AD Biogas Plant. Not. Bot. Horti. Agrobot. Cluj-Napoca 2017, 45, 498–506. [Google Scholar] [CrossRef] [Green Version]
- Grown My Own Health Food. Available online: https://growmyownhealthfood.com/how-to-grow-chicken-of-the-woods-indoors/ (accessed on 13 March 2023).
- Field and Forest Products. Available online: https://www.fieldforest.net/product/chicken-of-the-woods-on-logs-instruction-sheet/instruction-sheets. (accessed on 13 March 2023).
- Dong, W.; Wang, Z.; Feng, X.; Zhang, R.; Shen, D.; Du, S.; Gao, J.; Qia, J. Chromosome-Level Genome Sequences, Comparative Genomic Analyses, and Secondary-Metabolite Biosynthesis Evaluation of the Medicinal Edible Mushroom Laetiporus sulphureus. Microbiol. Spectr. 2022, 10, e02439-22. [Google Scholar] [CrossRef]
- Wright, R.; Woof, K.; Douglas, B.; Gaya, E. The genome sequence of the chicken of the woods fungus, Laetiporus sulphureus (Bull.) Murrill, 1920. Wellcome Open Res. 2022, 7, 83. [Google Scholar] [CrossRef]
- Ayaz, F.A.; Torun, H.; Özel, A.; Col, M.; Duran, C.; Sesli, E.; Colak, A. Nutritional value of some wild edible mushrooms from Black Sea Region (Turkey). Turk. J. Biochem./Turk Biyokim. Derg. 2011, 36, 213–221. [Google Scholar]
- Petrović, J.; Sojković, D.S.; Reis, F.S.; Barros, L.; Glamočlija, J.; Ćirić, A.; Ferreira, I.C.F.R.; Soković, M. Study on chemical, bioactive and food preserving properties of Laetiporus sulphureus (Bull.: Fr.) Murr. Food Funct. 2014, 5, 1441–1451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teke, A.N.; Bi, M.E.; Ndam, L.M.; Kinge, T.R. Nutrient and mineral components of wild edible mushrooms from the Kilum-Ijim forest, Cameroon. Afr. J. Food Sci. 2021, 15, 152–161. [Google Scholar] [CrossRef]
- Saha, D.; Sundriyal, M.; Sundriyal, R.C. Diversity of food composition and nutritive analysis of edible wild plants in a multi-ethnic tribal land, Northeast India: An important facet for food supply. Indian J. Tradit. Knowl. 2014, 13, 698–705. [Google Scholar]
- Florczak, J.; Karmańska, A.; Karwowski, B. Niektóre składniki żółciaka siarkowego Laetiporus sulfureus (Bull.) Murrill. Bromat. Chem. Toksykol. 2015, 48, 210–215. [Google Scholar]
- Luangharn, T.; Hyde, K.D.; Chukeatirote, E. Proximate Analysis and Mineral Content of Laetiporus sulphureus Strain MFLUCC 12-0546 from Northern Thailand. Chiang Mai J. Sci. 2014, 41, 765–770. [Google Scholar]
- Olennikov, D.N.; Agafonova, S.V.; Borovskii, G.B.; Penzina, T.A.; Rokhin, A.V. Water-soluble endopolysaccharides from the fruiting bodies of Laetiporus sulphureus (Bull.: Fr.) Murr. Appl. Biochem. Microbiol. 2009, 45, 536–543. [Google Scholar] [CrossRef]
- Zhao, H.; Lan, Y.; Liu, H.; Zhu, Y.; Liu, W.; Zhang, J.; Jia, L. Antioxidant and Hepatoprotective Activities of Polysaccharides from Spent Mushroom Substrates (Laetiporus sulphureus) in Acute Alcohol-Induced Mice. Oxid. Med. Cell. Longev. 2017, 2017, 5863523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ChemSpider Search and Share Chemistry. Available online: http://www.chemspider.com/ (accessed on 23 February 2023).
- PubChem Explore Chemistry. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 6 January 2023).
- Khatua, S.; Ghosh, S.; Acharya, K. Laetiporus sulphureus (Bull.: Fr.) Murr. as Food as Medicine. Pharmacogn. J. 2017, 9, s1–s15. [Google Scholar] [CrossRef] [Green Version]
- Turfan, N.; Pekşen, A.; Kibar, B.; Űnal, S. Determination of natural and bioactive properties in soma selected wild growing and cultivated mushrooms from Tukey. Acta Sci. Pol. Hortorum Cultus 2018, 17, 57–72. [Google Scholar] [CrossRef]
- Sułkowska-Ziaja, K.; Muszyńska, B.; Gawalska, A.; Sałaciak, K. Laetiporus sulphureus–chemical composition and medicinal value. Acta Sci. Pol. Hortorum Cultus 2018, 17, 87–96. [Google Scholar] [CrossRef]
- Younis, A.M.; Yosri, M.; Stewart, J.K. In vitro evaluation of pleiotropic properties of wild mushroom Laetiporus sulphureus. Ann. Agric. Sci. 2019, 64, 79–87. [Google Scholar] [CrossRef]
- Bengu, A.S. Some elements and fatty acid profiles of three different wild edible mushrooms from Tokat province in Turkey. Prog. Nutr. 2019, 21, 189–193. [Google Scholar] [CrossRef]
- Woldegiorgis, A.Z.; Abate, D.; Haki, G.D.; Ziegler, G.R.; Harvatine, K.J. Fatty Acid Profile of Wild and Cultivated Edible Mushrooms Collected from Ethiopia. J. Nutr. Food Sci. 2015, 5, 360. [Google Scholar] [CrossRef] [Green Version]
- Agafanova, S.V.; Olennikov, D.N.; Borovskii, G.B.; Penzina, T.A. Chemical composition of fruiting bodies from two strains of Laetiporus sulphureus. Chem. Nat. Compd. 2007, 43, 687–688. [Google Scholar] [CrossRef]
- Palazzolo, E.; Gargano, M.L.; Venturella, G. The nutritional composition of selected wild edible mushrooms from Sicily (southern Italy). Int. J. Food Sci. 2012, 63, 79–83. [Google Scholar] [CrossRef]
- Sinanoglou, V.J.; Zoumpoulakis, P.; Heropoulos, G.; Proestos, C.; Ćirić, A.; Petrovic, J.; Glamoclija, J.; Sokovic, M. Lipid and fatty acid profile of the edible fungus Laetiporus sulphurous. Antifungal and antibacterial properties. J. Food Sci. Technol. 2014, 52, 3264–3272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yilmaz, H.C.; Işik, H.; Bengü, A.S.; Türkekul, I. Fatty acid contents of two edible mushroom species (Cyclocybe aegerita and Hygrophorus eburneus) collected from Tokat Region. Middle East J. Sci. 2020, 6, 37–43. [Google Scholar] [CrossRef]
- Petrović, J.; Glamočlija, J.; Sojković, D.S.; Ćirić, A.; Nikolić, M.; Bukvički, D.; Guerzoni, M.E.; Soković, M.D. Laetiporus sulphureus, edible mushroom from Serbia: Investigation on volatile compounds, in vitro antimicrobial activity and in situ control of Aspergillus flavus in tomato paste. Food Chem. Toxicol. 2013, 59, 297–302. [Google Scholar] [CrossRef]
- Weber, R.W.S.; Mucci, A.; Davoli, P. Laetiporic acid, a new polyene pigment from the wood-rotting basidiomycete Laetiporus sulphureus (Polyporales, Fungi). Tetrahedron Lett. 2004, 45, 1075–1078. [Google Scholar] [CrossRef]
- Zschätzsch, M.; Steudler, S.; Reinhardt, O.; Bergmann, P.; Ersoy, F.; Stange, S.; Wagenführ, A.; Walther, T.; Berger, R.G.; Werner, A. Production of natural colorants by liquid fermentation with Chlorociboria aeruginascens and Laetiporus sulphureus and prospective applications. Eng. Life Sci. 2021, 21, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Davoli, P.; Mucci, A.; Schenetti, L.; Weber, R. Laetiporic acids, a family of non-carotenoid polyene pigments from fruit-bodies and liquid cultures of Laetiporus sulphureus (Polyporales, Fungi). Phytochemistry 2005, 66, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.-Y.; Yin, X.; Li, Z.-H.; Li, Y.; Liu, J.-K.; Feng, T.; Zhao, B.-H. Mycophenolic acid derivatives from cultures of the mushroom Laetiporus sulphureus. Chin. J. Nat. Med. 2014, 12, 685–688. [Google Scholar] [PubMed]
- Wang, Y.; Wu, B.; Shao, J.; Jia, J.; Tian, Y.; Shu, X.; Ren, X.; Guan, Y. Extraction, purification and physicochemical properties of a novel lectin from Laetiporus sulphureus mushroom. LWT-Food Sci. Technol. 2018, 91, 151–159. [Google Scholar] [CrossRef]
- Quintero-Cabello, K.P.; Lugo-Flores, M.A.; Rivera-Palafox, P.; Silva-Espinoza, B.A.; González-Aguilar, G.A.; Esqueda, M.; Gaitán-Hernández, R.; Ayala-Zavala, J.F. Antioxidant Properties and Industrial Uses of Edible Polyporales. J. Fungi 2021, 7, 196. [Google Scholar] [CrossRef]
- Sułkowska-Ziaja, K.; Grabowska, K.; Apola, A.; Kryczyk-Poprawa, A.; Muszyńska, B. Mycelial culture extracts of selected wood-decay mushrooms as a source of skin-protecting factors. Biotechnol. Lett. 2021, 43, 1051–1061. [Google Scholar] [CrossRef]
- Sigma Aldrich. Karta Charakterystyki Zgodnie z Rozporządzeniem WE 1907/2006. Available online: https://www.sigmaaldrich.com/PL/pl/sds/sigma/c3641 (accessed on 15 January 2023).
- Klaus, A.; Kozarski, M.; Niksic, M.; Jakovljevic, D.; Todorovic, N.; Stefanoska, I.; van Griensven, L.J.L.D. The edible mushroom Laetiporus sulphureus as potential source of natural antioxidants. Int. J. Food Sci. Nutr. 2013, 64, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Acharya, K.; Ghosh, S.; Khauta, S.; Mitra, P. Pharmacognostic standardization and antioxidant capacity of an edible mushroom Laetiporus sulphureus. J. Verbrauch. Lebensm. 2016, 11, 33–42. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Agafonova, S.V.; Nazarova, A.V.; Borovskii, G.B.; Penzina, T.A. Organic acids and carbohydrates from Laetiporus sulphureus fruiting bodies. Brief Communications. Chem. Nat. Compd. 2008, 44, 762–763. [Google Scholar] [CrossRef]
- Bari, E.; Sistani, A.; Morrell, J.J.; Pizzi, A.; Akbari, M.R.; Ribera, J. Current Strategies for the Production of Sustainable Biopolymer Composites. Polymers 2021, 13, 2878. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Tankhaeva, L.M.; Agafonova, S.V. Antioxidant Components of Laetiporus sulphureus (Bull.: Fr.) Murr. Fruit Bodies. Appl. Biochem. Microbiol. 2011, 47, 419–425. [Google Scholar] [CrossRef]
- Nowacka, N.; Nowak, R.; Drozd, M.; Olech, M.; Los, R.; Malm, A. Analysis of phenolic constituents, antiradical and antimicrobial activity of edible mushrooms growing wild in Poland. LWT-Food Sci. Technol. 2014, 59, 689–694. [Google Scholar] [CrossRef]
- Karaman, M.; Jovin, E.; Malbaša, R.; Matavuly, M.; Popowić, M. Medicinal and Edible Lignicolous Fungi as Natural Sources of Antioxidative and Antibacterial Agents. Phytother. Res 2010, 24, 1473–1481. [Google Scholar] [CrossRef]
- Sułkowska-Ziaja, K.; Muszyńska, B.; Motyl, P.; Pasko, P.; Ekiert, H. Phenolic Compounds and antioxidant activity in some species of polyporoid mushrooms from Poland. Int. J. Med. Mushrooms 2012, 14, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Lear, M.J.; Simon, O.; Foley, T.L.; Burkart, M.D.; Baiga, T.J.; Noel, J.P.; DiPasuale, A.G.; Rheingold, A.L.; La Clair, J.L. Laetirobin from the Parasitic Growth of Laetiporus sulphureus on Robinia pseudoacacia. J. Nat. Prod. 2009, 72, 1980–1987. [Google Scholar] [CrossRef] [PubMed]
- Ericsson, D.; Ivonne, J. Sterol composition of the macromycete fungus Laetiporus sulphureus. Chem. Nat. Compd. 2009, 45, 193–196. [Google Scholar] [CrossRef]
- Chepkirui, C.; Matasyoh, J.C.; Decock, C.; Stadlera, M. Two cytotoxic triterpenes from cultures of a Kenyan Laetiporus sp. (Basidiomycota). Phytochem. Lett. 2017, 20, 106–110. [Google Scholar] [CrossRef]
- Hassan, K.; Kemkuignou, M.B.; Stadler, M. Two New Triterpenes from Basidiomata of the Medicinal and Edible Mushroom, Laetiporus sulphureus. Molecules 2021, 26, 7090. [Google Scholar] [CrossRef]
- Khalilov, Q.; Numonov, S.; Sukhrobov, P.; Bobakulov, K.; Sharopov, F.; Habasi, M.; Zhao, J.; Yuan, T.; Aisa, H.A. New Triterpenoids from the Fruiting Bodies of Laetiporus sulphureus and Their Anti-Inflammatory Activity. ACS Omega 2022, 7, 27272–27277. [Google Scholar] [CrossRef]
- HMDB: The Human Metabolome Database. Available online: https://hmdb.ca (accessed on 13 January 2023).
- Szczepkowski, A. Grzyby nadrzewne w innym świetle–użytkowanie owocników. Stud. Mater. CEPL Rogowie 2012, 32, 171–189. [Google Scholar]
- Chemical Book. Available online: www.chemicalbook.com (accessed on 15 January 2023).
- Feng, W.; Yang, J.; Xu, X.; Liu, Q. Quantitative Determination of Lanostane Triterpenes in Fomes officinalis and their Fragmentation Study by HPLC-ESI. Phytochem. Anal. 2010, 21, 531–538. [Google Scholar] [CrossRef] [PubMed]
- SpectraBase. Open access Spectral Database. Available online: https://spectrabase.com/ (accessed on 16 January 2023).
- Saba, E.; Son, Y.; Jeon, B.R.; Kim, S.-E.; Lee, I.-K.; Yun, B.-S.; Rhee, M.H. Acetyl Eburicoic Acid from Laetiporus sulphureus var. miniatus Suppresses Inflammation in Murine Macrophage RAW 264.7 Cells. Mycobiology 2015, 43, 131–136. [Google Scholar] [CrossRef] [Green Version]
- Duan, Y.; Qi, J.; Gao, J.; Liu, C. Bioactive components of Laetiporus species and their pharmacological effects. Appl. Microbiol. Biotechnol. 2022, 106, 5929–5944. [Google Scholar] [CrossRef] [PubMed]
- He, J.-B.; Tao, J.; Miao, X.-S.; Bu, W.; Zhang, S.; Dong, Z.-J.; Li, Z.-H.; Feng, T.; Liu, J.-K. Seven new drimane-type sesquiterpenoids from cultures of fungus Laetiporus sulphureus. Fitoterapia 2015, 102, 102. [Google Scholar] [CrossRef]
- Petrovska, B.B. Protein Fraction in Edible Macedonian Mushrooms. Eur. Food Res. Technol. 2001, 212, 469–472. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Agafonova, S.V.; Borovskii, G.B.; Penzina, T.A.; Rokhin, A.V. Alkali-Soluble Polysaccharides of Laetiporus Sulphureus (Bull.: Fr.) Murr Fruit Bodies. Appl. Biochem. Microbiol. 2009, 45, 626–630. [Google Scholar] [CrossRef]
- Alquini, G.; Carbonero, E.R.; Rosado, F.R.; Cosentino, C.; Iacomini, M. Polysaccharides from the fruit bodies of the basidiomycete Laetiporus sulphureus (Bull.: Fr.) Murr. FEMS Microbiol. Lett. 2004, 230, 47–52. [Google Scholar] [CrossRef] [Green Version]
- Wiater, A.; Pleszczyńska, M.; Szczodrak, J.; Próchniak, K. α-(1→3)-Glukany ściany komórkowej żółciaka siarkowego-Laetiporus sulphureus (Bull.: Fr.) Murrill-izolacja, charakterystyka i zastosowanie do indukcji syntezy mutanazy. Biotechnologia 2008, 2, 174–189. [Google Scholar]
- Wiater, A.; Pleszczyńska, M.; Szczodrak, J.; Janusz, G. Comparative Studies on the Induction of Trichoderma harzianum Mutanase by α-(1→3)-Glucan-Rich Fruiting Bodies and Mycelia of Laetiporus sulphureus. Int. J. Mol. Sci. 2012, 13, 9584–9598. [Google Scholar] [CrossRef] [Green Version]
- Wiater, A.; Waśko, A.; Adamczyk, P.; Gustaw, K.; Pleszczyńska, M.; Wlizło, K.; Skowronek, M.; Tomczyk, M.; Szczodrak, J. Prebiotic Potential of Oligosaccharides Obtained by Acid Hydrolysis of α-(1→3)-Glucan from Laetiporus sulphureus: A Pilot Study. Molecules 2020, 25, 5542. [Google Scholar] [CrossRef]
- Dimopoulou, M.; Kolonas, A.; Mourtakos, S.; Androutsos, O.; Gortzi, O. Nutritional Composition and Biological Properties of Sixteen Edible Mushroom Species. Appl. Sci. 2022, 12, 8074. [Google Scholar] [CrossRef]
- Teke, A.N.; Bi, M.E.; Ndam, L.M.; Kinge, T.R. Nutrient and Mineral Contents of Wild Edible Mushrooms from the Kilum-Ijim Forest, Cameroon. Nutr. Food Sci. J. 2020, 3, 128. [Google Scholar]
- Ćirić, A.; Kruljević, I.; Stojković, D.; Fernandes, A.; Barros, L.; Calhelha, R.C.C.; Ferreira, I.C.F.R.; Soković, M.; Glamočlija, J. Comparative investigation on edible mushrooms Macrolepiota mastoidea, M. rhacodes and M. procera: Functional foods with diverse biological activities. Food Funct. 2019, 10, 7678–7686. [Google Scholar] [CrossRef]
- Bulam, S.; Karadeniz, M.; Bakir, T.K.; Ünal, S. Assessment of total phenolic, total flavonoid, metal contents and antioxidant activities of Trametes versicolor and Laetiporus sulphureus. Acta Sci. Pol. Hortorum Cultus 2022, 21, 39–47. [Google Scholar] [CrossRef]
- Sevindik, M.; Akgul, H.; Dogan, M.; Akata, I.; Selamoglu, Z. Determination of antioxidant, antimicrobial, DNA protective activity and heavy metals content of Laetiporus sulphureus. Fresenius Environ. Bull. 2018, 27, 1946–1952. [Google Scholar]
- Barros, L.; Ferreira, M.-J.; Queirós, B.; Ferreira, I.C.F.R.; Baptista, P. Total phenols, ascorbic acid, carotene and lycopene in Portuguese wild edible mushrooms and their antioxidant activities. Food Chem. 2007, 103, 413–419. [Google Scholar] [CrossRef]
- Ferreira, I.; Barros, L.; Abreu, R. Antioxidants in wild mushrooms. Curr. Med. Chem. 2009, 16, 1543–1560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vamanu, E.; Nita, S. Bioactive Compounds, Antioxidant and Anti-inflammatory Activities of Extracts from Cantharellus cibarius. Rev. Chim 2014, 65, 372–379. [Google Scholar]
- Popa, G.; Cornea, C.P.; Luta, G.; Gherghina, E.; Israel-Roming, F.; Bubueanu, C.; Toma, R. Antioxidant and antimicrobial properties of Laetiporus sulphureus (Bull.) Murrill. AgroLife Sci. J. 2016, 5, 168–173. [Google Scholar]
- Zhang, J.; Lv, J.; Zhao, L.; Shui, X.; Wang, L.-A. Antioxidant and antimicrobial activities and chemical composition of submerged cultivated mycelia of Laetiporus sulphureus. Chem. Nat. Compd. 2018, 54, 1187–1188. [Google Scholar] [CrossRef]
- Shelest, A. Methods of Increasing Biosynthetic Activity of the Strain LS-0917 Laetiporus sulphureus (Bull.) Murril–Carotenoid Producer. Master’s Thesis, Vytautas Magnus University, Kaunas, Lithuania, 2020. [Google Scholar]
- Frljak, J.; Mulabećirović, A.H.; Isaković, S.; Karahmet, E.; Toroman, A. Biological Active Components of Selected Medical Fungi. Open J. Prev. Med. 2021, 11, 9–22. [Google Scholar] [CrossRef]
- Sevindik, M.; Akgul, H.; Selmoglu, Z.; Braidy, N. Antioxidant, antimicrobial and neuroprotective effects of Octaviania asterosperma in vitro. Mycology 2021, 12, 128–138. [Google Scholar] [CrossRef]
- Nicolcioiu, M.B.; Popa, G.; Matei, F. Biochemical investigations of different mushroom species for their biotechnological potential. Proc. Conf. Agric. Life Life Agric. 2018, 1, 562–567. [Google Scholar] [CrossRef] [Green Version]
- Florczak, J.; Karmańska, A.; Karwowski, B. Badanie zawartości związków polifenolowych oraz aktywności przeciwutleniającej niektórych jadalnych gatunków grzybów wielkoowocnikowych. Bromat. Chem. Toksykol. 2016, 49, 719–724. [Google Scholar]
- Lung, M.-Y.; Huang, W.-Z. Antioxidant properties of polysaccharides from Laetiporus sulphureus in submerged cultures. Afr. J. Biotechnol. 2012, 11, 6350–6358. [Google Scholar] [CrossRef]
- Mahmoud, O.A.; Abdel-Hadi, S.Y. Extraction and Purification of Lovastatin from the Edible Mushroom Laetiporus sulphureus and its Antioxidant Activity. Egypt. J. Bot. 2022, 62, 169–175. [Google Scholar] [CrossRef]
- Lin, W.C.; Lee, T.T. The Laetiporus sulphureus Fermented Product Enhances the Antioxidant Status, Intestinal Tight Junction, and Morphology of Broiler Chickens. Animals 2021, 11, 149. [Google Scholar] [CrossRef]
- de Carvalho, M.P.; Türck, P.; Abraham, W.-R. Secondary Metabolites Control the Associated Bacterial Communities of Saprophytic Basidiomycotina Fungi. Microbes Environ. 2015, 30, 196–198. [Google Scholar] [CrossRef] [Green Version]
- Čuvalová, A.; Strapáč, I.; Handrová, L.; Kmeť, V. Antibiofilm activity of mushroom extracts against Staphylococcus aureus F. J. Rosen. Ann. Univ. Paedagog. Crac. Stud. Nat. 2018, 3, 17–23. [Google Scholar] [CrossRef]
- Turkoglu, A.; Duru, M.E.; Mercan, N.; Kivrak, I.; Gezer, K. Antioxidant and antimicrobial activities of Laetiporus sulphureus (Bull.) Murrill. Food Chem. 2007, 101, 267–273. [Google Scholar] [CrossRef]
- Kolundzić, M.D.; Grozdanić, N.D.; Stanojković, T.P.; Milenković, M.T.; Dinić, M.R.; Golić, N.E.; Kojić, M.; Kundaković, T.D. Antimicrobial and Cytotoxic Activities of the Sulphur Shelf Medicinal Mushroom, Laetiporus sulphurous (Agaricomycetes), from Serbia. Int. J. Med. Mushrooms 2016, 18, 469–476. [Google Scholar] [CrossRef]
- Gavryushina, I.A.; Gromovykh, T.I.; Feldman, N.B.; Lutsenko, S.V.; Ponomarenko, V.I.; Kisil, O.V.; Sadykova, V.S. Antimikrobnyje cbojstwa wodorastworimych polisacharidow i spirtowych ekstraktow micelija Laetiporus sulphureus (Bull.) Murrill i razrabotka biotechnołogii jego połuczenija w immobilizowannoj kulturie na bakterioalnoj celljulozje. Antibiot. Chim. 2020, 65, 10–14. [Google Scholar]
- Pârvu, M.; Andrei, A.-S.; Roşca-Casian, O. Antifungal activity of Laetiporus sulphureus mushroom extract. Contrib. Bot. 2010, 45, 65–70. [Google Scholar]
- Sevindik, M. Mushrooms as natural antiviral sources and supplements foods against coronavirus (COVID-19). J. Bacteriol. Mycol. 2021, 9, 73–76. [Google Scholar] [CrossRef]
- Zhang, J.-W.; Wen, G.-L.; Zhang, L.; Duan, D.-M.; Ren, Z.-H. Sulphureuine B, a drimane type sesquiterpenoid isolated from Laetiporus sulphureus induces apoptosis in glioma cells. Bangladesh J. Pharmacol. 2015, 10, 844–853. [Google Scholar] [CrossRef] [Green Version]
- Petrović, J.; Glamočlija, J.; Ilić-Tomić, T.; Sojković, M.; Robajac, D.; Nedić, O.; Pavić, A. Lectin from Laetiporus sulphureus effectively inhibits angiogenesis and tumor development in the zebrafish xenograft models of colorectal carcinoma and melanoma. Int. J. Biol. Macromol. 2020, 148, 129–139. [Google Scholar] [CrossRef]
- Kim, E.-J.; Yoo, K.-H.; Kim, Y.-S.; Seok, S.-J.; Kim, J.-H. Hexane and Chloroform Fractions of Laetiporus sulphrueus var. miniatus Inhibit Thrombin-treated Matrix Metalloproteinase-2/9 Expression in Human Oral Squamous Carcinoma YD-10B Cells. Kor. J. Mycol. 2017, 45, 175–187. [Google Scholar] [CrossRef] [Green Version]
- Pecić, K.; Jovanović, M.; Arsenijević, D.; Pavić, J.; Grujović, M.; Mladenović, K.; Virijević, K.; Živanović, M.; Šeklić, D. Laetiporus sulphureus Affects Migration and Superoxide Anion Radical Levels in HeLa Cervical Cancer Cells. Biol. Life Sci. Forum 2022, 18, 16. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Mayakrishnan, V.; Al-Ghamdi, S.; Alsaidan, M.; Geddawy, A.; Abdelaziz, M.A.; Mohideen, A.P.; Bahakim, N.O.; Ramesh, T.; Ayyakannu, U.R.N. Investigation of phytochemical profile and in vivo anti-proliferative effect of Laetiporus versisporus (Lloyd) Imazeki mushroom against diethylnitrosamine-induced hepatocellular carcinoma. J. King Saud Univ. Sci. 2021, 33, 101551. [Google Scholar] [CrossRef]
- Jovanović, M.M.; Virijević, K.; Grujić, J.; Živanović, M.; Šeklić, D.S. Extract of Edible Mushroom Laetiporus sulphureus Affects the Redox Status and Motility of Colorectal and Cervical Cancer Cell Lines. Biol. Life Sci. Forum 2021, 6, 82. [Google Scholar] [CrossRef]
- Badalyan, S.; Rapior, S. Agaricomycetes mushrooms (Basidiomycota) as potential neuroprotectants. IJM-Ital. J. Mycol. 2021, 50, 30–43. [Google Scholar] [CrossRef]
- Khalilov, Q.; Li, L.; Liu, Y.; Liu, W.; Numonov, S.; Aisa, H.A.; Yuan, T. Brassinosteroid analogues from the fruiting bodies of Laetiporus sulphureus and their anti-inflammatory activity. Steroids 2019, 151, 108468. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.C.; Lee, M.T.; Lin, L.J.; Chng, S.C.; Lee, T.T. Immunomodulation Properties of Solid-State Fermented Laetiporus sulphureus Ethanol Extracts in Chicken Peripheral Blood Monocytes In Vitro. Braz. J. Poult. Sci. 2019, 21, 001–010. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhang, Y.; Shao, J.; Wu, B.; Li, B. Potential immunomodulatory activities of a lectin from the mushroom Latiporus sulphureus. Int. J. Biol. Macromol. 2019, 130, 399–406. [Google Scholar] [CrossRef]
- Ćilerdžić, J.; Galić, M.; Vukojević, J.; Stajic, M. Pleurotus ostreatus and Laetiporus sulphureus (Agaricomycetes): Possible Agents against Alzheimer and Parkinson Diseases. Int. J. Med. Mushrooms 2019, 21, 275–289. [Google Scholar] [CrossRef]
- Das, A.; Chen, C.-M.; Mu, S.-C.; Yang, S.-H.; Ju, Y.-M.; Li, S.-C. Medicinal Components in Edible Mushrooms on Diabetes Mellitus Treatment. Pharmaceutics 2022, 14, 436. [Google Scholar] [CrossRef]
- Pavic, A.; Ilic-Tomic, T.; Glamočlija, J. Unravelling Anti-Melanogenic Potency of Edible Mushrooms Laetiporus sulphureus and Agaricus silvaticus In Vivo Using the Zebrafish Model. J. Fungi 2021, 7, 834. [Google Scholar] [CrossRef]
- Badalyan, S.; Barkhudaryan, A.; Rapior, S. The Cardioprotective Properties of Agaricomycetes Mushrooms Growing in the Territory of Armenia (Review). Int. J. Med. Mushrooms 2021, 23, 21–31. [Google Scholar] [CrossRef]
- Šiljegović, J.; Stojković, D.S.; Nikolić, M.M.; Glamočlija, J.M.; Soković, M.D.; Ćirić, A.M. Antimicorbial activity of aqueous extract of Laetiporus sulphureus (Bull.:Fr.) Murill. Proc. Nat. Sci. Matica Srpska Novi Sad. 2011, 120, 297–303. [Google Scholar]
- Hassan, F.; Ni, S.; Becker, T.L.; Kinstedt, C.M.; Abdul-Samad, J.L.; Actis, L.A.; Kennedy, M.A. Evaluation of the Antibacterial Activity of 75 Mushrooms Collected in the Vicinity of Oxford, Ohio (USA). Int. J. Med. Mushrooms 2019, 21, 131–141. [Google Scholar] [CrossRef]
- Martinez-Medina, G.A.; Chavez-González, M.L.; Verma, D.K.; Prado-Barragan, L.A.; Martínez-Hernandez, J.L.; Flores-Gallegos, A.C.; Thakur, M.; Srivastav, P.P.; Aguilar, C.N. Bio-funcional components in mushrooms, a health opportunity: Ergothionine and huitlacohe as recent trends. J. Funct. Foods 2021, 77, 104326. [Google Scholar] [CrossRef]
- Mtui, G.; Masalu, R. Extracellular enzymes from brown-rot fungus Laetiporus sulphureus isolated from mangrove forests of coastal Tanzania. J. Sci. Res. Essay 2008, 3, 154–161. [Google Scholar]
- de Figueiredo, F.L.; de Oliveira, A.C.P.; Terrasan, C.R.F.; Gonçalves, T.A.; Gerhardt, J.A.; Tomazetto, G.; Persinoti, G.F.; Rubio, M.V.; Peña, J.A.T.; Araújo, M.F.; et al. Multi-omics analysis provides insights into lignocellulosic biomass degradation by Laetiporus sulphureus ATCC 52600. Biotechnol Biofuels 2021, 14, 96. [Google Scholar] [CrossRef] [PubMed]
- Beug, M.W. North American Mushroom Poisonings and Adverse Reactions to Mushrooms 2018–2020. Fungi 2021, 14, 17–22. [Google Scholar]
- Łuczaj, Ł.; Wilde, M.; Townsend, L. The Ethnobiology of Contemporary British Foragers: Foods They Teach, Their Sources of Inspiration and Impact. Sustainability 2021, 13, 3478. [Google Scholar] [CrossRef]
- First Nature: Laetiporus sulphureus (Bull.) Murrill-Chicken-of-the-Woods. Available online: www.first-nature.com/fungi/laetiporus-sulphureus.php (accessed on 3 November 2022).
- Woodland Trust: Chicken on the woods. Available online: https://www.woodlandtrust.org.uk/trees-woods-and-wildlife/fungi-and-lichens/chicken-of-the-woods/ (accessed on 7 December 2022).
- Regulation of the Minister of Health of 3 November 2022 amending the regulation on mushrooms admitted to trading or production of mushroom preserves, foodstuffs containing mushrooms and the qualifications of a mushroom classifier and mushroom expert. J. Laws Repub. Pol. 2022, 2365.
- Klich, M.A. Pathogen profile. Aspergillus flavus: The major producer of aflatoxin. Mol. Plant Pathol. 2007, 8, 713–722. [Google Scholar] [CrossRef]
- Mariutti, L.R.B.; Valente Soares, L.M. Survey of aflatoxins in tomato products. Cienc. Tecnol. Aliment. Camp. 2009, 29, 431–434. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Gupta, A.; Mahato, D.K.; Pandhi, S.; Pandey, A.K.; Kargwal, R.; Mishra, S.; Suhag, R.; Sharma, N.; Saurabh, V.; et al. Aflatoxins in Cereals and Cereal-Based Products: Occurrence, Toxicity, Impact on Human Health, and Their Detoxification and Management Strategies. Toxins 2022, 14, 687. [Google Scholar] [CrossRef] [PubMed]





| Component | Source of Information about Presence of Component | Chemical Group | Molecular Formula | Molecular Weight (g/mol) | Source of Information about Properties of Component |
|---|---|---|---|---|---|
| Carbohydrates and their derivatives | |||||
| Arabinose | [26,27] |
| C5H10O5 | 150.13 | [28,29] |
| Fructose | [30] | C6H12O6 | 180.16 | [28,29] | |
| Galactose | [27] | C6H12O6 | 180.16 | [28,29] | |
| Glucose | [8,26,27,31] | C6H12O6 | 180.16 | [28,29] | |
| Mannose | [26,27] | C6H12O6 | 180.16 | [28,29] | |
| Xylose | [26,27] | C5H10O5 | 150.13 | [28,29] | |
| Fucose | [26,27] |
| C6H12O5 | 164.16 | [28,29] |
| Rhamnose | [26] | C6H12O5 | 164.16 | [28,29] | |
| Sucrose | [8,31] |
| C12H22O11 | 342.30 | [28,29] |
| Trehalose | [14,30] | C12H22O11 | 342.30 | [28,29] | |
| Laminaran | [26] |
| C18H32O16 | 504.44 | [28,29] |
| Mannitol | [21,30] |
| C6H14O6 | 182.17 | [28,29] |
| Matsutakic acid (masutakic acid) | [32] | acetylenic acids | C10H16O4 | 200.23 | [29] |
| Egonol glucoside | Yoshikawa et al., 2001 after: [33] | glucosides | C25H28O10 | 488.48 | [28,29] |
| Lipids | |||||
| Linoleic acid | [21,30,34,35,36,37,38] |
| C18H32O2 | 280.45 | [28,29] |
| Oleic acid | [21,30,33,34,35,36,37,38,39] | C18H34O2 | 282.46 | [28,29] | |
| Palmitoleic acid | [34] | C16H30O2 | 254.41 | [28,29] | |
| Isovaleric acid | [40] |
| C5H10O2 | 102.13 | [28,29] |
| Myristic acid | [34] | C14H28O2 | 228.37 | [28,29] | |
| Palmitic acid | [21,30,34,35,36,37,38] | C16H32O2 | 256.42 | [28,29] | |
| Stearic acid | [34,37] | C18H36O2 | 284.48 | [28,29] | |
| 9-Octadecenoic acid (Z)-, 2-hydroxy-1-(hydroxymethyl)ethyl ester; (2-Oleoylglycerol) | [33] |
| C21H40O4 | 356.54 | [28] |
| Ethyl cholate | [33] |
| C26H44O5 | 436.63 | [28] |
| Laetiporic acid | [41,42] | polyenes | C27H32O4 | 420.50 | [28,29] |
| 2-dehydro-3-deoxylaetiporic acid A | [43] | C27H30O3 | 402.53 | [28,29] | |
| 6-((2E, 6E)-3, 7-dimethyldeca-2, 6-dienyl)-7-hydroxy-5-methoxy-4-methylphtanlan-1-one | [44] | C22H30O5 | 374.21 | [44] | |
| Amino acids and peptides | |||||
| Cysteine | [45] |
| C3H7NO2S | 121.16 | [28,29] |
| Ergothioneine | [46] | C9H15N3O2S | 229.30 | [28,29] | |
| L-Histidine | [30,36] | C6H9N3O2 | 155.15 | [28,29] | |
| Isoleucine | [30,36] | C6H13NO2 | 131.17 | [28,29] | |
| Leucine | [30,36] | C6H13NO2 | 131.17 | [28,29] | |
| Lysine | [30,45] | C6H14N2O2 | 146.19 | [28,29] | |
| Methionine | [30,36] | C5H11NO2S | 149.21 | [28,29] | |
| Phenylalanine | [45] | C9H11NO2 | 165.19 | [28,29] | |
| Threonine | [30,36] | C4H9NO3 | 119.12 | [28,29] | |
| Tryptophan | [36,47] | C11H12N2O2 | 204.23 | [28,29] | |
| Valine | [45] | C5H11NO2 | 117.15 | [28,29] | |
| 5-hydroxy-L-tryptophan | [47] | C11H12N2O3 | 220.22 | [28,29,48] | |
| Alanine | [45] |
| C3H7NO2 | 89.09 | [28,29] |
| Arginine | [36] | C6H14N4O2 | 174.20 | [28,29] | |
| Aspartic acid | [45] | C4H7NO4 | 133.10 | [28,29] | |
| Glycine | [45] | C₂H₅NO₂ | 75.07 | [28,29] | |
| Proline | [45] | C5H9NO2 | 115.13 | [28,29] | |
| Serine | [45] | C3H7NO3 | 105.09 | [28,29] | |
| Beauvericin | Del et al., 1978 after: [32] | peptides | C45H57N3O9 | 783.95 | [28,29,48] |
| Carboxylic acids | |||||
| Ascorbic acid | [32,46,49,50] | C6H8O6 | 176.13 | [28,29] | |
| Cinnamic acid | [3,21] | C9H8O2 | 148.16 | [28,29] | |
| Citric acid | [3,20,21,32,51] | C6H8O7 | 192.12 | [28,29] | |
| Enoxolone (glycyrrhetinic acid) | [36] | C30H46O4 | 470.68 | [28,29,48] | |
| Fumaric acid | [21] | C4H4O4 | 116.07 | [28,29] | |
| Lactic acid (polylactic acid PLA) | [52] | C3H6O3 | 90.08 | [28] | |
| Malic acid | [20,32] | C4H6O5 | 134.09 | [28,29] | |
| Malonic acid | [51] | C3H4O4 | 104.06 | [28,29] | |
| Oxalic acid | [3,21] | C2H2O4 | 90.03 | [28,29] | |
| Quinic acid | [21] | C7H12O6 | 192.17 | [28,29] | |
| Tartaric acid | [51] | C4H6O6 | 150.09 | [28,29] | |
| Vitamins | |||||
| Cholecalciferol | [30] | D3 | C27H44O | 384.64 | [28,29] |
| Cyanocobalamin | [8] | B12 | C₆₃H₈₈CoN₁₄O₁₄P | 1 355.38 | [29] |
| ʆ-tocopherol | [3,30,46,49] | E | C29H50O2 | 430.71 | [28,29] |
| β-tocopherol | [3,30,46] | C28H48O2 | 416.68 | [28,29] | |
| ɣ-tocopherol | [3,46] | C28H48O2 | 416.68 | [28,29] | |
| Caffeic acid | [53] | Phenolic acids | C9H8O4 | 180.16 | [28,29] |
| Chlorogenic acid | [53] | C16H18O9 | 354.31 | [28,29] | |
| p-Coumaric acid | [53,54] | C9H8O3 | 164.16 | [28,29] | |
| Gallic acid | [47,53,55] | C7H6O5 | 170.12 | [28,29] | |
| 4-Hydroxybenzoic acid | [3,21,47,54] | C7H6O3 | 138.12 | [28,48] | |
| Kojic acid | [47] | C6H6O4 | 142.11 | [28,29] | |
| Protocatechuic acid | [47,54,55,56] | C7H6O4 | 154.12 | [28] | |
| Salicylic acid | [54] | C7H6O3 | 138.12 | [28] | |
| Egonol | Yoshikawa et al., 2001 after: [33] | Benzofurans and derivates | C19H18O5 | 326.34 | [28,29] |
| Demethoxyegonol | Yoshikawa et al., 2001 after: [33] | C18H16O4 | 296.32 | [28,29] | |
| Egonol gentiobioside | Yoshikawa et al., 2001 after: [33] | C31H38O15 | 650.6 | [29] | |
| Matsutakeside I | [32] | C30H36O14 | 620.60 | [29] | |
| (±)-Laetirobin (Laetiporina) | [57] | C44H32O12 | 752.72 | [28,29] | |
| Ergosterol (Provitamin D2) | [46] | Sterols | C28H44O | 396.65 | [28,29] |
| Dehydroergosterol | [58] | C28H42O | 394.63 | [28,29] | |
| Ergost-7-en-3-ol | [58] | C28H48O | 400.68 | [28,29] | |
| Ergost-3,5,7,9(11),22-pentaen | [58] | C28H40 | 376.60 | [29] | |
| 24-methylenelanost-8-en-3-ol (obtusifoldienol) | [58] | C31H52O | 440.74 | [28,29] | |
| 4,4-dimethylergost-24-en-3-ol | [58] | C30H52O | 428.70 | [29] | |
| 4-methylergost-5,7,25-trien-3-ol | [58] | C29H46O | 410.70 | [29] | |
| 4-methylergost-7,14,25-trien-3-ol | [58] | C29H46O | 410.70 | [29] | |
| Ergosterol peroxide | [58] | C28H44O3 | 428.65 | [28,29] | |
| Ergosta-7,22-dien-3,5,6- triol (cerevisterol) | [58] | C28H46O3 | 430.66 | [28,29] | |
| Laetiporin A | [59] | Triterpenoids | C31H49O3 | 469.37 | [59] |
| Laetiporin B | [59] | C34H53O7 | 573.38 | [59] | |
| Laetiporin C | [60] | C31H50NaO5 | 525.35 | [60] | |
| Laetiporin D | [60] | C31H48NaO5 | 523.34 | [60] | |
| Fomefficinic acid | [60] | C31H48O4 | 484.70 | [29] | |
| Eburicoic acid | [60,61] | C31H50O3 | 470.73 | [29,61] | |
| Dehydroeburicoic acid | [59] | C31H48O3 | 468.71 | [28,29] | |
| 15 α-hydroxytrametenolic acid | [60] | C30H48O4 | 472.7 | [29] | |
| Trametenolic acid | [60] | C30H48O3 | 456.70 | [28,62] | |
| Sulphurenic acid | [63] | C31H50O4 | 486.73 | [28,29] | |
| Sulphurenoid A | [61] | C27H42O5 | 445.30 | [61] | |
| Sulphurenoid B | [61] | C27H40O5 | 443.27 | [61] | |
| Sulphurenoid C | [61] | C27H44O5 | 447.31 | [61] | |
| Sulphurenoid D | [61] | C30H44O4 | 467.32 | [61] | |
| 15α-hydroxy-3-oxolanosta-8,24-dien-21-oic acid | [61] | C30H46O3 | 454.68 | [64] | |
| 3-keto-dehydrosulfurenic acid | [61] | C31H46O4 | 481.3 | [65] | |
| 3-oxolanosta-8,24-dien-21- oic acid (pinicolic acid A) | [61] | C30H46O3 | 454.70 | [29,66] | |
| 5α-hydroxytrametenolic acid | [61] | C30H48O4 | 472.7 | [63] | |
| 3- oxosulfurenic acid | [61] | C31H48O4 | 484.7 | [66] | |
| Dehydrosulphurenic acid | [61] | C31H48O4 | 484.7 | [66] | |
| Acetyl eburicoic acid (LSM-H7) | Leon et al., 2004 after: [33,67] | C33H52O4 | 512.8 | [29,67] | |
| Acetyl trametenolic acid | León et al., 2004 after: [68] | C32H50O4 | 498.70 | [29] | |
| Versisponic acid A | Yoshikawa et al., 2000 after: [68] | C30H48O5 | 488.70 | [28,29] | |
| Versisponic acid B | Yoshikawa et al., 2000 after: [68] | C32H48O5 | 512.72 | [28,29] | |
| Versisponic acid C | Yoshikawa et al., 2000 after: [68] | C33H50O5 | 526.75 | [28,29] | |
| Versisponic acid D | Yoshikawa et al., 2000 after: [68] | C33H52O5 | 528.76 | [28,29] | |
| Versisponic acid E | Yoshikawa et al., 2000 after: [68] | C35H54O5 | 554.80 | [28] | |
| 3β-hydroxylanosta-8,24-dien-21-oic acid | [61] | C30H48O3 | 456.70 | [28] | |
| laricinolic acid | [61] | Sesquiterpenoids | C15H24O3 | 252.35 | [28] |
| Sulphureuine A | [69] | C15H22O2 | 234.33 | [28,29,69] | |
| Sulphureuine B | [69] | C15H28O4 | 272.20 | [69] | |
| Sulphureuine C | [69] | C15H28O4 | 272.20 | [69] | |
| Sulphureuine D | [69] | C15H26O3 | 254.19 | [69] | |
| Sulphureuine E | [69] | C15H24O4 | 268.17 | [69] | |
| Sulphureuine F | [69] | C15H24O3 | 252.17 | [69] | |
| Sulphureuine G | [69] | C15H28O3 | 256.20 | [69] | |
| Sulphureuine H | [69] | C15H26O3 | 254.18 | [69] | |
| Agripilol A | [69] | C15H28O4 | 272.38 | [28,29] | |
| Bulam et al. [79] (mg/kg dw) | Teke et al. [22] (mg/kg−1 dw) | Bengu [34] (mg/kg−1 dw) | Sevindik et al. [80] (mg/kg−1 dw) | Turfan et al. [31] (mg/kg−1 dw) | Florczak et al. [24] (mg/ kg−1 dw) | Kovacs and Vetter [1] (mg/kg−1 dw) | Luangharn et al. [25] (mg/kg dw) | |
|---|---|---|---|---|---|---|---|---|
| Ca | 0.49 ± 0.01 | 13.04 ± 0.11 | nd | nd | 18.78 ± 0.06 | 1.02 ± 0.77 | 765 ± 55.1 | 2.59 ± 0.01 |
| K | nd | 433.62 ± 4.28 | nd | nd | 5752.54 ± 8.32 | nd | 28,940 ± 2174 | nd |
| Mg | 4.59 ± 0.01 | 13.85 ± 0.79 | nd | nd | 16.86 ± 0.90 | 2.90 ± 0.45 | 1001 ± 15.5 | 1.09 |
| P | 24.52 ± 0.09 | 542.88 ± 4.26 | nd | nd | 1524.50 ± 4.32 | nd | 4890 ± 575 | nd |
| Na | 1.88 ± 0.01 | 4.20 ± 0.58 | nd | nd | 8.00 ± 0.30 | nd | 209.9 ± 141.0 | 11.01 ± 0.12 |
| Cu | 0.04 ± 0.001 | 1.15 ± 0.06 | 5.00 | 1.90 ± 1.22 | 14.35 ± 0.11 | 0.52 ± 0.093 | 9.72 ± 4.90 | 0.14 ± 0.01 |
| Fe | 0.49 ± 0.02 | 8.69 ± 0.46 | 162.92 | 138.44 ± 21.22 | 80.62 ± 0.54 | 2.88 ± 0.12 | 50.9 ± 17.30 | 2.28 ± 0.03 |
| Zn | 0.21 ± 0.001 | 2.66 ± 0.18 | 28.360 | 47.42 ± 6.60 | 113.63 ± 9.47 | 0.22 ± 0.012 | 56.5 ± 6.10 | 1.20 |
| Al | 0.89 ± 0.01 | nd | nd | nd | 27.96 ± 0.18 | nd | 34.57 ± 23.00 | nd |
| Ba | 0.07 ± 0.09 | nd | nd | nd | nd | nd | 3.04 ± 1.89 | nd |
| As | 0.01 ± 0.001 | nd | nd | nd | 2.04 ± 0.02 | nd | bd | nd |
| Mn | 0.03 ± 0.001 | nd | 19.360 | nd | 99.49 ± 0.41 | nd | 5.18 ± 1.10 | 0.35 ± 0.02 |
| B | 0.04 ± 0.001 | nd | nd | nd | nd | nd | nd | nd |
| Co | 0.001 ± 0.001 | nd | nd | nd | 1.76 ± 0.40 | nd | 0.33 ± 0.13 | nd |
| Cd | 0.003 ± 0.001 | nd | nd | nd | 0.41 ± 0.02 | nd | 1.79 ± 2.00 | nd |
| Pb | 0.004 ± 0.001 | nd | nd | 1.73 ± 0.89 | 2.45 ± 0.01 | nd | nd | nd |
| Ni | 0.042 ± 0.001 | nd | nd | 0.00 ± 00 | 9.54 ± 0.15 | nd | 1.36 ± 0.69 | nd |
| Cr | 0.008 ± 0.001 | nd | nd | nd | 4.03 ± 0.03 | nd | 0.55 ± 0.07 | nd |
| Body System (or Part) | Documented Effect | Source of Information |
|---|---|---|
| Protection: 1. Anti-aging effect |
| [1,21,31,56,79,80,84,85,88,89,90,91,92,93] |
| 2. Anti-cancer effect |
| [24,33,49,56,57,60,80,97,102,103,104,105,106,114] |
| Immunity |
| [24,33,38,40,54,60,63,80,94,95,96,97,98,100,111,115,116,117], Seibold et al., 2020 after: [42] |
| Metabolism |
| [49,112], Hwang et al., 2008 after: [42] |
| Digestive system |
| [35,75] |
| Circulatory system |
| [114] |
| Nervous system |
| [107,111] |
| Reproductive system |
| [35] |
| Skin |
| [47,107] |
| Dental prophylaxis |
| [73] |
| Possible industrial applications | ||
| Food technology |
| [8,21,40,79] |
| Material industry |
| [52] |
| Clothing and textile industry |
| [42,63] |
| Chemical industry (production of agents used to protect the environment) |
| [118,119], Lim et al. after: [90] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamska, I. The Possibility of Using Sulphur Shelf Fungus (Laetiporus sulphureus) in the Food Industry and in Medicine—A Review. Foods 2023, 12, 1539. https://doi.org/10.3390/foods12071539
Adamska I. The Possibility of Using Sulphur Shelf Fungus (Laetiporus sulphureus) in the Food Industry and in Medicine—A Review. Foods. 2023; 12(7):1539. https://doi.org/10.3390/foods12071539
Chicago/Turabian StyleAdamska, Iwona. 2023. "The Possibility of Using Sulphur Shelf Fungus (Laetiporus sulphureus) in the Food Industry and in Medicine—A Review" Foods 12, no. 7: 1539. https://doi.org/10.3390/foods12071539
APA StyleAdamska, I. (2023). The Possibility of Using Sulphur Shelf Fungus (Laetiporus sulphureus) in the Food Industry and in Medicine—A Review. Foods, 12(7), 1539. https://doi.org/10.3390/foods12071539