Development of New Antibodies and an ELISA System to Detect the Potato Alkaloids α-Solanine and α-Chaconine
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Sample Preparation
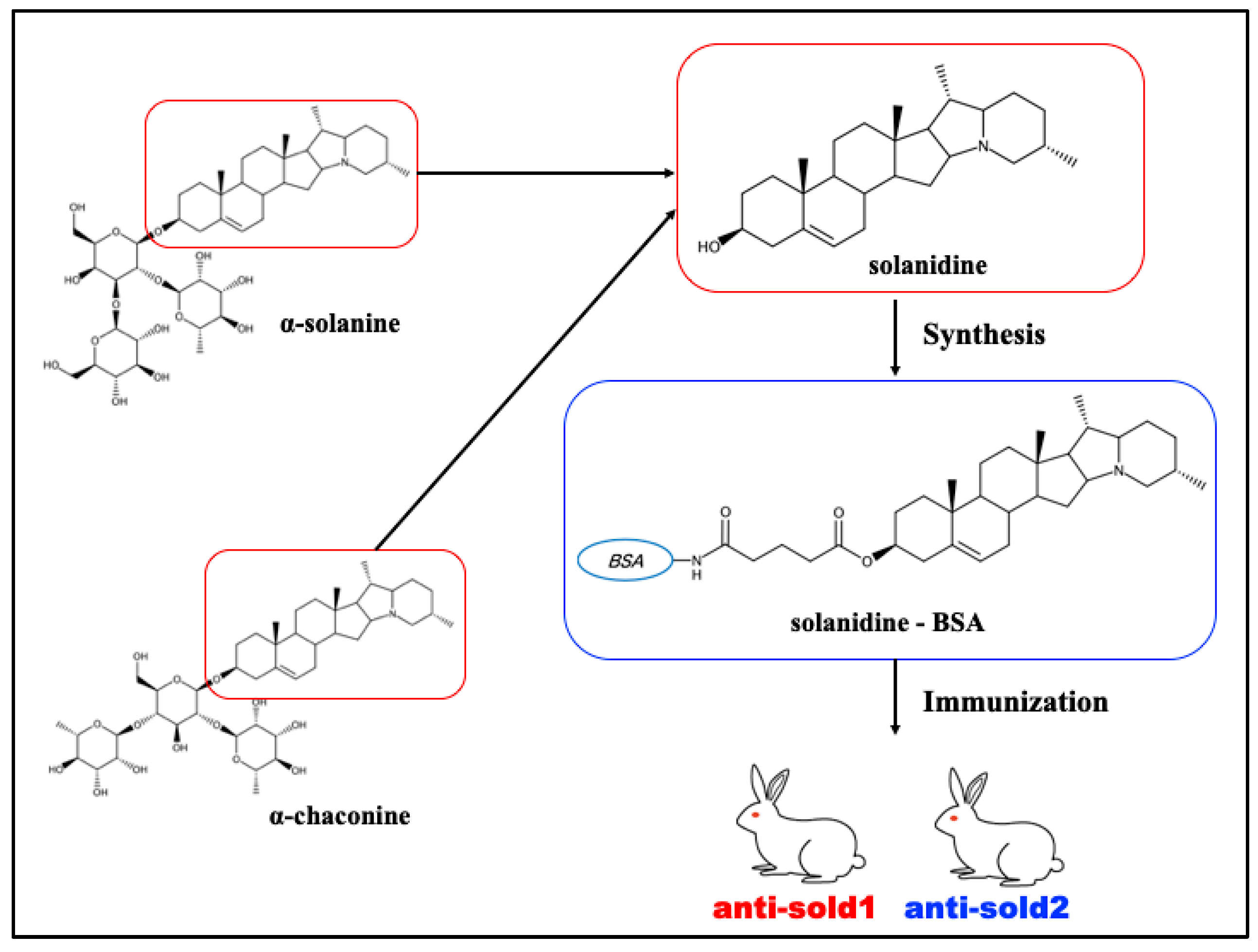
2.3. Development of Solanidine-Binding Antibodies
2.4. SDS-PAGE and Western Blotting
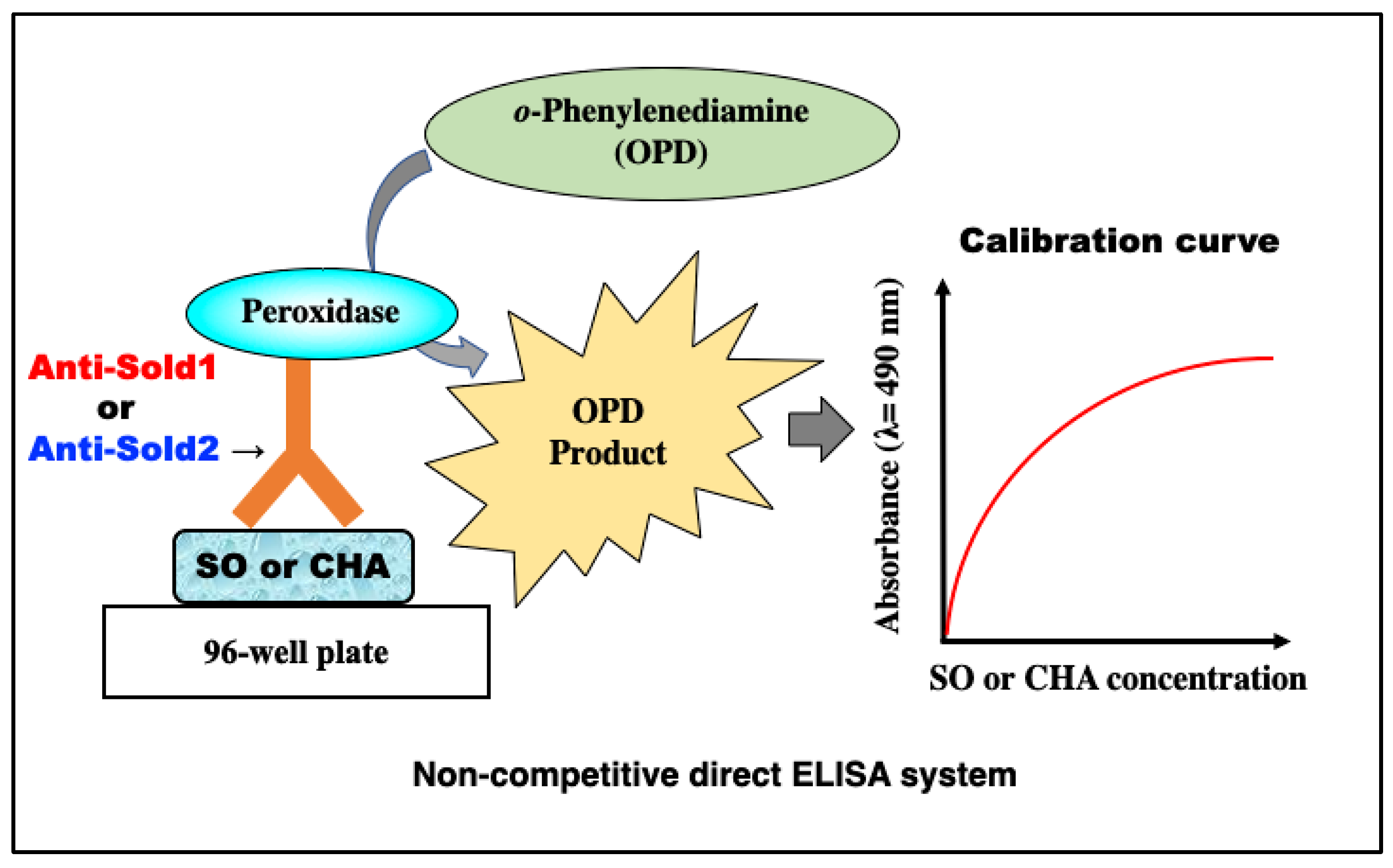
2.5. Construction of Non-Competitive Direct ELISAs
2.6. HPLC Analysis
2.7. Statistical Analysis
3. Results
3.1. Detection of Purified Polyclonal Antibodies
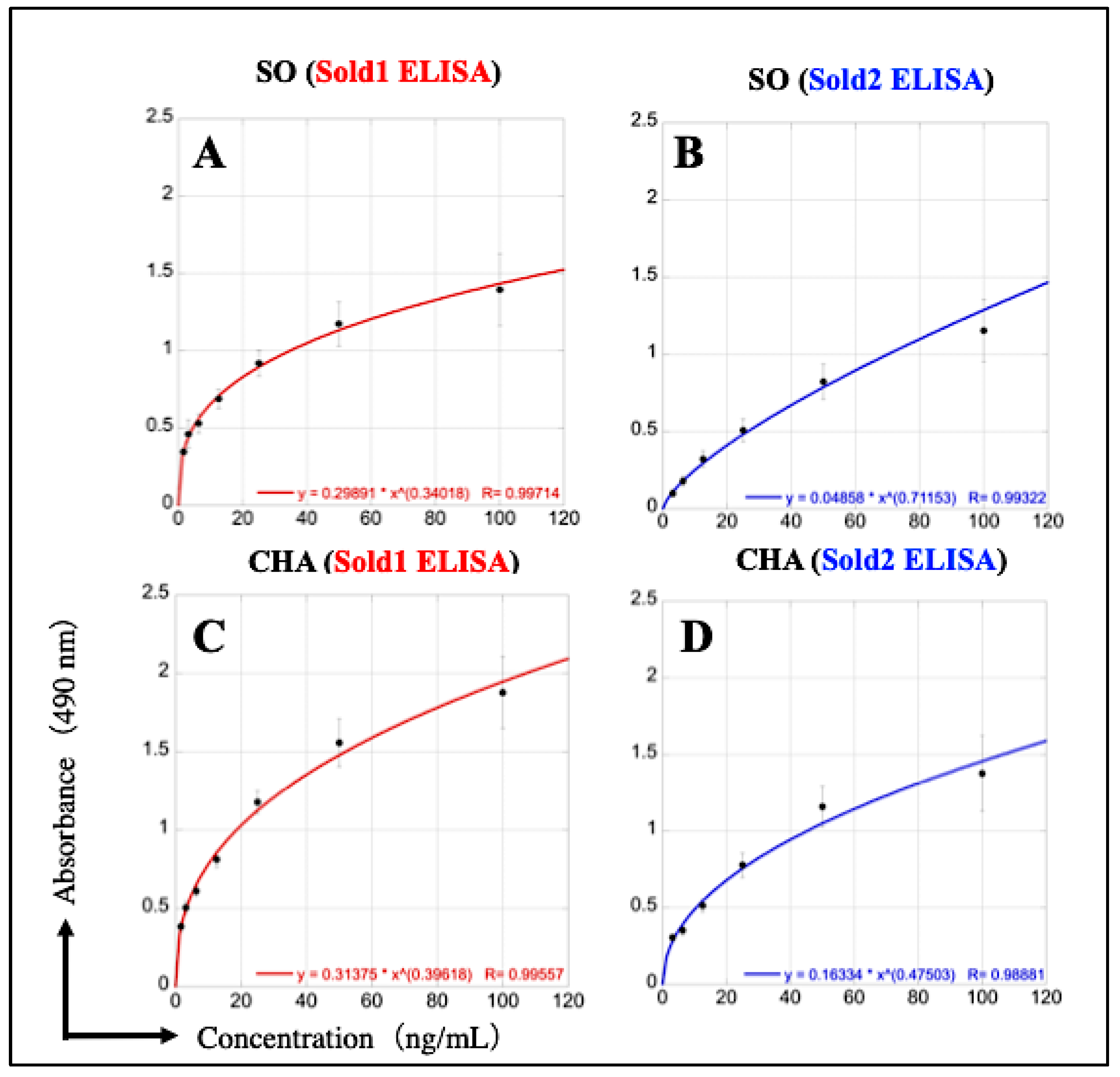
3.2. Construction of Two ELISAs to Detect SO and CHA
3.3. Verification of the Repeatability of Sold1 ELISA
3.4. Verification of the Repeatability of Sold2 ELISA
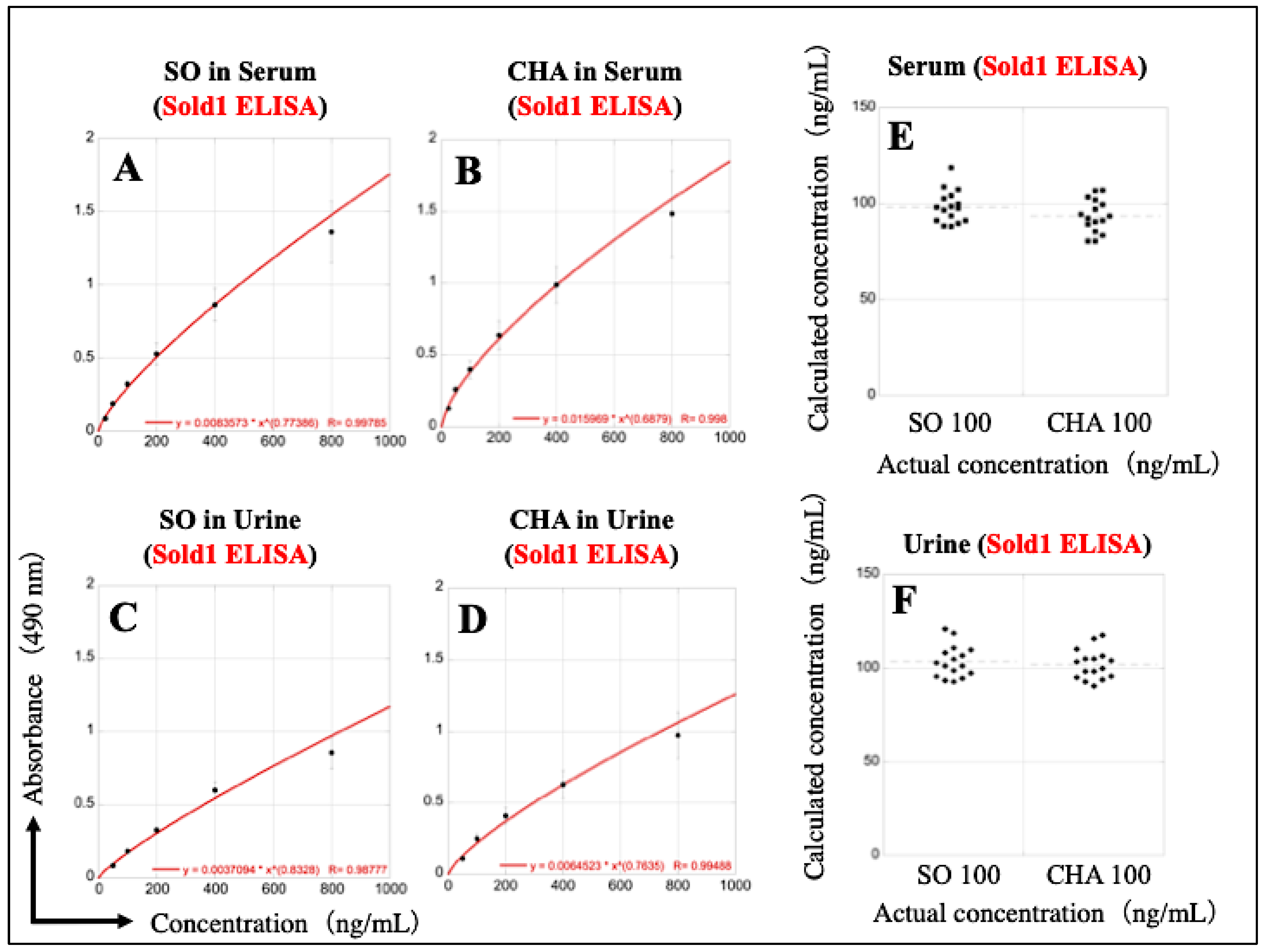
3.5. Reconstruction of Sold1 ELISA Calibration Curves Using the Dilution Series of SO and CHA in Serum and Urine
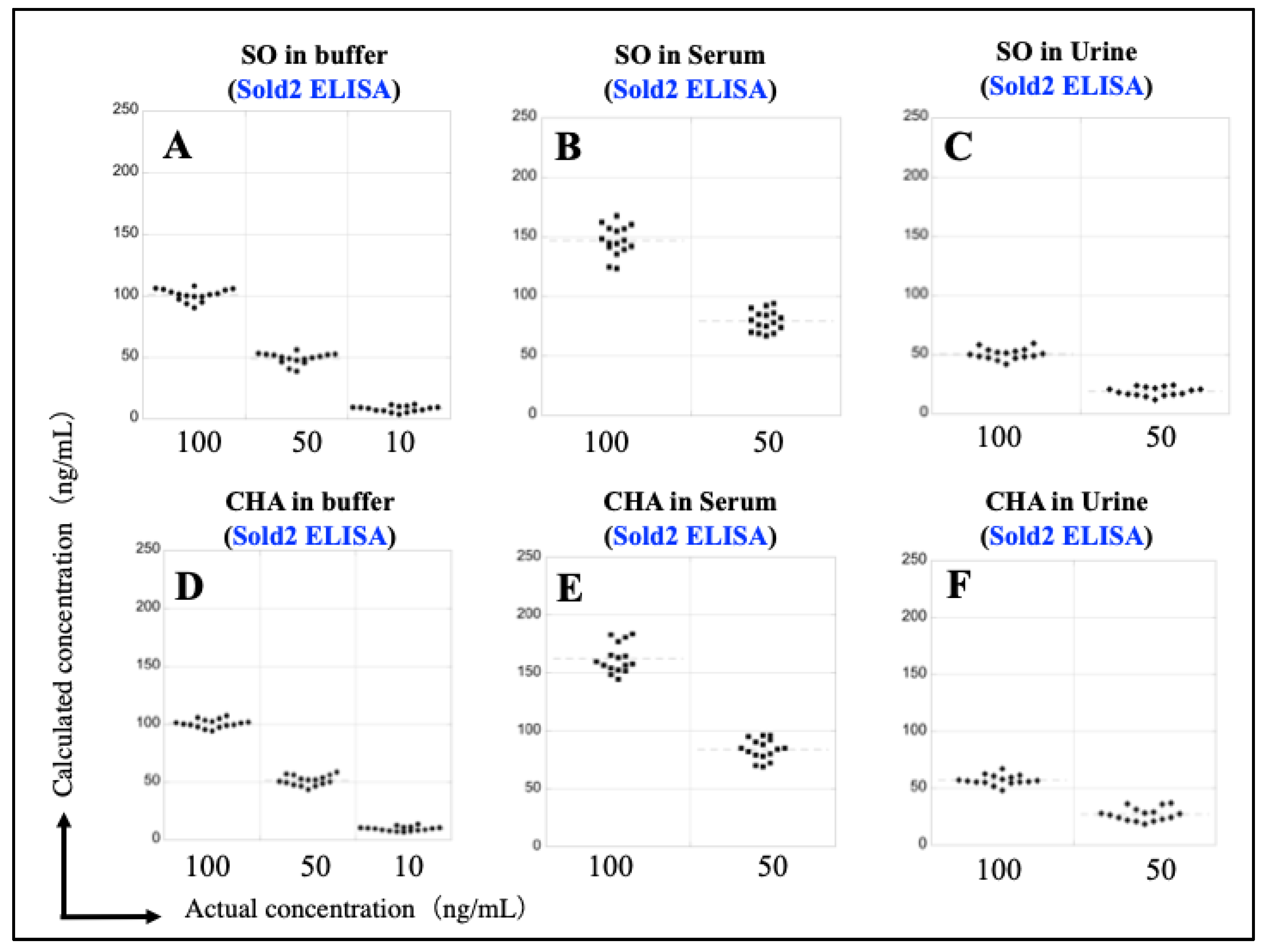
3.6. Reconstruction of Sold2 ELISA Calibration Curves Using the Dilution Series of SO and CHA in Serum and Urine
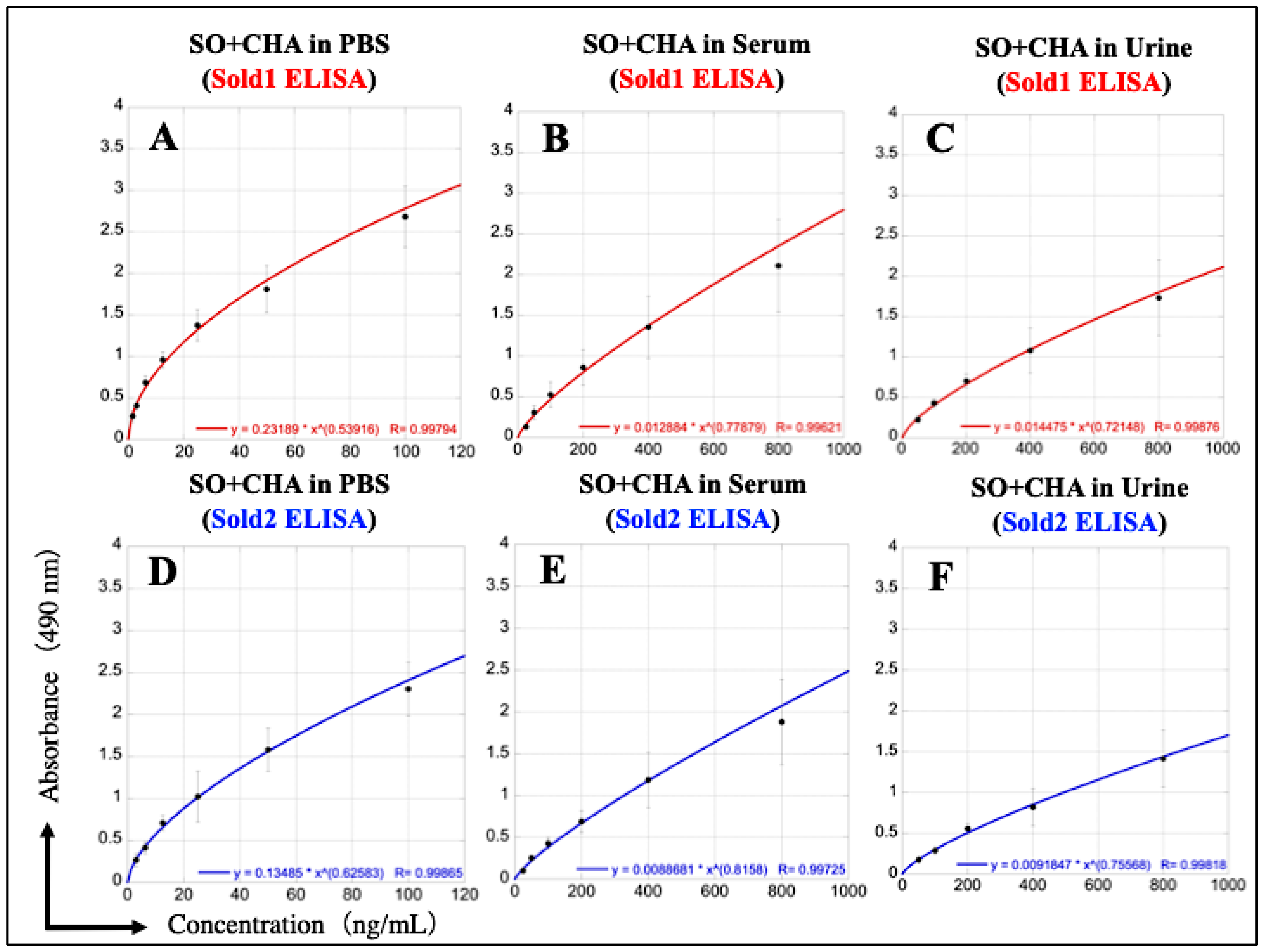
3.7. Construction of Calibration Curve for Simultaneous Measurement of SO and CHA
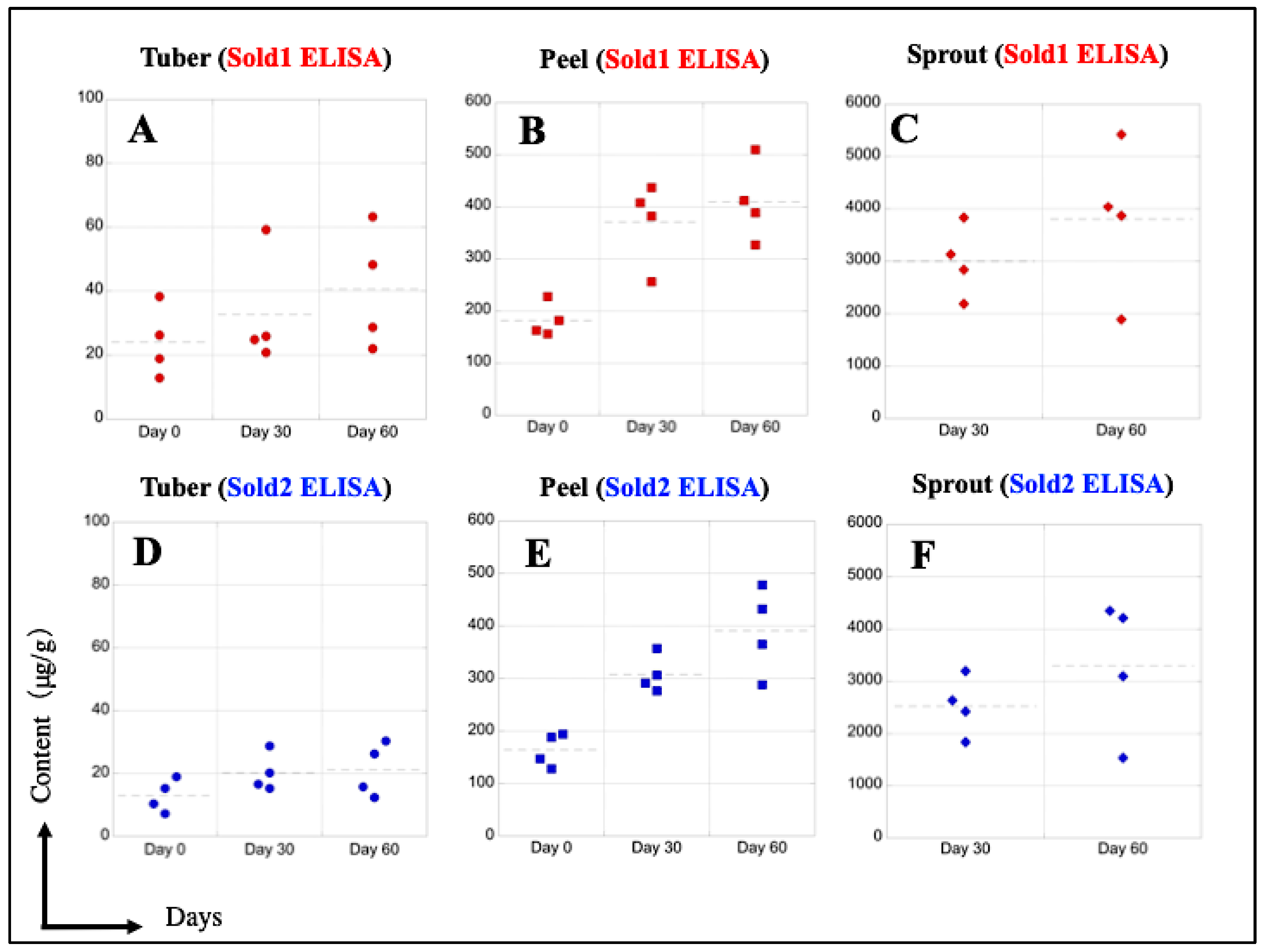
3.8. Detection Performance of SO and CHA in Potato Extracts
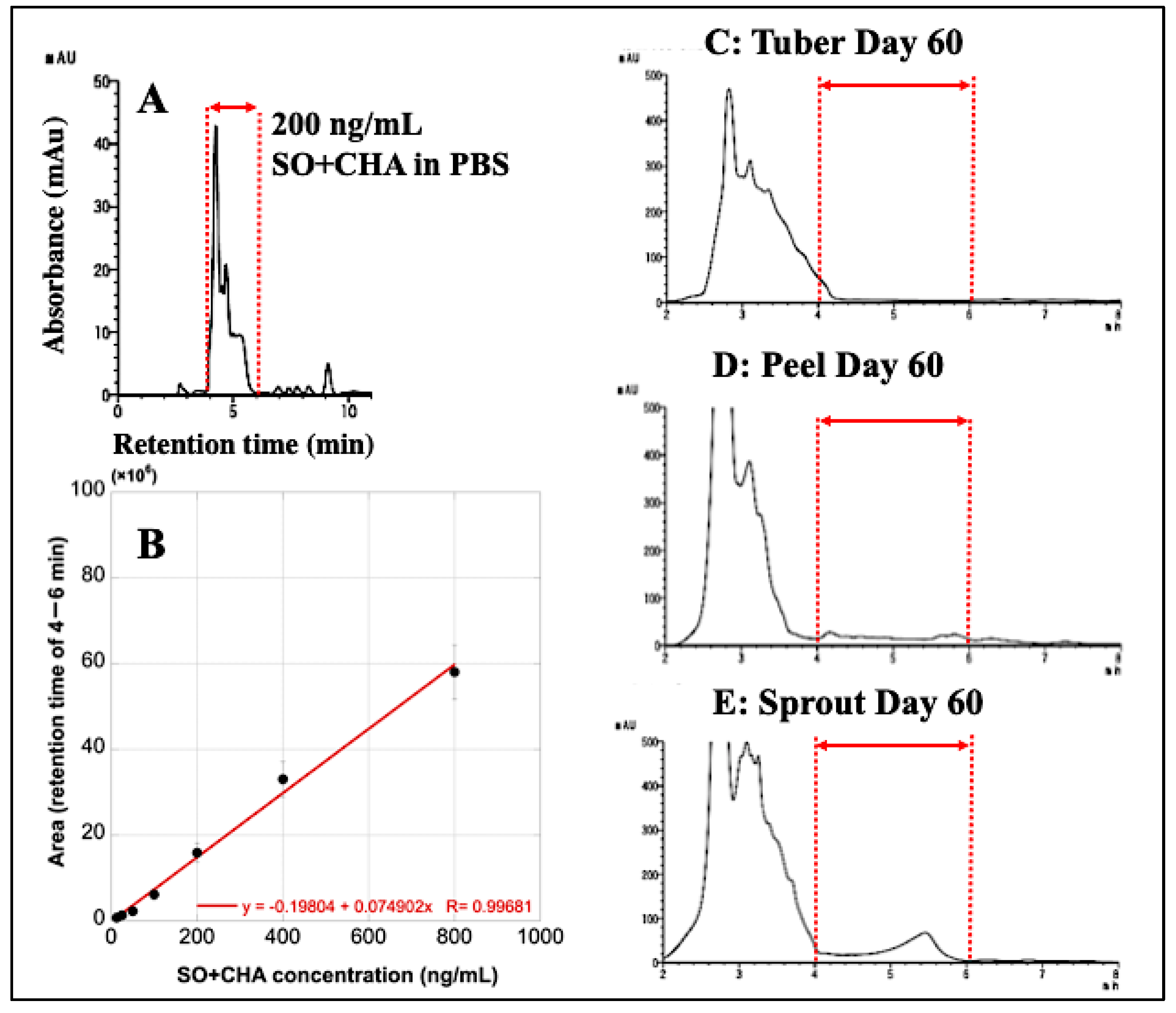
3.9. HPLC Analysis of SO and CHA in Potato Extracts
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Friedman, M. Potato Glycoalkaloids and Metabolites: Roles in the Plant and in the Diet. Mol. Nutr. Food Res. 2007, 51, 116–134. [Google Scholar] [CrossRef] [PubMed]
- Slanina, P. Solanine (glycoalkaloids) in potatoes—toxicological evaluation. Food Chem. Toxicol. 1990, 28, 759–761. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.; McDonald, G.M. Potato glycoalkaloids: Chemistry, analysis, safety, and plant physiology. Crit. Rev. Plant Sci. 1997, 16, 55–132. [Google Scholar] [CrossRef]
- Sinden, S.L.; Deahl, K.L.; Aulenbach, B.B. Effect of glycoalkaloids and phenolics on potato flavor. J. Food Sci. 1976, 41, 520–523. [Google Scholar] [CrossRef]
- Bushway, R.J.; Rathy, P. a-Chaconine and a-Solanine Content of Potato Products and Their Stability during Several Modes of Cooking. J. Agric. Food Chem. 1981, 29, 814–817. [Google Scholar] [CrossRef]
- Wilson, A.M.; McGann, D.F.; Bushway, R.J. Effect of Growth-Location and Length of Storage on Glycoalkaloid Content of Roadside-Stand Potatoes as Stored by Consumers. J. Food Prot. 1983, 46, 119–121. [Google Scholar] [CrossRef]
- Phillips, B.J.; Hughes, J.A.; Phillips, J.C.; Walters, D.G.; Anderson, D.; Tahourdin, C.S. A study of the toxic hazard that might be associated with the consumption of green potato tops. Food Chem. Toxicol. 1996, 34, 439–448. [Google Scholar] [CrossRef]
- Sotelo, A.; Serrano, B. High-performance liquid chromatographic determination of the glycoalkaloids α-solanine and α-chaconine in 12 commercial varieties of Mexican potato. J. Agric. Food Chem. 2000, 48, 2472–2475. [Google Scholar] [CrossRef]
- Stanker, L.H.; Kamps-Holtzapple, C.; Friedman, M. Development and characterization of monoclonal antibodies that differentiate between potato and tomato glycoalkaloids andaglycons. J. Agric. Food Chem. 1994, 42, 2360–2366. [Google Scholar] [CrossRef]
- Friedman, M.; Bautista, F.F.; Stanker, L.H.; Larkin, K.A. Analysis of Potato Glycoalkaloids by a New ELISA Kit. J. Agric. Food Chem. 1998, 46, 5097–5102. [Google Scholar] [CrossRef]
- Kodamatani, H.; Saito, K.; Niina, N.; Yamazaki, S.; Tanaka, Y. Simple and sensitive method for determination of glycoalkaloids in potato tubers by high-performance liquid chromatography with chemiluminescence detection. J. Chromatogr. A 2005, 1100, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Popova, I.; Sell, B.; Pillai, S.S.; Kuhl, J.; Dandurand, L.M. High-Performance Liquid Chromatography–Mass Spectrometry Analysis of Glycoalkaloids from Underexploited Solanum Species and Their Acetylcholinesterase Inhibition Activity. Plants 2022, 11, 269. [Google Scholar] [CrossRef] [PubMed]
- Hellenäs, K.E.; Nyman, A.; Slanina, P.; Lööf, L.; Gabrielsson, J. Determination of potato glycoalkaloids and their aglycone in blood serum by high-performance liquid chromatography. Application to pharmacokinetic studies in humans. J. Chromatogr. 1992, 573, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Mensinga, T.T.; Sips, A.J.; Rompelberg, C.J.; van Twillert, K.; Meulenbelt, J.; van den Top, H.J.; van Egmond, H.P. Potato glycoalkaloids and adverse effects in humans: An ascending dose study. Regul. Toxicol. Pharmacol. 2005, 41, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.A. Two fatal cases of potato poisoning. Science 1925, 61, 348–349. [Google Scholar] [CrossRef]
- McMillan, M.; Thompson, J.C. An outbreak of suspected solanine poisoning in school boys: An examination of criteria of solanine poisoning. Q. J. Med. 1979, 48, 227–243. [Google Scholar]
- Stephens, D.B.; Thomas, R.E.; Stanton, J.F.; Iverson, B.L. Polyclonal antibody catalytic variability. Biochem. J. 1998, 332, 127–134. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- PubChem Homepage. Available online: https://pubchem.ncbi.nlm.nih.gov (accessed on 30 March 2023).
- Epam/Ketcher Homepage. Available online: https://github.com/epam/ketcher/blob/master/LICENSE (accessed on 30 March 2023).
- Towbin, H.; Staehelin, T.; Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc. Natl. Acad. Sci. USA 1979, 76, 4350–4354. [Google Scholar] [CrossRef]
- Koike, A.; Arai, S.; Yamada, S.; Nagae, A.; Saita, N.; Itoh, H.; Uemoto, S.; Totani, M.; Ikemoto, M. Dynamic mobility of immunological cells expressing S100A8 and S100A9 in vivo: A variety of functional roles of the two proteins as regulators in acute inflammatory reaction. Inflammation 2012, 35, 409–419. [Google Scholar] [CrossRef]
- Lim, M.; Erdman, P.; Cho, S.; Mathew, A.; Fleisher, M.; Thoren, K.L. Evaluation of CisBio ELISA for Chromogranin A Measurement. J. Appl. Lab. Med. 2019, 4, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Terato, K.; Do, C.T.; Cutler, D.; Waritani, T.; Shionoya, H. Preventing intense false positive and negative reactions attributed to the principle of ELISA to re-investigate antibody studies in autoimmune diseases. J. Immunol. Methods 2014, 407, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Song, M.F.; Li, Y.S.; Ootsuyama, Y.; Kasai, H.; Kawai, K.; Ohta, M.; Eguchi, Y.; Yamato, H.; Matsumoto, Y.; Yoshida, R.; et al. Urea, the most abundant component in urine, cross-reacts with a commercial 8-OH-dG ELISA kit and contributes to overestimation of urinary 8-OH-dG. Free. Radic. Biol. Med. 2009, 47, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Barceloux, D.G. Potatoes, Tomatoes, and Solanine Toxicity (Solanum tuberosum L., Solanum lycopersicum L.). Dis. Mon. 2009, 55, 391–402. [Google Scholar] [CrossRef]
- Lachman, J.; Hamouz, K.; Orsak, M.; Pivec, V. Potato glycoalkaloids and their significance in plant protection and human nutrition-Review. Rostl. Vyroba. 2001, 47, 181–191. [Google Scholar]
- McGill, C.R.; Kurilich, A.C.; Davignon, J. The role of potatoes and potato components in cardiometabolic health: A review. Ann. Med. 2013, 45, 467–473. [Google Scholar] [CrossRef]
- Tabatabaei, M.S.; Ahmed, M. Enzyme-Linked Immunosorbent Assay (ELISA). Methods Mol. Biol. 2022, 2508, 115–134. [Google Scholar]




| Sold1 ELISA | 100 (ng/mL) | 50 (ng/mL) | 10 (ng/mL) | Sold1 ELISA | 100 (ng/mL) | 50 (ng/mL) | 10 (ng/mL) | ||
|---|---|---|---|---|---|---|---|---|---|
| SO in PBS | Mean (ng/mL) | 98.49 | 51.74 | 10.89 | CHA in PBS | Mean (ng/mL) | 101.70 | 52.76 | 9.64 |
| SD | 5.58 | 4.50 | 1.72 | SD | 5.62 | 3.45 | 1.32 | ||
| CV (%) | 5.66 | 8.70 | 15.80 | CV (%) | 5.53 | 6.55 | 13.71 | ||
| Recovery (%) | 97.46 | 95.20 | 89.20 | Recovery (%) | 105.28 | 101.58 | 98.20 | ||
| LOD (ng/mL) | 1.38 | LOD (ng/mL) | 1.08 | ||||||
| SO in serum | Mean (ng/mL) | 196.95 | 102.38 | CHA in serum | Mean (ng/mL) | 208.83 | 114.44 | ||
| SD | 16.85 | 14.30 | SD | 15.29 | 14.27 | ||||
| CV (%) | 8.55 | 13.96 | CV (%) | 7.32 | 12.47 | ||||
| Recovery (%) | 122.18 | 119.82 | Recovery (%) | 120.52 | 126.84 | ||||
| SO in urine | Mean (ng/mL) | 60.73 | 22.16 | CHA in urine | Mean (ng/mL) | 78.53 | 39.85 | ||
| SD | 5.14 | 3.77 | SD | 4.66 | 5.08 | ||||
| CV (%) | 8.47 | 17.03 | CV (%) | 5.93 | 12.74 | ||||
| Recovery (%) | 78.26 | 68.56 | Recovery (%) | 84.18 | 81.65 | ||||
| Sold2 ELISA | 100 (ng/mL) | 50 (ng/mL) | 10 (ng/mL) | Sold2 ELISA | 100 (ng/mL) | 50 (ng/mL) | 10 (ng/mL) | ||
|---|---|---|---|---|---|---|---|---|---|
| SO in PBS | Mean (ng/mL) | 101.01 | 49.23 | 8.14 | CHA in PBS | Mean (ng/mL) | 100.89 | 51.45 | 9.67 |
| SD | 4.88 | 4.55 | 2.36 | SD | 3.63 | 4.22 | 1.88 | ||
| CV (%) | 4.83 | 9.24 | 28.96 | CV (%) | 3.60 | 8.21 | 19.48 | ||
| Recovery (%) | 102.25 | 97.68 | 90.18 | Recovery (%) | 101.95 | 104.25 | 96.42 | ||
| LOD (ng/mL) | 2.95 | LOD (ng/mL) | 2.76 | ||||||
| SO in serum | Mean (ng/mL) | 146.98 | 84.44 | CHA in serum | Mean (ng/mL) | 162.35 | 90.88 | ||
| SD | 12.63 | 13.26 | SD | 12.47 | 11.70 | ||||
| CV (%) | 8.59 | 15.71 | CV (%) | 7.68 | 12.88 | ||||
| Recovery (%) | 114.56 | 125.82 | Recovery (%) | 120.88 | 129.27 | ||||
| SO in urine | Mean (ng/mL) | 50.79 | 19.11 | CHA in urine | Mean (ng/mL) | 57.01 | 26.87 | ||
| SD | 4.54 | 3.65 | SD | 4.47 | 4.76 | ||||
| CV (%) | 8.95 | 19.11 | CV (%) | 7.83 | 17.71 | ||||
| Recovery (%) | 77.25 | 68.28 | Recovery (%) | 78.82 | 77.16 | ||||
| Sold1 ELISA | 100 (ng/mL) | Sold2 ELISA | 100 (ng/mL) | |
|---|---|---|---|---|
| SO in serum | Mean (ng/mL) | 98.48 | Mean (ng/mL) | 96.55 |
| SD | 8.42 | SD | 8.62 | |
| CV (%) | 8.55 | CV (%) | 8.92 | |
| Recovery (%) | 96.58 | Recovery (%) | 94.15 | |
| LOD (ng/mL) | 15.25 | LOD (ng/mL) | 19.41 | |
| SO in urine | Mean (ng/mL) | 93.59 | Mean (ng/mL) | 102.20 |
| SD | 8.65 | SD | 9.43 | |
| CV (%) | 9.24 | CV (%) | 9.23 | |
| Recovery (%) | 90.88 | Recovery (%) | 105.64 | |
| LOD (ng/mL) | 30.28 | LOD (ng/mL) | 45.16 | |
| CHA in serum | Mean (ng/mL) | 103.60 | Mean (ng/mL) | 98.97 |
| SD | 8.50 | SD | 9.29 | |
| CV (%) | 8.20 | CV (%) | 9.38 | |
| Recovery (%) | 106.62 | Recovery (%) | 96.67 | |
| LOD (ng/mL) | 13.48 | LOD (ng/mL) | 16.92 | |
| CHA in urine | Mean (ng/mL) | 102.02 | Mean (ng/mL) | 103.69 |
| SD | 7.97 | SD | 8.22 | |
| CV (%) | 7.81 | CV (%) | 7.93 | |
| Recovery (%) | 105.74 | Recovery (%) | 106.58 | |
| LOD (ng/mL) | 27.92 | LOD (ng/mL) | 38.15 |
| Calculated Content (μg/g) Using Sold1 ELISA | ||||
|---|---|---|---|---|
| (n = 4) | Tuber | Peel | Sprout | |
| Potato on Day 60 | No.1 | 22.04 | 389.0 | 1892 |
| No.2 | 63.28 | 510.2 | 3868 | |
| No.3 | 48.20 | 327.1 | 5423 | |
| No.4 | 28.68 | 412.4 | 4038 | |
| Calculated Content (μg/g) Using Sold2 ELISA | ||||
| (n = 4) | Tuber | Peel | Sprout | |
| Potato on Day 60 | No.1 | 15.68 | 365.1 | 1538 |
| No.2 | 30.28 | 478.4 | 3102 | |
| No.3 | 26.22 | 288.0 | 4348 | |
| No.4 | 12.25 | 432.8 | 4212 | |
| Calculated Content (μg/g) Using HPLC | ||||
| (n = 4) | Tuber | Peel | Sprout | |
| Potato on Day 60 | No.1 | 8.27 | 170.3 | 1065 |
| No.2 | 13.95 | 209.8 | 1982 | |
| No.3 | 10.16 | 152.7 | 2643 | |
| No.4 | 8.66 | 202.0 | 2129 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okada, K.; Matsuo, K. Development of New Antibodies and an ELISA System to Detect the Potato Alkaloids α-Solanine and α-Chaconine. Foods 2023, 12, 1621. https://doi.org/10.3390/foods12081621
Okada K, Matsuo K. Development of New Antibodies and an ELISA System to Detect the Potato Alkaloids α-Solanine and α-Chaconine. Foods. 2023; 12(8):1621. https://doi.org/10.3390/foods12081621
Chicago/Turabian StyleOkada, Kohki, and Kano Matsuo. 2023. "Development of New Antibodies and an ELISA System to Detect the Potato Alkaloids α-Solanine and α-Chaconine" Foods 12, no. 8: 1621. https://doi.org/10.3390/foods12081621
APA StyleOkada, K., & Matsuo, K. (2023). Development of New Antibodies and an ELISA System to Detect the Potato Alkaloids α-Solanine and α-Chaconine. Foods, 12(8), 1621. https://doi.org/10.3390/foods12081621





