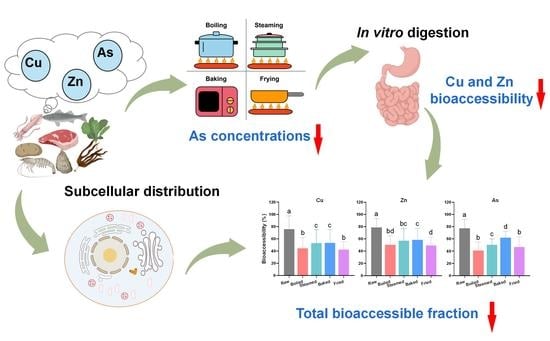
Effects of Culinary Procedures on Concentrations and Bioaccessibility of Cu, Zn, and As in Different Food Ingredients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection and Preparation of Food Ingredients
2.2. Culinary Procedures
2.3. In Vitro Digestion
2.4. Subcellular Distribution
2.5. Measurement of Cu, Zn, and As Concentrations
2.6. Calculation of the Retention Rate and Bioaccessibility
2.7. Nutrition and Risk Assessment
2.8. Statistical Analyses
3. Results
3.1. The Concentrations and Subcellular Distribution of Cu, Zn, and As in Raw Ingredients
3.2. The Retention Rate of Cu, Zn, and As in Ingredients during Culinary Procedures
3.3. The Bioaccessibility of Cu, Zn, and As during In Vitro Digestion
3.4. The TBF of Cu, Zn, and As in Food Ingredients
3.5. Nutrition and Risk Assessment
4. Discussion
4.1. The Concentrations and Subcellular Distribution of Cu, Zn, and As
4.2. The Retention Rate of Cu, Zn, and As
4.3. The Bioaccessibility of Cu, Zn, and As
4.4. The TBF of Cu, Zn, and As in Food Ingredients
4.5. The Nutrition and Risk Assessments
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Amiard, J.-C.; Amiard-Triquet, C.; Charbonnier, L.; Mesnil, A.; Rainbow, P.S.; Wang, W.-X. Bioaccessibility of essential and non-essential metals in commercial shellfish from Western Europe and Asia. Food Chem. Toxicol. 2008, 46, 2010–2022. [Google Scholar] [CrossRef]
- Clemens, S. Zn and Fe biofortification: The right chemical environment for human bioavailability. Plant Sci. 2014, 225, 52–57. [Google Scholar] [CrossRef]
- Fu, Y.; Yin, N.; Cai, X.; Du, H.; Wang, P.; Sultana, M.S.; Sun, G.; Cui, Y. Arsenic speciation and bioaccessibility in raw and cooked seafood: Influence of seafood species and gut microbiota. Environ. Pollut. 2021, 280, 116958. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, Z.; Chen, J.; Liang, H.; Chen, X.; Qin, Y.; Shohag, M.; Wei, Y.; Gu, M. Comparative evaluation of in vivo relative bioavailability and in vitro bioaccessibility of arsenic in leafy vegetables and its implication in human exposure assessment. J. Hazard. Mater. 2022, 423, 126909. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-X.; Yang, Y.; Guo, X.; He, M.; Guo, F.; Ke, C. Copper and zinc contamination in oysters: Subcellular distribution and detoxification. Environ. Toxicol. Chem. 2011, 30, 1767–1774. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sato, T.; Xing, B.; Tao, S. Health risks of heavy metals to the general public in Tianjin, China via consumption of vegetables and fish. Sci. Total. Environ. 2005, 350, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Olmedo, P.; Hernández, A.; Pla, A.; Femia, P.; Navas-Acien, A.; Gil, F. Determination of essential elements (copper, manganese, selenium and zinc) in fish and shellfish samples. Risk and nutritional assessment and mercury–selenium balance. Food Chem. Toxicol. 2013, 62, 299–307. [Google Scholar] [CrossRef]
- USEPA. Risk-Based Concentration Table. April 2005; U.S. EPA: Philadelphia, PA, USA, 2005. Available online: https://www.epa.gov/sites/default/files/2020-07/iris_downloads_database_export_200608.xlsx (accessed on 1 January 2023).
- Liu, B.; Lv, L.; An, M.; Wang, T.; Li, M.; Yu, Y. Heavy metals in marine food web from Laizhou Bay, China: Levels, trophic magnification, and health risk assessment. Sci. Total. Environ. 2022, 841, 156818. [Google Scholar] [CrossRef]
- Maulvault, A.L.; Machado, R.; Afonso, C.; Lourenço, H.M.; Nunes, M.L.; Coelho, I.; Langerholc, T.; Marques, A. Bioaccessibility of Hg, Cd and As in cooked black scabbard fish and edible crab. Food Chem. Toxicol. 2011, 49, 2808–2815. [Google Scholar] [CrossRef]
- Fernández-Caliani, J.C.; Giráldez, M.I.; Barba-Brioso, C. Oral bioaccessibility and human health risk assessment of trace elements in agricultural soils impacted by acid mine drainage. Chemosphere 2019, 237, 124441. [Google Scholar] [CrossRef]
- Chávez-Capilla, T.; Beshai, M.; Maher, W.; Kelly, T.; Foster, S. Bioaccessibility and degradation of naturally occurring arsenic species from food in the human gastrointestinal tract. Food Chem. 2016, 212, 189–197. [Google Scholar] [CrossRef]
- Karadaş, C.; Kara, D. In vitro gastro-intestinal method for the assessment of heavy metal bioavailability in contaminated soils. Environ. Sci. Pollut. Res. Int. 2011, 18, 620–628. [Google Scholar] [CrossRef]
- Cámara-Martos, F.; Obregón-Cano, S.; de Haro-Bailón, A. Glucosinolates, Ca, Se Contents, and Bioaccessibility in Brassica rapa Vegetables Obtained by Organic and Conventional Cropping Systems. Foods 2022, 11, 350. [Google Scholar] [CrossRef] [PubMed]
- Ciudad-Mulero, M.; Pinela, J.; Carvalho, A.M.; Barros, L.; Fernández-Ruiz, V.; Ferreira, I.C.F.R.; Sánchez-Mata, M.D.C.; Morales, P. Bioaccessibility of Macrominerals and Trace Elements from Tomato (Solanum lycopersicum L.) Farmers’ Varieties. Foods 2022, 11, 1968. [Google Scholar] [CrossRef] [PubMed]
- Feitosa, S.; Greiner, R.; Meinhardt, A.-K.; Müller, A.; Almeida, D.T.; Posten, C. Effect of Traditional Household Processes on Iron, Zinc and Copper Bioaccessibility in Black Bean (Phaseolus vulgaris L.). Foods 2018, 7, 123. [Google Scholar] [CrossRef]
- Kalogeropoulos, N.; Karavoltsos, S.; Sakellari, A.; Avramidou, S.; Dassenakis, M.; Scoullos, M. Heavy metals in raw, fried and grilled Mediterranean finfish and shellfish. Food Chem. Toxicol. 2012, 50, 3702–3708. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, H.; Muniz, J.A.; Bandarra, N.M.; Castanheira, I.; Coelho, I.R.; Delgado, I.; Gonçalves, S.; Lourenço, H.M.; Motta, C.; Duarte, M.P.; et al. Effects of Industrial Boiling on the Nutritional Profile of Common Octopus (Octopus vulgaris). Foods 2019, 8, 411. [Google Scholar] [CrossRef]
- Ulaganathan, A.; Robinson, J.S.; Rajendran, S.; Geevaretnam, J.; Pandurangan, P.; Durairaj, S. Effect of different thermal processing methods on potentially toxic metals in the seafood, Penaeus vannamei, and the related human health risk assessment. J. Food Compos. Anal. 2022, 105, 104259. [Google Scholar] [CrossRef]
- Hosseini, H.; Mahmoudzadeh, M.; Rezaei, M.; Mahmoudzadeh, L.; Khaksar, R.; Khosroshahi, N.K.; Babakhani, A. Effect of different cooking methods on minerals, vitamins and nutritional quality indices of kutum roach (Rutilus frisii kutum). Food Chem. 2014, 148, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Singhato, A.; Judprasong, K.; Sridonpai, P.; Laitip, N.; Ornthai, N.; Yafa, C.; Chimkerd, C. Effect of Different Cooking Methods on Selenium Content of Fish Commonly Consumed in Thailand. Foods 2022, 11, 1808. [Google Scholar] [CrossRef]
- Wallace, W.; Lee, B.; Luoma, S. Subcellular compartmentalization of Cd and Zn in two bivalves. I. Significance of metal-sensitive fractions (MSF) and biologically detoxified metal (BDM). Mar. Ecol. Prog. Ser. 2003, 249, 183–197. [Google Scholar] [CrossRef]
- He, M.; Ke, C.-H.; Wang, W.-X. Effects of Cooking and Subcellular Distribution on the Bioaccessibility of Trace Elements in Two Marine Fish Species. J. Agric. Food Chem. 2010, 58, 3517–3523. [Google Scholar] [CrossRef]
- He, M.; Wang, W.-X. Bioaccessibility of 12 trace elements in marine molluscs. Food Chem. Toxicol. 2013, 55, 627–636. [Google Scholar] [CrossRef]
- Ruby, M.V.; Davis, A.; Schoof, R.; Eberle, S.; Sellstone, C.M. Estimation of Lead and Arsenic Bioavailability Using a Physiologically Based Extraction Test. Environ. Sci. Technol. 1996, 30, 422–430. [Google Scholar] [CrossRef]
- Wallace, W.G.; Luoma, S.N. Subcellular compartmentalization of Cd and Zn in two bivalves. II. Significance of trophically available metal (TAM). Mar. Ecol. Prog. Ser. 2003, 257, 125–137. [Google Scholar] [CrossRef]
- Iaquinta, F.; Pistón, M.; Machado, I. In vitro bioaccessibility of Cu and Zn in cooked beef cuts. LWT-Food Sci. Technol. 2021, 150, 112027. [Google Scholar] [CrossRef]
- Alvanon. Most Extensive Chinese Body Measurement Study. 2008. Available online: http://www.alvanon.com/news/CHINASCAN.pdf (accessed on 10 June 2019).
- National Institute of Health (NIH). Recommended Dietary Allowances (RDAs) for Copper. 2021. Available online: https://ods.od.nih.gov/factsheets/Copper-HealthProfessional/ (accessed on 1 January 2023).
- National Institute of Health (NIH). Recommended Dietary Allowances (RDAs) for Zinc. 2021. Available online: https://ods.od.nih.gov/factsheets/Zinc-HealthProfessional/ (accessed on 1 January 2023).
- Liao, W.; Wang, G.; Li, K.; Zhao, W. Change of Arsenic Speciation in Shellfish after Cooking and Gastrointestinal Digestion. J. Agric. Food Chem. 2018, 66, 7805–7814. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Cao, X.; Yu, J.-J.; Sen-Chun, L.F.; Wang, X.-R. Determination of Arsenic and It’s Species in Dry Seafood by High Performance Liquid Chromatography-Inductively Coupled Plasma Mass Spectrometry. Chin. J. Anal. Chem. 2009, 37, 1738–1742. [Google Scholar] [CrossRef]
- Yu, X.-J.; Pan, K.; Liu, F.; Yan, Y.; Wang, W.-X. Spatial variation and subcellular binding of metals in oysters from a large estuary in China. Mar. Pollut. Bull. 2013, 70, 274–280. [Google Scholar] [CrossRef]
- Borak, J.; Hosgood, H.D. Seafood arsenic: Implications for human risk assessment. Regul. Toxicol. Pharmacol. 2007, 47, 204–212. [Google Scholar] [CrossRef]
- Alvis, A.; Vélez, C.; Rada-Mendoza, M.; Villamiel, M.; Villada, H.S. Heat transfer coefficient during deep-fat frying. Food Control 2009, 20, 321–325. [Google Scholar] [CrossRef]
- Iwegbue, C.M.; Osijaye, K.O.; Igbuku, U.A.; Egobueze, F.E.; Tesi, G.O.; Bassey, F.I.; Martincigh, B.S. Effect of the number of frying cycles on the composition, concentrations and risk of polycyclic aromatic hydrocarbons (PAHs) in vegetable oils and fried fish. J. Food Compos. Anal. 2020, 94, 103633. [Google Scholar] [CrossRef]
- Devesa, V.; Macho, M.L.; Jalón, M.; Urieta, I.; Muñoz, O.; Súñer, M.A.; López, F.; Vélez, D.; Montoro, R. Arsenic in Cooked Seafood Products: Study on the Effect of Cooking on Total and Inorganic Arsenic Contents. J. Agric. Food Chem. 2001, 49, 4132–4140. [Google Scholar] [CrossRef]
- Vélez, D.; Ybáñez, N.; Montoro, R. Migration of Arsenobetaine from Canned Seafood to Brine. J. Agric. Food Chem. 1997, 45, 449–453. [Google Scholar] [CrossRef]
- Alves, R.N.; Maulvault, A.L.; Barbosa, V.L.; Fernandez-Tejedor, M.; Tediosi, A.; Kotterman, M.; Heuvel, F.H.V.D.; Robbens, J.; Fernandes, J.O.; Rasmussen, R.R.; et al. Oral bioaccessibility of toxic and essential elements in raw and cooked commercial seafood species available in European markets. Food Chem. 2018, 267, 15–27. [Google Scholar] [CrossRef]
- Laparra, J.M.; Vélez, D.; Barberá, R.; Montoro, R.; Farré, R. Bioaccessibility and Transport by Caco-2 Cells of Organoarsenical Species Present in Seafood. J. Agric. Food Chem. 2007, 55, 5892–5897. [Google Scholar] [CrossRef]
- Laparra, J.M.; Vélez, D.; Montoro, R.; Barberá, R.; Farré, R. Estimation of Arsenic Bioaccessibility in Edible Seaweed by an in Vitro Digestion Method. J. Agric. Food Chem. 2003, 51, 6080–6085. [Google Scholar] [CrossRef]
- Almela, C.; Laparra, J.M.; Vélez, D.; Barberá, R.; Farré, R.; Montoro, R. Arsenosugars in Raw and Cooked Edible Seaweed: Characterization and Bioaccessibility. J. Agric. Food Chem. 2005, 53, 7344–7351. [Google Scholar] [CrossRef]
- Kulp, K.S.; Fortson, S.L.; Knize, M.G.; Felton, J.S. An in vitro model system to predict the bioaccessibility of heterocyclic amines from a cooked meat matrix. Food Chem. Toxicol. 2003, 41, 1701–1710. [Google Scholar] [CrossRef]
- Gao, S.; Wang, W.-X. Oral bioaccessibility of toxic metals in contaminated oysters and relationships with metal internal sequestration. Ecotoxicol. Environ. Saf. 2014, 110, 261–268. [Google Scholar] [CrossRef]
- Niu, F.; Yu, J.; Fan, J.; Zhang, B.; Ritzoulis, C.; Pan, W. The role of glycerol on the thermal gelation of myofibrillar protein from giant squid (Dosidicus gigas) mince. Food Chem. 2022, 371, 131149. [Google Scholar] [CrossRef] [PubMed]
- Luvonga, C.; Rimmer, C.A.; Yu, L.L.; Lee, S.B. Determination of total arsenic and hydrophilic arsenic species in seafood. J. Food Compos. Anal. 2020, 96, 103729. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-W.; Liang, C.-P.; Huang, F.M.; Hsueh, Y.-M. Assessing the human health risks from exposure of inorganic arsenic through oyster (Crassostrea gigas) consumption in Taiwan. Sci. Total. Environ. 2006, 361, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Guo, Z.; Song, D.; Du, S.; Zhang, L. Arsenic speciation in wild marine organisms and a health risk assessment in a subtropical bay of China. Sci. Total. Environ. 2018, 626, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Hackethal, C.; Kopp, J.F.; Sarvan, I.; Schwerdtle, T.; Lindtner, O. Total arsenic and water-soluble arsenic species in foods of the first German total diet study (BfR MEAL Study). Food Chem. 2021, 346, 128913. [Google Scholar] [CrossRef]
- Menezes, E.A.; Oliveira, A.F.; França, C.J.; Souza, G.B.; Nogueira, A.R.A. Bioaccessibility of Ca, Cu, Fe, Mg, Zn, and crude protein in beef, pork and chicken after thermal processing. Food Chem. 2018, 240, 75–83. [Google Scholar] [CrossRef]






| RDA (μg day−1) | RfD (μg kg−1 day−1) | Sample | BC (μg g−1 ww) | EDI (μg kg−1 day−1) | RDA Contribution (%) | HQ | |
|---|---|---|---|---|---|---|---|
| Cu | 900 | 40 | oysters | 7.1–13 | 23–43 | 157–290 | 0.58–1.1 |
| prawns | 0.8–1.7 | 2.6–5.5 | 18–37 | 0.07–0.14 | |||
| water spinach | 0.24–0.83 | 0.77–2.7 | 5.2–18 | 0.02–0.07 | |||
| Zn | 11,000 | 300 | oysters | 48–76 | 156–248 | 87–138 | 0.52–0.83 |
| beef | 21–33 | 68–109 | 38–61 | 0.23–0.36 | |||
| squid | 7.8–11 | 25–34 | 14–19 | 0.08–0.11 | |||
| inorganic As | 0.3 | oysters | 0.06–0.10 | 0.20–0.31 | 0.65–1.1 | ||
| clams | 0.02–0.03 | 0.05–0.10 | 0.18–0.32 | ||||
| kelp | 0.01–0.02 | 0.03–0.06 | 0.11–0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Miao, X.; Du, S.; Zhang, T.; Chen, L.; Liu, Y.; Zhang, L. Effects of Culinary Procedures on Concentrations and Bioaccessibility of Cu, Zn, and As in Different Food Ingredients. Foods 2023, 12, 1653. https://doi.org/10.3390/foods12081653
Zhang C, Miao X, Du S, Zhang T, Chen L, Liu Y, Zhang L. Effects of Culinary Procedures on Concentrations and Bioaccessibility of Cu, Zn, and As in Different Food Ingredients. Foods. 2023; 12(8):1653. https://doi.org/10.3390/foods12081653
Chicago/Turabian StyleZhang, Canchuan, Xi Miao, Sen Du, Ting Zhang, Lizhao Chen, Yang Liu, and Li Zhang. 2023. "Effects of Culinary Procedures on Concentrations and Bioaccessibility of Cu, Zn, and As in Different Food Ingredients" Foods 12, no. 8: 1653. https://doi.org/10.3390/foods12081653