Establishment of a Sonotrode Extraction Method and Evaluation of the Antioxidant, Antimicrobial and Anticancer Potential of an Optimized Vaccinium myrtillus L. Leaves Extract as Functional Ingredient
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Plant Material
2.2. Extraction of Phenolic Compounds from Bilberry Leaves by Sonotrode
2.3. Experimental Design
2.4. TPC and Antioxidant Capacity Assays (FRAP and DPPH)
2.5. HPLC-ESI-TOF-MS Analysis
2.6. Antimicrobial Analysis
2.7. Cell Cultures and In Vitro Studies
2.7.1. Cell Lines and Culture
2.7.2. In Vitro Antiproliferative Assay
2.8. Statistical Analysis
3. Results and Discussion
3.1. Fitting the Model
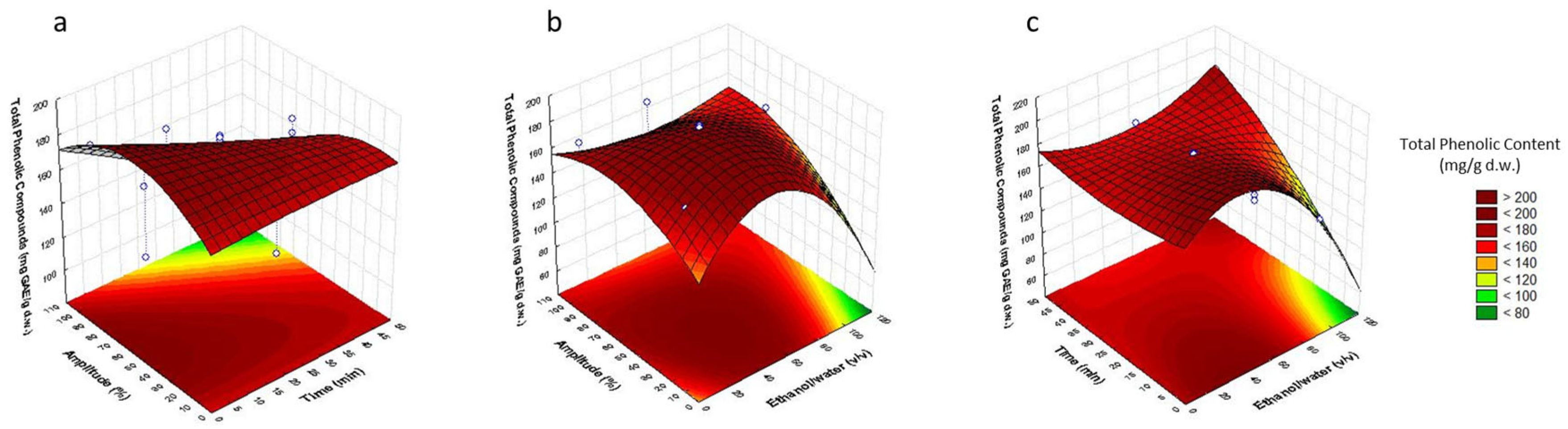
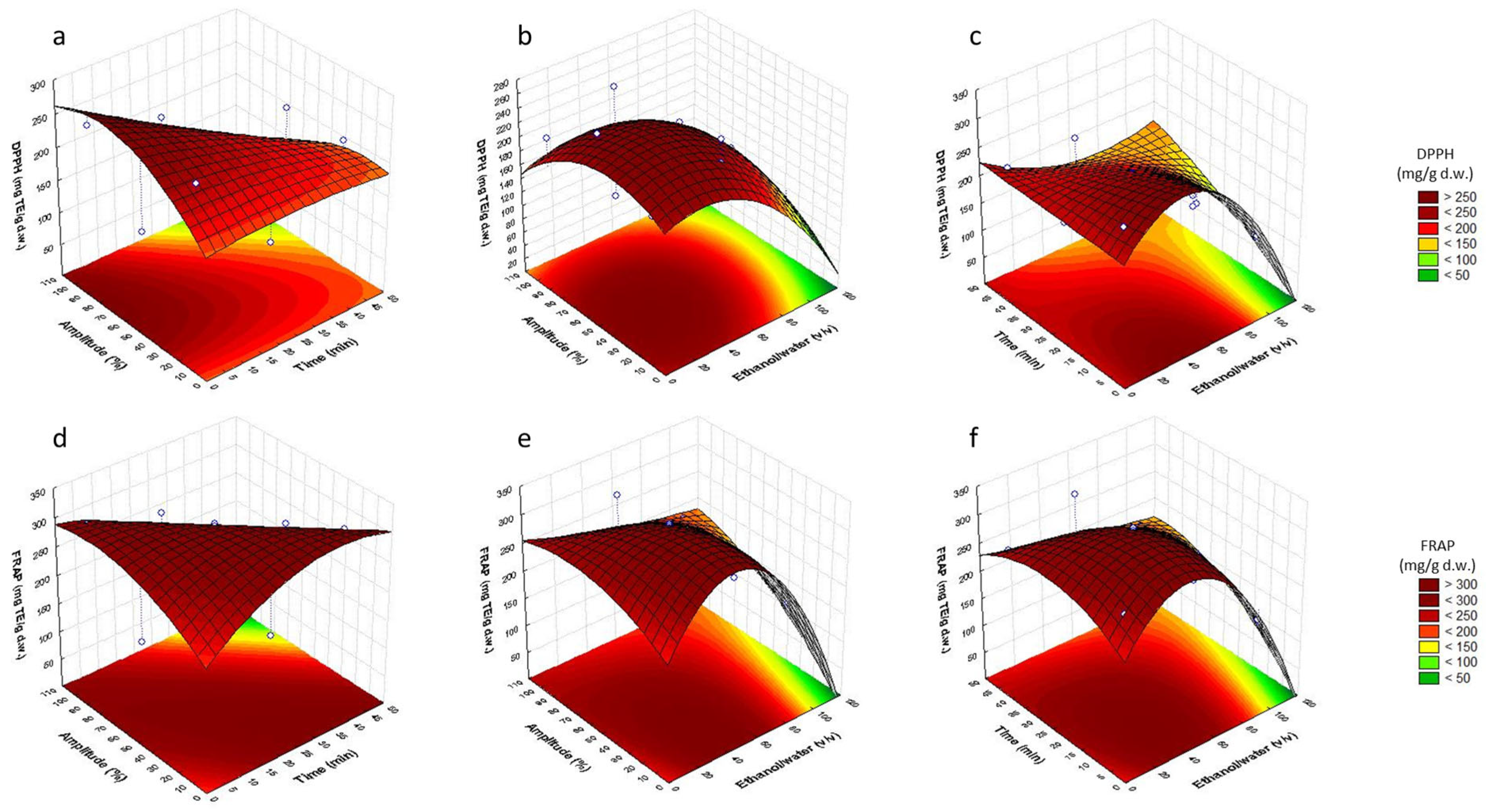
3.2. Analysis of Response Surfaces
3.3. Optimization of Sonotrode Parameters
3.4. Determination of Phenolic Compounds by HPLC-ESI-TOF-MS
3.5. Antimicrobial Activity of V. myrtillus L. Leaves Extract
3.6. Antitumor Activity of V. myrtillus L. Leaves Extract
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlemi, A.V.; Lamari, F.N. Berry Leaves: An Alternative Source of Bioactive Natural Products of Nutritional and Medicinal Value. Antioxidants 2016, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Ștefănescu, B.E.; Călinoiu, L.F.; Ranga, F.; Fetea, F.; Mocan, A.; Vodnar, D.C.; Crișan, G. Chemical Composition and Biological Activities of the Nord-West Romanian Wild Bilberry (Vaccinium myrtillus L.) and Lingonberry (Vaccinium vitis-idaea L.) Leaves. Antioxidants 2020, 9, 495. [Google Scholar] [CrossRef]
- Helmstädter, A.; Schuster, N. Vaccinium myrtillus as an Antidiabetic Medicinal Plant—Research through the Ages. Pharmazie 2010, 65, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Ziemlewska, A.; Zagórska-Dziok, M.; Nizioł-Łukaszewska, Z. Assessment of Cytotoxicity and Antioxidant Properties of Berry Leaves as By-Products with Potential Application in Cosmetic and Pharmaceutical Products. Sci. Rep. 2021, 11, 3240. [Google Scholar] [CrossRef] [PubMed]
- Stanoeva, J.P.; Stefova, M.; Andonovska, K.B.; Vankova, A.; Stafilov, T. Phenolics and Mineral Content in Bilberry and Bog Bilberry from Macedonia. Int. J. Food Prop. 2017, 20, S863–S883. [Google Scholar] [CrossRef]
- Ropiak, H.M.; Ramsay, A.; Mueller-Harvey, I. Condensed Tannins in Extracts from European Medicinal Plants and Herbal Products. J. Pharm. Biomed. Anal. 2016, 121, 225–231. [Google Scholar] [CrossRef]
- Fernandez-Panchon, M.S.; Villano, D.; Troncoso, A.M.; Garcia-Parrilla, M.C. Antioxidant Activity of Phenolic Compounds: From in Vitro Results to in Vivo Evidence. Crit. Rev. Food Sci. Nutr. 2008, 48, 649–671. [Google Scholar] [CrossRef]
- Kwon, N.; Vinayagam, R.; Do, G.S.; Lee, K.E.; Kang, S.G. Protective Effects of Fermented Houttuynia cordata Against UVA and H2O2-Induced Oxidative Stress in Human Skin Keratinocytes. Appl. Biochem. Biotechnol. 2022, 1, 1–20. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef]
- Tatipamula, V.B.; Kukavica, B. Phenolic Compounds as Antidiabetic, Anti-Inflammatory, and Anticancer Agents and Improvement of Their Bioavailability by Liposomes. Cell Biochem. Funct. 2021, 39, 926–944. [Google Scholar] [CrossRef]
- Bouarab-Chibane, L.; Forquet, V.; Lantéri, P.; Clément, Y.; Léonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial Properties of Polyphenols: Characterization and QSAR (Quantitative Structure-Activity Relationship) Models. Front. Microbiol. 2019, 10, 829. [Google Scholar] [CrossRef] [PubMed]
- Lucchini, J.J.; Corre, J.; Cremieux, A. Antibacterial Activity of Phenolic Compounds and Aromatic Alcohols. Res. Microbiol. 1990, 141, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Lee, K.E.; Vinayagam, R.; Kang, S.G. Antioxidant and Antibacterial Profiling of Pomegranate-Pericarp Extract Functionalized-Zinc Oxide Nanocomposite. Biotechnol. Bioprocess Eng. 2021, 26, 728–737. [Google Scholar] [CrossRef] [PubMed]
- Bljajić, K.; Petlevski, R.; Vujić, L.; Čačić, A.; Šoštarić, N.; Jablan, J.; De Carvalho, I.S.; Končić, M.Z. Chemical Composition, Antioxidant and α-Glucosidase-Inhibiting Activities of the Aqueous and Hydroethanolic Extracts of Vaccinium myrtillus Leaves. Molecules 2017, 22, 703. [Google Scholar] [CrossRef] [PubMed]
- Vamvakas, S.S.; Chroni, M.; Genneos, F.; Gizeli, S. Vaccinium myrtillus L. Dry Leaf Aqueous Extracts Suppress Aflatoxins Biosynthesis by Aspergillus Flavus: Bilberry Leaf Extracts Suppress Aflatoxins Biosynthesis. Food Biosci. 2021, 39, 100790. [Google Scholar] [CrossRef]
- Mäkinen, S.; Hellström, J.; Mäki, M.; Korpinen, R.; Mattila, P.H. Bilberry and Sea Buckthorn Leaves and Their Subcritical Water Extracts Prevent Lipid Oxidation in Meat Products. Foods 2020, 9, 265. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of Total Phenolic Content and Other Oxidation Substrates in Plant Tissues Using Folin-Ciocalteu Reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Martín-García, B.; Aznar-Ramos, M.J.; Verardo, V.; Gómez-Caravaca, A.M. Development of an Effective Sonotrode Based Extraction Technique for the Recovery of Phenolic Compounds with Antioxidant Activities in Cherimoya Leaves. Plants 2022, 11, 2034. [Google Scholar] [CrossRef]
- CLSI. Methods for Dilution Antimicrobial. Susceptibility Tests for Bacteria That Grow, 11th ed.; CLSI: St. Louis, MO, USA, 2018. [Google Scholar]
- CLSI. Method Broth Dilution Antifung. Susceptibility Test. Filamentous Fungi, 3rd ed.; CLSI: St. Louis, MO, USA, 2017. [Google Scholar]
- Vichai, V.; Kirtikara, K. Sulforhodamine B Colorimetric Assay for Cytotoxicity Screening. Nat. Protoc. 2016, 1, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- Razola-Díaz, M.D.C.; Guerra-Hernández, E.J.; Rodríguez-Pérez, C.; Gómez-Caravaca, A.M.; García-Villanova, B.; Verardo, V. Optimization of Ultrasound-Assisted Extraction via Sonotrode of Phenolic Compounds from Orange by-Products. Foods 2021, 10, 1120. [Google Scholar] [CrossRef] [PubMed]
- Martz, F.; Jaakola, L.; Julkunen-Tiitto, R.; Stark, S. Phenolic Composition and Antioxidant Capacity of Bilberry (Vaccinium myrtillus) Leaves in Northern Europe Following Foliar Development and Along Environmental Gradients. J. Chem. Ecol. 2010, 36, 1017–1028. [Google Scholar] [CrossRef] [PubMed]
- Bujor, O.C.; Le Bourvellec, C.; Volf, I.; Popa, V.I.; Dufour, C. Seasonal Variations of the Phenolic Constituents in Bilberry (Vaccinium myrtillus L.) Leaves, Stems and Fruits, and Their Antioxidant Activity. Food Chem. 2016, 213, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Aita, S.E.; Capriotti, A.L.; Cavaliere, C.; Cerrato, A.; Giannelli Moneta, B.; Montone, C.M.; Piovesana, S.; Laganà, A. Andean Blueberry of the Genus Disterigma: A High-Resolution Mass Spectrometric Approach for the Comprehensive Characterization of Phenolic Compounds. Separations 2021, 8, 58. [Google Scholar] [CrossRef]
- Ammar, S.; Del Mar Contreras, M.; Belguith-Hadrich, O.; Segura-Carretero, A.; Bouaziz, M. Assessment of the Distribution of Phenolic Compounds and Contribution to the Antioxidant Activity in Tunisian Fig Leaves, Fruits, Skins and Pulps Using Mass Spectrometry-Based Analysis. Food Funct. 2015, 6, 3663–3677. [Google Scholar] [CrossRef]
- Sun, L.; Tao, S.; Zhang, S. Characterization and Quantification of Polyphenols and Triterpenoids in Thinned Young Fruits of Ten Pear Varieties by UPLC-Q TRAP-MS/MS. Molecules 2019, 24, 159. [Google Scholar] [CrossRef]
- Tian, Y.; Liimatainen, J.; Alanne, A.L.; Lindstedt, A.; Liu, P.; Sinkkonen, J.; Kallio, H.; Yang, B. Phenolic Compounds Extracted by Acidic Aqueous Ethanol from Berries and Leaves of Different Berry Plants. Food Chem. 2017, 220, 266–281. [Google Scholar] [CrossRef]
- Choe, U.; Li, Y.; Gao, B.; Yu, L.; Wang, T.T.Y.; Sun, J.; Chen, P.; Yu, L. The Chemical Composition of a Cold-Pressed Milk Thistle Seed Flour Extract, and Its Potential Health Beneficial Properties. Food Funct. 2019, 10, 2461–2470. [Google Scholar] [CrossRef]
- Tazaki, H.; Ito, M.; Miyoshi, M.; Kawabata, J.; Fukushi, E.; Fujita, T.; Motouri, M.; Furuki, T.; Nabeta, K. Subulatin, an Antioxidic Caffeic Acid Derivative Isolated from the in Vitro Cultured Liverworts, Jungermannia Subulata, Lophocolea Heterophylla, and Scapania Parvitexta. Biosci. Biotechnol. Biochem. 2002, 66, 255–261. [Google Scholar] [CrossRef]
- Oszmiański, J.; Wojdyło, A.; Gorzelany, J.; Kapusta, I. Identification and Characterization of Low Molecular Weight Polyphenols in Berry Leaf Extracts by HPLC-DAD and LC-ESI/MS. J. Agric. Food Chem. 2011, 59, 12830–12835. [Google Scholar] [CrossRef] [PubMed]
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The Potential Effects of Chlorogenic Acid, the Main Phenolic Components in Coffee, on Health: A Comprehensive Review of the Literature. Eur. J. Nutr. 2017, 56, 2215–2244. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Atanasov, A.G.; Li, Y.; Kumar, N.; Bishayee, A. Chlorogenic Acid for Cancer Prevention and Therapy: Current Status on Efficacy and Mechanisms of Action. Pharmacol. Res. 2022, 186, 106505. [Google Scholar] [CrossRef]
- Liu, P.; Lindstedt, A.; Markkinen, N.; Sinkkonen, J.; Suomela, J.P.; Yang, B. Characterization of Metabolite Profiles of Leaves of Bilberry (Vaccinium myrtillus L.) and Lingonberry (Vaccinium vitis-idaea L.). J. Agric. Food Chem. 2014, 62, 12015–12026. [Google Scholar] [CrossRef] [PubMed]
- Brasanac-Vukanovic, S.; Mutic, J.; Stankovic, D.M.; Arsic, I.; Blagojevic, N.; Vukasinovic-Pesic, V.; Tadic, V.M. Wild Bilberry (Vaccinium myrtillus L., Ericaceae) from Montenegro as a Source of Antioxidants for Use in the Production of Nutraceuticals. Molecules 2018, 23, 1864. [Google Scholar] [CrossRef]
- Subbiah, V.; Zhong, B.; Nawaz, M.A.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. Screening of Phenolic Compounds in Australian Grown Berries by LC-ESI-QTOF-MS/MS and Determination of Their Antioxidant Potential. Antioxidants 2020, 10, 26. [Google Scholar] [CrossRef]
- Suvanto, J.; Nohynek, L.; Seppänen-Laakso, T.; Rischer, H.; Salminen, J.P.; Puupponen-Pimiä, R. Variability in the Production of Tannins and Other Polyphenols in Cell Cultures of 12 Nordic Plant Species. Planta 2017, 246, 227–241. [Google Scholar] [CrossRef]
- Hokkanen, J.; Mattila, S.; Jaakola, L.; Pirttilä, A.M.; Tolonen, A. Identification of Phenolic Compounds from Lingonberry (Vaccinium vitis-idaea L.), Bilberry (Vaccinium myrtillus L.) AndHybrid Bilberry (Vaccinium x intermedium Ruthe L.) Leaves. J. Agric. Food Chem. 2009, 57, 9437–9447. [Google Scholar] [CrossRef]
- Tadić, V.M.; Nešić, I.; Martinović, M.; Rój, E.; Brašanac-Vukanović, S.; Maksimović, S.; Žugić, A. Old Plant, New Possibilities: Wild Bilberry (Vaccinium myrtillus L., Ericaceae) in Topical Skin Preparation. Antioxidants 2021, 10, 465. [Google Scholar] [CrossRef]
- Xu, J.G.; Hu, Q.P.; Liu, Y. Antioxidant and DNA-Protective Activities of Chlorogenic Acid Isomers. J. Agric. Food Chem. 2012, 60, 11625–11630. [Google Scholar] [CrossRef] [PubMed]
- Lesjak, M.; Beara, I.; Simin, N.; Pintać, D.; Majkić, T.; Bekvalac, K.; Orčić, D.; Mimica-Dukić, N. Antioxidant and Anti-Inflammatory Activities of Quercetin and Its Derivatives. J. Funct. Foods 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Wood, J.E.; Senthilmohan, S.T.; Peskin, A.V. Antioxidant Activity of Procyanidin-Containing Plant Extracts at Different PHs. Food Chem. 2002, 77, 155–161. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid Antioxidants: Chemistry, Metabolism and Structure-Activity Relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Puganen, A.; Alakomi, H.L.; Uusitupa, A.; Saarela, M.; Yang, B. Antioxidative and Antibacterial Activities of Aqueous Ethanol Extracts of Berries, Leaves, and Branches of Berry Plants. Food Res. Int. 2018, 106, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Dragana, M.V.; Miroslav, R.P.; Branka, B.R.G.; Olgica, D.S.; Sava, M.V.; Ljiljana, R.Č. Antibacterial and Antioxidant Activities of Bilberry (Vaccinium myrtillus L.) In Vitro. African J. Microbiol. Res. 2013, 7, 5130–5136. [Google Scholar] [CrossRef]
- Katsube, N.; Iwashita, K.; Tsushida, T.; Yamaki, K.; Kobori, M. Induction of Apoptosis in Cancer Cells by Bilberry (Vaccinium myrtillus) and the Anthocyanins. J. Agric. Food Chem. 2003, 51, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.K.; Koponen, J.M.; Mykkänen, H.M.; Törrönen, A.R. Berry Phenolic Extracts Modulate the Expression of P21WAF1 and Bax but Not Bcl-2 in HT-29 Colon Cancer Cells. J. Agric. Food Chem. 2007, 55, 1156–1163. [Google Scholar] [CrossRef]
- Šavikin, K.; Zdunić, G.; Janković, T.; Godevac, D.; Stanojković, T.; Pljevljakušić, D. Berry Fruit Teas: Phenolic Composition and Cytotoxic Activity. Food Res. Int. 2014, 62, 677–683. [Google Scholar] [CrossRef]
- Minker, C.; Duban, L.; Karas, D.; Järvinen, P.; Lobstein, A.; Muller, C.D. Impact of Procyanidins from Different Berries on Caspase 8 Activation in Colon Cancer. Oxid. Med. Cell. Longev. 2015, 2015, 154164. [Google Scholar] [CrossRef]
- Mudd, A.M.; Gu, T.; Munagala, R.; Jeyabalan, J.; Egilmez, N.K.; Gupta, R.C. Chemoprevention of Colorectal Cancer by Anthocyanidins and Mitigation of Metabolic Shifts Induced by Dysbiosis of the Gut Microbiome. Cancer Prev. Res. 2020, 13, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Mechikova, G.Y.; Kuzmich, A.S.; Ponomarenko, L.P.; Kalinovsky, A.I.; Stepanova, T.A.; Fedorov, S.N.; Stonik, V.A. Cancer-Preventive Activities of Secondary Metabolites from Leaves of the Bilberry Vaccinium Smallii A. Gray. Phyther. Res. 2010, 24, 1730–1732. [Google Scholar] [CrossRef] [PubMed]
- Gil-Sánchez, I.; Cueva, C.; Tamargo, A.; Quintela, J.C.; de la Fuente, E.; Walker, A.W.; Moreno-Arribas, M.V.; Bartolomé, B. Application of the Dynamic Gastrointestinal Simulator (Simgi®) to Assess the Impact of Probiotic Supplementation in the Metabolism of Grape Polyphenols. Food Res. Int. 2020, 129, 108790. [Google Scholar] [CrossRef] [PubMed]
| Independent Factors | Dependent Factors | |||||
|---|---|---|---|---|---|---|
| No | X1 | X2 | X3 | TPC (mg GAE/g d.w.) | DPPH (mg TE/g d.w.) | FRAP (mg TE/g d.w.) |
| 1 | 10 (−1) | 5 (−1) | 60 (0) | 171.82 | 253.76 | 267.28 |
| 2 | 100 (1) | 5 (−1) | 60 (0) | 130.69 | 126.96 | 148.01 |
| 3 | 10 (−1) | 45 (1) | 60 (0) | 164.93 | 218.73 | 243.48 |
| 4 | 100 (1) | 45 (1) | 60 (0) | 173.61 | 151.99 | 184.86 |
| 5 | 10 (−1) | 25 (0) | 20 (−1) | 170.77 | 190.50 | 272.97 |
| 6 | 100 (1) | 25 (0) | 20 (−1) | 135.15 | 116.10 | 164.67 |
| 7 | 10 (−1) | 25 (0) | 100 (1) | 165.60 | 200.43 | 257.46 |
| 8 | 100 (1) | 25 (0) | 100 (1) | 145.44 | 142.78 | 195.94 |
| 9 | 55 (0) | 5 (−1) | 20 (−1) | 179.51 | 253.43 | 271.62 |
| 10 | 55 (0) | 45 (1) | 20 (−1) | 175.30 | 220.19 | 291.60 |
| 11 | 55 (0) | 5 (−1) | 100 (1) | 174.69 | 234.33 | 289.50 |
| 12 | 55 (0) | 45 (1) | 100 (1) | 119.82 | 73.73 | 114.33 |
| 13 | 55 (0) | 25 (0) | 60 (0) | 181.86 | 220.20 | 295.80 |
| 14 | 55 (0) | 25 (0) | 60 (0) | 179.02 | 224.76 | 291.70 |
| 15 | 55 (0) | 25 (0) | 60 (0) | 180.80 | 222.77 | 288.05 |
| Regression Coefficients | TPC (mg GAE/g d.w.) | DPPH (mg TE/g d.w.) | FRAP (mg TE/g d.w.) | |||
|---|---|---|---|---|---|---|
| Effect | p-Value | Effect | p-Value | Effect | p-Value | |
| β0 | 158.9447 | 0.0000 * | 181.9114 | 0.0000 * | 225.1434 | 0.0000 * |
| Linear | ||||||
| β1 | −20.1117 | 0.0028 * | −86.5233 | 0.0004 * | −87.6007 | 0.0011 * |
| β2 | 2.1615 | 0.1814 | −35.6410 | 0.0023 * | −21.5165 | 0.0176 * |
| β3 | −8.3473 | 0.0161 * | −15.3924 | 0.0120 * | −21.3091 | 0.0179 * |
| Crossed | ||||||
| β12 | 24.9041 | 0.0033 * | 30.0333 | 0.0057 * | 30.3200 | 0.0160 * |
| β13 | 7.7287 | 0.0329 * | 8.3744 | 0.0669 | 23.3861 | 0.0264 * |
| β23 | −25.3309 | 0.0032 * | −63.6782 | 0.0013 * | −97.5754 | 0.0016 * |
| Quadratic | ||||||
| β11 | 14.1936 | 0.0028 * | 33.8420 | 0.0012 * | 49.9700 | 0.0016 * |
| β22 | 6.1028 | 0.0147 * | 0.8747 | 0.5381 | 30.9723 | 0.0042 * |
| β33 | 12.1282 | 0.0038 * | 26.2820 | 0.0020 * | 19.1176 | 0.0110 * |
| R2 | 0.9978 | 0.9980 | 0.9992 | |||
| p Model | 0.0018 * | 0.0065 * | 0.0024 * | |||
| p Lack of fit | 0.1814 | 0.1249 | 0.5379 | |||
| Parameter | Optimal Conditions | ||
|---|---|---|---|
| Ethanol (%) | 30 | ||
| Time (min) | 5 | ||
| Amplitude (%) | 55 | ||
| TPC | DPPH | FRAP | |
| Predicted value (mg/g d.w.) | 195.59 ± 6.76 | 276.85 ± 10.98 | 301.55 ± 18.96 |
| Empirical value (mg/g d.w.) | 217.03 ± 4.92 | 271.13 ± 5.84 | 312.21 ± 9.30 |
| Coefficient of variation (%) | 7.35 | 1.48 | 2.46 |
| Peak No. | Retention Time (min) | m/z Exp. | m/z Calc. | Molecular Formula | Error (ppm) | Score | Proposed Compound | Quantification (mg/g d.w.) |
|---|---|---|---|---|---|---|---|---|
| Phenolic acids and derivatives | ||||||||
| 2 | 3.61 | 285.0608 | 285.0610 | C12H14O8 | −0.7 | 99.26 | Dihydroxybenzoic acid pentose | 0.30 ± 0.03 |
| 3 | 4.42 | 343.1034 | 343.1029 | C15H20O9 | 1.5 | 99.98 | Dihydro-caffeoyl-O-hexoside | 1.01 ± 0.07 |
| 4 | 4.61 | 341.0869 | 341.0873 | C15H18O9 | −1.2 | 93.96 | Caffeoyl-O-hexoside | <LOQ |
| 5 | 4.68 | 515.1406 | 515.1401 | C22H28O14 | 1 | 96.08 | Chlorogenoyl hexose | 0.51 ± 0.04 |
| 6 | 4.96 | 433.0981 | 433.0982 | C17H22O13 | −0.2 | 99.21 | Gallic acid di-pentoside I | <LOQ |
| 8 | 5.02 | 417.1037 | 417.1033 | C17H22O12 | 1 | 99.99 | Dihydroxybenzoic acid di-pentoside isomer a | <LOQ |
| 9 | 5.05 | 417.1034 | 417.1033 | C17H22O12 | −0.2 | 99.95 | Dihydroxybenzoic acid di-pentoside isomer b | <LOQ |
| 10 | 5.12 | 707.1827 | 707.1823 | C32H36O18 | 0.6 | 99.20 | Chlorogenic acid dimer isomer a | 2.54 ± 0.07 |
| 11 | 5.25 | 707.1826 | 707.1823 | C32H36O19 | 0.4 | 93.54 | Chlorogenic acid dimer isomer b | 7.49 ± 0.14 |
| 12 | 5.68 | 353.0862 | 353.0873 | C16H18O9 | −3.1 | 100 | Chlorogenic acid * | 90.66 ± 0.40 |
| 13 | 5.95 | 707.1813 | 707.1823 | C32H36O18 | −1.4 | 95.65 | Chlorogenic acid dimer isomer c | 14.20 ± 0.24 |
| 14 | 6.26 | 707.1829 | 707.1823 | C32H36O18 | 0.9 | 99.90 | Chlorogenic acid dimer isomer d | 2.26 ± 0.06 |
| 15 | 6.38 | 691.1888 | 691.1874 | C32H36O17 | 2 | 94.75 | Methyl 5-(6-caffeoyl-glucopyranosyl)-caffeoylquinic acid | 1.67 ± 0.09 |
| 18 | 7.05 | 337.0909 | 337.0923 | C16H18O8 | −4.2 | 100 | Coumaroylquinic acid isomer a | 1.85 ± 0.10 |
| 19 | 7.28 | 337.0908 | 337.0923 | C16H18O8 | −4.4 | 99.80 | Coumaroylquinic acid isomer b | 2.75 ± 0.13 |
| 21 | 7.70 | 367.1023 | 367.1029 | C17H20O9 | −1.6 | 99.70 | Feruloylquinic acid | <LOQ |
| 24 | 8.33 | 551.1400 | 551.1401 | C25H28O14 | −0.2 | 99.86 | Caffeoyl hexosyl trihydroxymethoxyphenyl propanoic acid | 1.70 ± 0.10 |
| 27 | 9.00 | 705.1643 | 705.1651 | C32H34O18 | −2.3 | 98.26 | Subulatin | <LOQ |
| 43 | 11.81 | 411.1650 | 411.1655 | C20H28O9 | −1.2 | 99.94 | Coumaric acid-malonyl-hexoside | 7.35 ± 0.21 |
| Flavonoids and derivatives | ||||||||
| 7 | 5.00 | 305.0660 | 305.0661 | C15H14O7 | −0.3 | 99.70 | Epigallocatechin | 0.87 ± 0.08 |
| 26 | 8.64 | 531.1337 | 531.1339 | C25H24O13 | −0.4 | 99.98 | 6′′-O-Malonylglycitin | <LOQ |
| 32 | 9.77 | 595.1298 | 595.1299 | C26H28O16 | −0.2 | 94.74 | Quercetin3-O-arabinosylgalactoside | <LOQ |
| 34 | 10.09 | 447.0934 | 447.0927 | C21H20O11 | 1.6 | 96.17 | Kaempferol 3-O-glucoside | <LOQ |
| 35 | 10.13 | 609.1462 | 609.1456 | C27H30O16 | 1 | 100 | Quercetin-rutinoside isomer a * | <LOQ |
| 36 | 10.29 | 609.1461 | 609.1456 | C27H30O16 | 0.8 | 99.73 | Quercetin-rutinoside isomer b * | 3.59 ± 0.27 |
| 37 | 10.39 | 463.0885 | 463.0877 | C21H20O12 | 1.7 | 99.84 | Quercetin 3-O-galactoside isomer a | 3.53 ± 0.36 |
| 38 | 10.53 | 463.0885 | 463.0877 | C21H20O12 | 0.9 | 99.55 | Quercetin 3-O-galactoside isomer b | 3.73 ± 0.40 |
| 39 | 10.83 | 477.0645 | 477.0669 | C21H18O13 | 5 | 99.99 | Quercetin-3-glucuronide | 7.27 ± 0.50 |
| 40 | 11.24 | 433.0751 | 433.0771 | C20H18O11 | −4.6 | 99.46 | Quercetin-3-arabinoside | 1.86 ± 0.13 |
| 41 | 11.41 | 505.0970 | 505.0982 | C23H22O13 | −2.4 | 99.95 | Quercetin 3-(2″-acetylgalactoside) isomer a | 4.37 ± 0.22 |
| 42 | 11.66 | 447.0921 | 447.0927 | C21H20O11 | −1.3 | 99.72 | Quercetin-3-O-rhamnoside | <LOQ |
| 44 | 11.90 | 505.0966 | 505.0982 | C23H22O13 | −3.2 | 80.48 | Quercetin 3-(2″-acetylgalactoside) isomer b | 0.28± 0.01 |
| 45 | 11.95 | 491.0803 | 491.0826 | C22H20O13 | −4.7 | 90.78 | Isorhamnetin-glucuronide | 0.21± 0.05 |
| 46 | 11.99 | 579.1350 | 579.1350 | C26H28O15 | 0 | 95.15 | Quercetin-3-O-R-arabinofuranoside | 0.17 ± 0.01 |
| 47 | 12.15 | 505.0988 | 505.0982 | C23H22O13 | 1.2 | 99.99 | Quercetin 3-(2″-acetylgalactoside) isomer c | 0.12 ± 0.01 |
| 48 | 12.49 | 489.1040 | 489.1033 | C23H22O12 | 1.4 | 97.29 | Kaempferol 3-O-acetyl-glucoside | 0.89 ± 0.03 |
| 49 | 12.71 | 519.1134 | 519.1139 | C24H24O13 | −1 | 99.66 | Isorhamnetin-acylated-hexoside | <LOQ |
| 50 | 13.35 | 591.1363 | 591.1350 | C27H28O15 | 2.2 | 99.98 | Quercetin-HMG-rhamnoside | 2.94 ± 0.04 |
| 51 | 15.09 | 329.0653 | 329.0661 | C17H14O7 | −2.4 | 99.78 | 3′.7-Dimethylquercetin | <LOQ |
| Condensed tannins | ||||||||
| 16 | 6.72 | 577.1337 | 577.1346 | C30H26O12 | −1.6 | 99.98 | Procyanidin dimer | 2.51 ± 0.18 |
| 22 | 7.75 | 879.1778 | 879.1773 | C45H36O19 | 0.6 | 89.69 | Procyanidin-prodelphinidin trimer(1 A-type bond) | <LOQ |
| 23 | 8.29 | 865.1976 | 865.1980 | C45H38O18 | −0.5 | 91.43 | Procyanidin trimer | 1.15 ± 0.09 |
| 25 | 8.57 | 863.1823 | 863.1825 | C45H36O18 | 0.2 | 90.32 | Procyanidin trimer (1A-type bond) | 1.33 ± 0.10 |
| 28 | 9.11 | 739.1647 | 739.1663 | C39H32O15 | −2.2 | 93.04 | Cinchonain II isomer a | <LOQ |
| 29 | 9.35 | 739.1680 | 739.1663 | C39H32O15 | 2.3 | 99.20 | Cinchonain II isomer b | 0.05 ± 0.06 |
| 30 | 9.43 | 451.1025 | 451.1029 | C24H20O9 | −0.9 | 99.97 | Cinchonain I a | 0.31 ± 0.12 |
| 33 | 9.86 | 577.1337 | 577.1346 | C30H26O12 | 0.9 | 88.65 | Procyanidin dimer | 0.60 ± 0.06 |
| Lignans | ||||||||
| 17 | 6.95 | 553.1544 | 553.1557 | C25H30O14 | −2.4 | 98.82 | Ligustrosidic acid | |
| 20 | 7.56 | 553.1555 | 553.1557 | C25H30O14 | −0.4 | 99.80 | Ligustrosidic acid | |
| Other compounds | ||||||||
| 1 | 0.45–0.60 | 191.0545 | 191.0556 | C7H12O6 | −4.8 | 99.00 | Quinic acid | |
| 31 | 9.57 | 535.1448 | 535.1452 | C25H28O13 | −0.7 | 98.95 | Coumaroyl iridoid (I) | |
| Sum of phenolic acids | 134.28 ± 2.73 | |||||||
| Sum of flavonoids | 35.79 ± 2.11 | |||||||
| Sum of phenolic compounds | 170.07 ± 4.84 | |||||||
| Strain Type | Bilberry Leaves Extract | Sorbic Acid (E-200) | |||
|---|---|---|---|---|---|
| MIC * (mg/mL) | MBC/MFC * (mg/mL) | MIC * (mg/mL) | MBC/MFC * (mg/mL) | ||
| Gram-positive | L. monocytogenes | 3.12 | 6.25 | 3.12 | 6.25 |
| L. innocua | 3.12 | 6.25 | 3.12 | 6.25 | |
| S. aureus | 0.4 | 0.8 | 0.8 | 1.56 | |
| E. faecalis | 3.12 | 6.25 | 3.12 | 6.25 | |
| B. cereus | 0.4 | 0.8 | 1.56 | 3.12 | |
| Gram-negative | S. enterica | 25 | 50 | 3.12 | 6.25 |
| E. coli | 25 | 50 | 3.12 | 6.25 | |
| S. sonnei | 25 | 50 | 6.25 | 12.5 | |
| P. aeruginosa | 25 | 50 | 1.56 | 3.12 | |
| Fungi | C. sake | 25 | 50 | 12.5 | 25 |
| Z. bailii | 50 | >50 | 6.25 | 12.5 | |
| P. expansum | 50 | >50 | 6.25 | 12.5 | |
| A. niger | 50 | >50 | 6.25 | 12.5 | |
| Cellular Line | IC50 (μg/mL) | |
|---|---|---|
| Bilberry Leaves Extract | 5-Fluorouracil | |
| HT-29 (Human grade II colorectal adenocarcinoma) | 213.2 ± 2.5 | 10.3 ± 0.2 |
| T-84 (Human colorectal carcinoma) | 1140.3 ± 5.2 | 27.0 ± 1.6 |
| SW-837 (Human grade IV rectum adenocarcinoma) | 936.5 ± 4.6 | 19.4 ± 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gil-Martínez, L.; Aznar-Ramos, M.J.; del Carmen Razola-Diaz, M.; Mut-Salud, N.; Falcón-Piñeiro, A.; Baños, A.; Guillamón, E.; Gómez-Caravaca, A.M.; Verardo, V. Establishment of a Sonotrode Extraction Method and Evaluation of the Antioxidant, Antimicrobial and Anticancer Potential of an Optimized Vaccinium myrtillus L. Leaves Extract as Functional Ingredient. Foods 2023, 12, 1688. https://doi.org/10.3390/foods12081688
Gil-Martínez L, Aznar-Ramos MJ, del Carmen Razola-Diaz M, Mut-Salud N, Falcón-Piñeiro A, Baños A, Guillamón E, Gómez-Caravaca AM, Verardo V. Establishment of a Sonotrode Extraction Method and Evaluation of the Antioxidant, Antimicrobial and Anticancer Potential of an Optimized Vaccinium myrtillus L. Leaves Extract as Functional Ingredient. Foods. 2023; 12(8):1688. https://doi.org/10.3390/foods12081688
Chicago/Turabian StyleGil-Martínez, Lidia, María José Aznar-Ramos, Maria del Carmen Razola-Diaz, Nuria Mut-Salud, Ana Falcón-Piñeiro, Alberto Baños, Enrique Guillamón, Ana María Gómez-Caravaca, and Vito Verardo. 2023. "Establishment of a Sonotrode Extraction Method and Evaluation of the Antioxidant, Antimicrobial and Anticancer Potential of an Optimized Vaccinium myrtillus L. Leaves Extract as Functional Ingredient" Foods 12, no. 8: 1688. https://doi.org/10.3390/foods12081688






