Influence of Fermentation on Functional Properties and Bioactivities of Different Cowpea Leaf Smoothies during In Vitro Digestion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Samples
2.3. Preparation and Fermentation of Smoothie
2.4. Reactivation of the Ltp. plantarum 75 Cultures and Fermentation of Cowpea Smoothies
2.5. Physicochemical Properties of Fermented and Unfermented Cowpea Smoothies
2.6. Organoleptic Properties of Unfermented and Fermented Cowpea Smoothie
2.7. Total Sugars of Cowpea Smoothies
2.8. Carotenoid Extraction, Identification, and Quantification
2.9. In Vitro Digestion of Cowpea Smoothies
2.10. Antioxidant Properties
2.11. Inhibition of Carbohydrate Hydrolysing Enzymes (α-Amylase and α-Glucosidase)
2.12. Glucose Uptake Assay
2.13. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Properties of Cowpea Smoothies Fermented Using Ltp. plantarum 75
3.2. Effect of Fermentation on the Ascorbic Acid Content of Three Different Cowpea Cultivar Leaf Smoothies
3.3. Colour Changes in Cowpea Leaf Smoothies after Fermentation
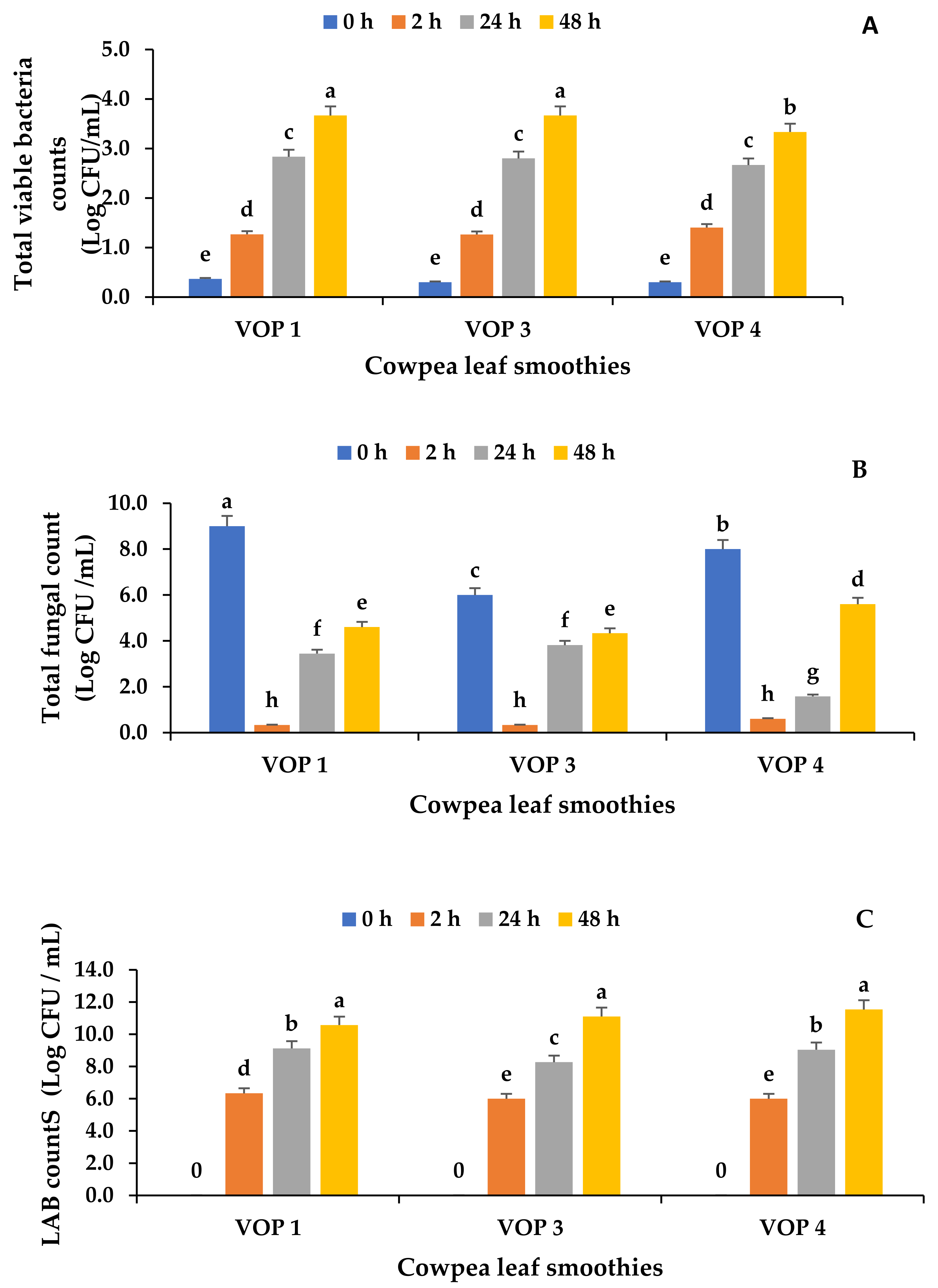
3.4. Microbial Counts in Fermented and Unfermented Cowpea Leaf Smoothies
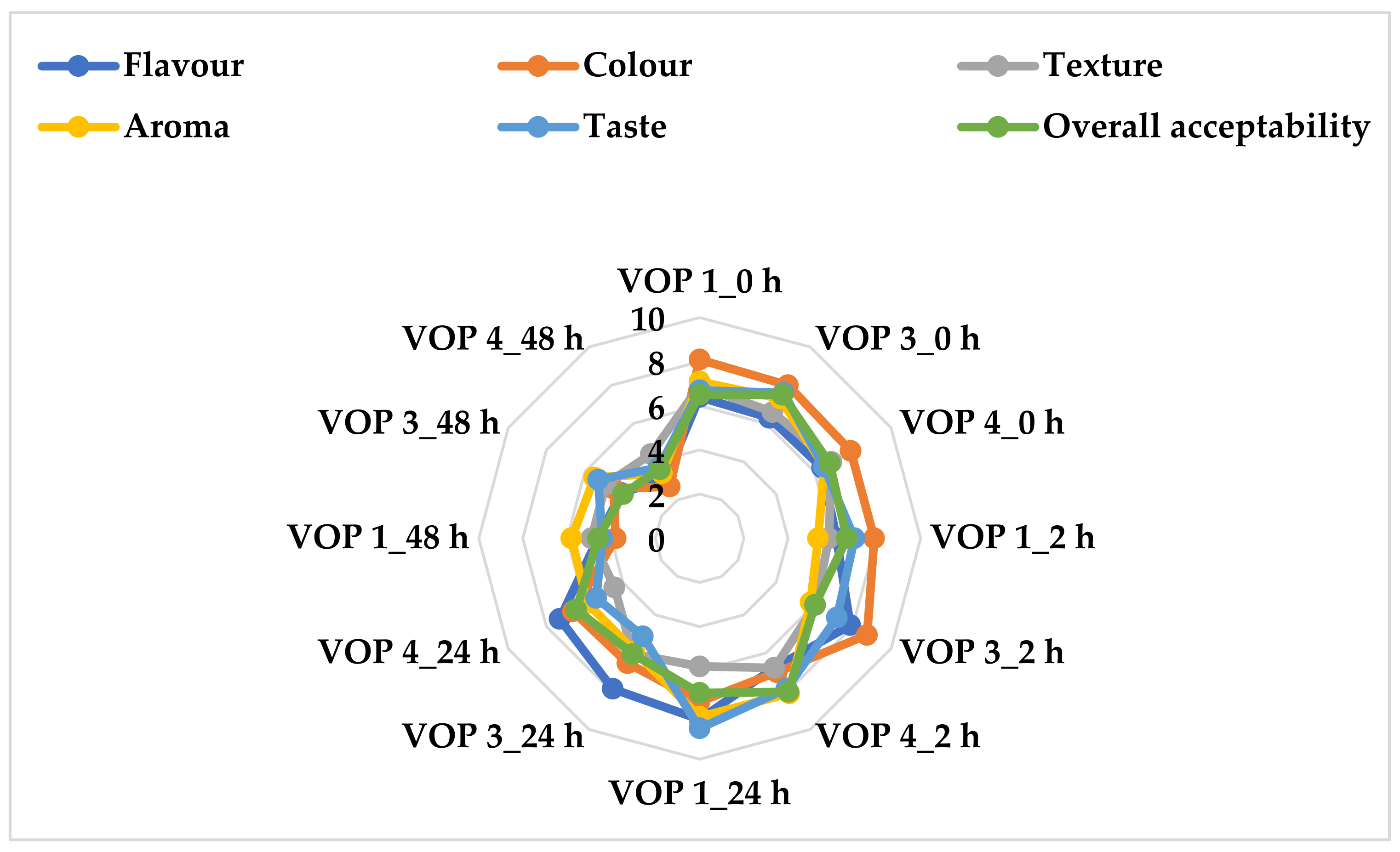
3.5. Sensory Evaluation of Unfermented and Fermented Cowpea Leaf Smoothies
3.6. Total Phenolic Content (TPC) and Antioxidant Activities of Unfermented and Fermented Cowpea Leaf Smoothies
3.7. Effect of Fermentation on the Carotenoid’s Profiles in Cowpea Leaf Smoothies
3.8. Effect of In Vitro Digestion on Total Phenolic Content and Antioxidant Activity of VOP 1 Fermented Cowpea Smoothie
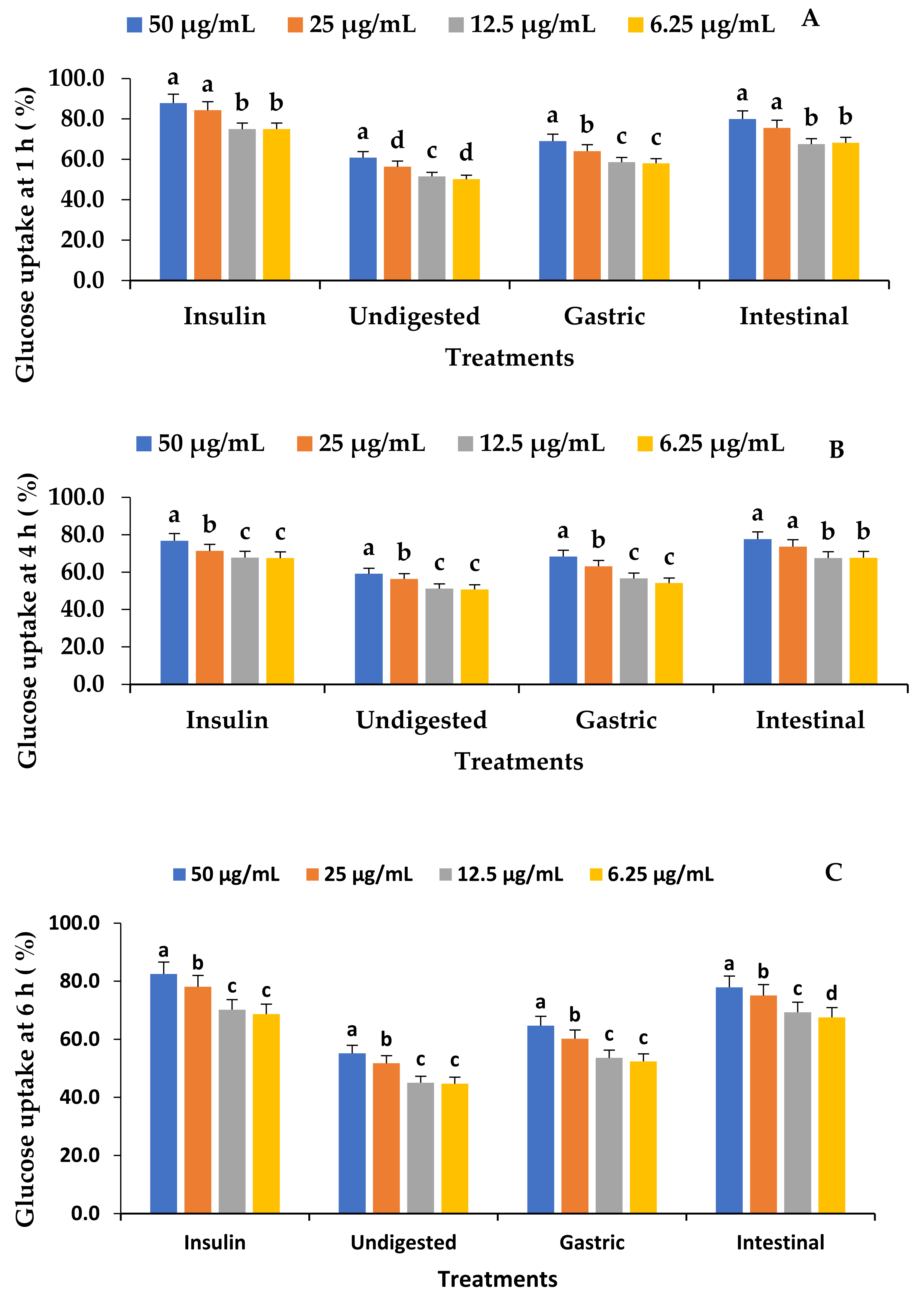
3.9. Effect of In Vitro Digestion on the C2C12 Glucose Uptake of Fermented VOP 1 Cowpea Leaf Smoothies
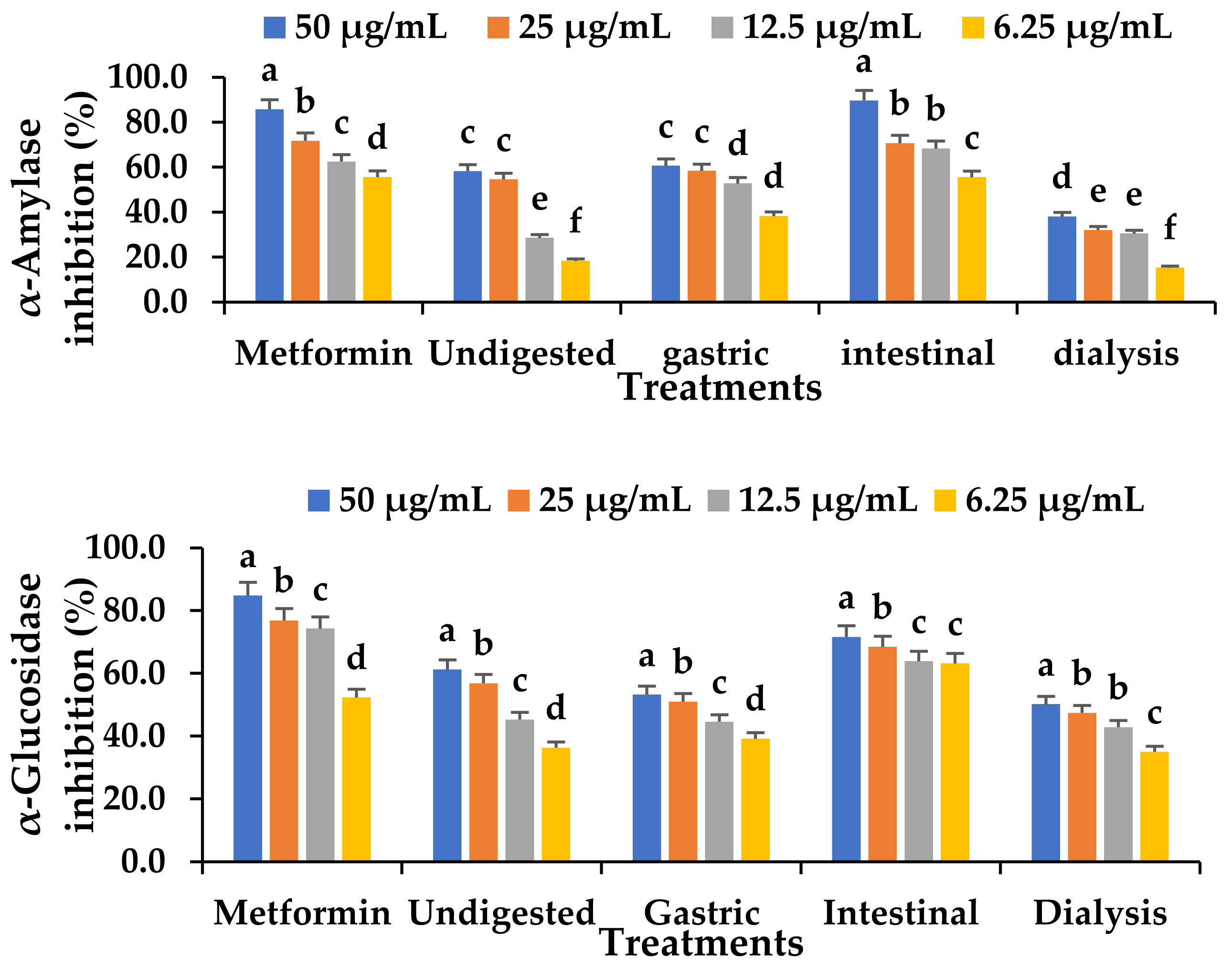
3.10. Effect of In Vitro Digestion on the α-Glucosidase and α-Amylase Inhibitory Capacity of Fermented VOP 1 Cowpea Leaf Smoothies
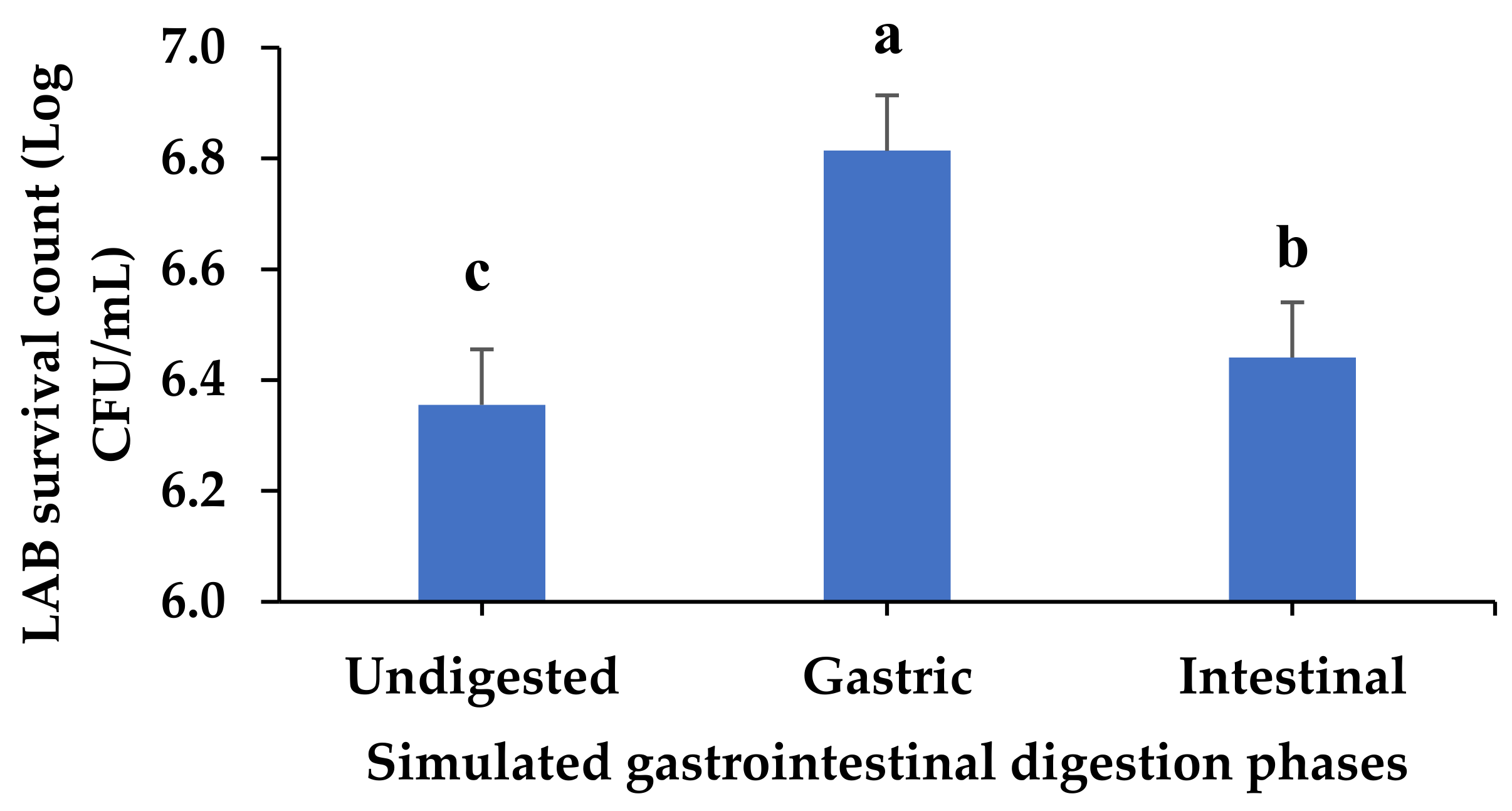
3.11. Evolution of Lactic Acid Bacteria in Fermented VOP 1 Cowpea Leaf Smoothie after Simulated Gastrointestinal Digestion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moloto, M.R.; Phan, A.D.T.; Shai, J.L.; Sultanbawa, Y.; Sivakumar, D. Comparison of phenolic compounds, carotenoids, amino acid composition, in vitro antioxidant and anti-diabetic activities in the leaves of seven cowpea (Vigna unguiculata) cultivars. Foods 2020, 9, 1285. [Google Scholar] [CrossRef] [PubMed]
- Seke, F.; Moloto, M.R.; Shoko, T.; Sultanbawa, Y.; Sivakumar, D. Comparative study of the functional compounds and antioxidant properties of different cowpea (Vigna unguiculata) leaf cultivars after in vitro digestion. Int. J. Food Sci. Technol. 2022, 58, 1089–1097. [Google Scholar] [CrossRef]
- Kitinoja, L.; Saran, S.; Roy, S.K.; Kader, A.A. Postharvest technology for developing countries: Challenges and opportunities in research, outreach and advocacy. J. Sci. Food Agric. 2011, 91, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Guiné, R. The drying of foods and its effect on the physical-chemical, sensorial and nutritional properties. Int. J. Food Eng. 2018, 2, 93–100. [Google Scholar] [CrossRef]
- Kasangi, D.M.; Shitandi, A.A.; Shalo, P.L.; Mbugua, S.K. Effect of spontaneous fermentation of cowpea leaves (Vigna unguiculata) on proximate composition, mineral content, chlorophyll content and beta-carotene content. Int. Food Res. J. 2010, 17, 721–732. [Google Scholar]
- Calín-Sánchez, Á.; Lipan, L.; Cano-Lamadrid, M.; Kharaghani, A.; Masztalerz, K.; Carbonell-Barrachina, Á.A.; Figiel, A. Comparison of traditional and novel drying techniques and its effect on quality of fruits, vegetables and aromatic herbs. Foods 2020, 9, 1261. [Google Scholar] [CrossRef]
- Tang, Z.; Zhao, Z.; Wu, X.; Lin, W.; Qin, Y.; Chen, H.; Wang, Y.; Zhou, C.; Bu, T.; Xiao, Y.; et al. A Review on fruit and vegetable fermented beverage-benefits of microbes and beneficial effects. Food Rev. Int. 2022, 1–38. [Google Scholar] [CrossRef]
- Oguntoyinbo, F.A.; Fusco, V.; Cho, G.S.; Kabisch, J.; Neve, H.; Bockelmann, W.; Huch, M.; Frommherz, L.; Trierweiler, B.; Franz, C.M.; et al. Produce from Africa’s gardens: Potential for leafy vegetable and fruit fermentations. Front. Microbiol. 2016, 7, 981. [Google Scholar] [CrossRef]
- Franz, C.M.; Huch, M.; Mathara, J.M.; Abriouel, H.; Benomar, N.; Reid, G.; Galvez, A.; Holzapfel, W.H. African fermented foods and probiotics. Int. J. Food Microbio. 2014, 190, 84–96. [Google Scholar] [CrossRef]
- Fessard, A.; Kapoor, A.; Patche, J.; Assemat, S.; Hoarau, M.; Bourdon, E.; Bahorun, T.; Remize, F. Lactic fermentation as an efficient tool to enhance the antioxidant activity of tropical fruit juices and teas. Microorganisms 2017, 5, 23. [Google Scholar] [CrossRef]
- Blana, V.A.; Grounta, A.; Tassou, C.C.; Nychas, G.J.E.; Panagou, E.Z. Inoculated fermentation of green olives with potential probiotic Lactobacillus pentosus and Lactobacillus plantarum starter cultures isolated from industrially fermented olives. Food Microbiol. 2014, 38, 208–218. [Google Scholar] [CrossRef]
- Bartkiene, E.; Vidmantiene, D.; Juodeikiene, G.; Viskelis, P.; Urbonaviciene, D. Lactic acid fermentation of tomato: Effects on cis/trans lycopene isomer ratio, β-carotene mass fraction and formation of L (+)-and D (–)-lactic acid. Food Technol. Biotechnol. 2013, 51, 471–478. [Google Scholar]
- Failla, M.L.; Chitchumroonchokchai, C.; Ishida, B.K. In vitro micellarization and intestinal cell uptake of cis isomers of lycopene exceed those of all-trans lycopene. J. Nutr. 2008, 138, 482–486. [Google Scholar] [CrossRef]
- Kiczorowski, P.; Kiczorowska, B.; Samolińska, W.; Szmigielski, M.; Winiarska-Mieczan, A. Effect of fermentation of chosen vegetables on the nutrient, mineral, and biocomponent profile in human and animal nutrition. Sci. Rep. 2022, 12, 13422. [Google Scholar] [CrossRef]
- Degrain, A.; Manhivi, V.; Remize, F.; Garcia, C.; Sivakumar, D. Effect of lactic acid fermentation on color, phenolic compounds and antioxidant activity in African nightshade. Microorg 2020, 8, 1324. [Google Scholar] [CrossRef]
- Managa, M.G.; Akinola, S.A.; Remize, F.; Garcia, C.; Sivakumar, D. Physicochemical parameters and bioaccessibility of lactic acid bacteria fermented chayote Leaf (Sechium edule) and pineapple (Ananas comosus) smoothies. Front. Nutr. 2021, 8, 649189. [Google Scholar] [CrossRef]
- Gao, H.; Wen, J.J.; Hu, J.L.; Nie, Q.X.; Chen, H.H.; Xiong, T.; Ning, S.; Xie, M.Y. Fermented Momordica charantia L. juice modulates hyperglycemia, lipid profile, and gut microbiota in type 2 diabetic rats. Int. Food Res. J. 2019, 121, 367–378. [Google Scholar] [CrossRef]
- Fujita, A.; Sarkar, D.; Genovese, M.I.; Shetty, K. Improving anti-hyperglycemic and anti-hypertensive properties of camu-camu (Myriciaria dubia Mc. Vaugh) using lactic acid bacterial fermentation. Process Biochem. 2017, 59, 133–140. [Google Scholar] [CrossRef]
- Mashitoa, F.M.; Akinola, S.A.; Manhevi, V.E.; Garcia, C.; Remize, F.; Slabbert, R.M.; Sivakumar, D. Influence of Fermentation of Pasteurised Papaya Puree with Different lactic acid Bacterial Strains on Quality and bioaccessibility of phenolic compounds during in vitro digestion. Foods 2021, 10, 962. [Google Scholar] [CrossRef]
- Chiou, Y.S.; Wu, J.C.; Huang, Q.; Shahidi, F.; Wang, Y.J.; Ho, C.T.; Pan, M.H. Metabolic and colonic microbiota transformation may enhance the bioactivities of dietary polyphenols. J. Funct. Food 2014, 7, 3–25. [Google Scholar] [CrossRef]
- Zhao, D.; Shah, N.P. Lactic acid bacterial fermentation modified phenolic composition in tea extracts and enhanced their antioxidant activity and cellular uptake of phenolic compounds following in vitro digestion. J. Funct. Food 2016, 20, 182–194. [Google Scholar] [CrossRef]
- Valero-Cases, E.; Nuncio-Jáuregui, N.; Frutos, M.J. Influence of fermentation with different lactic acid bacteria and in vitro digestion on the biotransformation of phenolic compounds in fermented pomegranate juices. J. Agric. Food Chem. 2017, 65, 6488–6496. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yuan, M.; Wang, Y.; Zhou, Y.; Sun, X. Influence of fermentation with different lactic acid bacteria and in vitro digestion on the change of phenolic compounds in fermented kiwifruit pulps. J. Food. Sci. Technol. 2022, 57, 2670–2679. [Google Scholar] [CrossRef]
- Yang, J.; Ji, Y.; Park, H.; Lee, J.; Park, S.; Yeo, S.; Shin, H.; Holzapfel, W.H. Selection of functional lactic acid bacteria as starter cultures for the fermentation of Korean leek (Allium tuberosum Rottler ex Sprengel.). Int. J. Food Microbiol. 2014, 191, 164–171. [Google Scholar] [CrossRef]
- Reddy, S.R.S.; Karnena, M.K.; Yalakala, S.; Saritha, V. Biological treatability of low total dissolved solids (LTDS) using SBR as a pre-treatment for reverse osmosis. J. Water Resour. Prot. 2020, 12, 135–154. [Google Scholar] [CrossRef]
- Nielsen, S.S. Introduction to food analysis. In Food Analysis; Springer: Berlin/Heidelberg, Germany, 2017; pp. 3–16. [Google Scholar] [CrossRef]
- Sagbo, I.J.; van de Venter, M.; Koekemoer, T.; Bradley, G. In vitro antidiabetic activity and mechanism of action of Brachylaena elliptica (Thunb.) DC. E. Based Complem. Altern. Med. eCAM 2018, 2018, 4170372. [Google Scholar] [CrossRef]
- Chauke, A.M.; Shai, L.J.; Mogale, M.A. Plants today drugs tomorrow: Cordia Grandicalyx A possible future anti-hypoglycaemic? J. Med. Plants By-Prod. 2022, in press. [Google Scholar] [CrossRef]
- Jabłońska-Ryś, E.; Sławińska, A.; Skrzypczak, K.; Goral, K. Dynamics of changes in pH and the contents of free sugars, organic acids and LAB in button mushrooms during controlled lactic fermentation. Foods 2022, 11, 1553. [Google Scholar] [CrossRef]
- Dimitrellou, D.; Kandylis, P.; Kokkinomagoulos, E.; Hatzikamari, M.; Bekatorou, A. Emmer-based beverage fortified with fruit juices. Appl. Sci. 2021, 11, 3116. [Google Scholar] [CrossRef]
- Magwaza, L.S.; Opara, U.L. Analytical methods for determination of sugars and sweetness of horticultural products—A review. Scie. Hortic. 2015, 184, 179–192. [Google Scholar] [CrossRef]
- Soibam, H.; Ayam, V.S.; Chakraborty, I. Preparation, and evaluation of wine from sugarcane and beet juice. Adv. Biol. Res. 2017, 8, 216–219. [Google Scholar]
- Cele, N.P.; Akinola, S.A.; Manhivi, V.E.; Shoko, T.; Remize, F.; Sivakumar, D. Influence of lactic acid bacterium strains on changes in quality, functional compounds and volatile compounds of mango juice from different cultivars during fermentation. Foods 2022, 11, 682. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, R.L.; Mukherjee, S. Effects of fruit juice blending ratios on kinnow juice preservation at ambient storage condition. Afr. J. Food Sci. 2011, 5, 281–286. [Google Scholar]
- Yang, X.; Zhou, J.; Fan, L.; Qin, Z.; Chen, Q.; Zhao, L. Antioxidant properties of a vegetable–fruit beverage fermented with two Lactobacillus plantarum strains. Food Sci. Biotech. 2018, 27, 1719–1726. [Google Scholar] [CrossRef] [PubMed]
- Kaprasob, R.; Kerdchoechuen, O.; Laohakunjit, N.; Sarkar, D.; Shetty, K. Fermentation-based biotransformation of bioactive phenolics and volatile compounds from cashew apple juice by select lactic acid bacteria. Process Biochem. 2017, 59, 141–149. [Google Scholar] [CrossRef]
- Yu, T.; Niu, L.; Iwahashi, H. High-pressure carbon dioxide used for pasteurization in food industry. Food Eng. Rev. 2020, 12, 364–380. [Google Scholar] [CrossRef]
- Znamirowska, A.; Szajnar, K.; Pawlos, M. Probiotic fermented milk with collagen. Dairy 2020, 1, 126–134. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Mousavi Khaneghah, A.; Barba, F.J.; Nemati, Z.; Sohrabi Shokofti, S.; Alizadeh, F. Fermented sweet lemon juice (Citrus limetta) using Lactobacillus plantarum LS5: Chemical composition, antioxidant and antibacterial activities. J. Food Funct. 2017, 38, 409–414. [Google Scholar] [CrossRef]
- Panda, S.K.; Behera, S.K.; Witness Qaku, X.W.; Sekar, S.; Ndinteh, D.T.; Nanjundaswamy, H.M.; Ray, R.C.; Kayitesi, E. Quality enhancement of prickly pears (Opuntia sp.) juice through probiotic fermentation using Lactobacillus fermentum-ATCC 9338. LWT 2017, 75, 453–459. [Google Scholar] [CrossRef]
- Chen, R.; Chen, W.; Chen, H.; Zhang, G.; Chen, W. Comparative evaluation of the antioxidant capacities, organic acids, and volatiles of papaya juices fermented by Lactobacillus acidophilus and Lactobacillus plantarum. J. Food Qual. 2018, 2018, 9490435. [Google Scholar] [CrossRef]
- Eddy, B.P.; Ingram, M. Interactions between ascorbic acid and bacteria. Bacteriol. Rev. 1953, 17, 93–107. [Google Scholar] [CrossRef]
- Abasi Joozdani, F.A.; Taghdir, M. Evaluation of transport mechanism of ascorbic acid through cyclic peptide-based nanotubes: A molecular dynamics study. J. Mol. Liq. 2022, 349, 118136. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, C.J.; Kunz, B. Identification of lactic acid bacteria isolated from kimchi and studies on their suitability for application as starter culture in the production of fermented sausages. Meat Sci. 2006, 72, 437–445. [Google Scholar] [CrossRef]
- Mellican, R.I.; Li, J.; Mehansho, H.; Nielsen, S.S. The role of iron and the factors affecting off-color development of polyphenols. J. Agric. Food Chem. 2003, 51, 2304–2316. [Google Scholar] [CrossRef]
- Takó, M.; Zambrano, C.; Kotogán, A.; Kerekes, E.B.; Papp, T.; Krisch, J.; Vágvölgyi, C. Fermentative and enzyme-assisted production of phenolic antioxidants from plant residues. In Microbial Fermentation and Enzyme Technology; CRC Press: Boca Raton, FL, USA, 2020; pp. 175–193. [Google Scholar]
- Cebeci, A.; Gürakan, C. Properties of potential probiotic Lactobacillus plantarum strains. Food Microbiol. 2003, 20, 511–518. [Google Scholar] [CrossRef]
- Panda, S.H.; Ray, R.C. Lactic acid fermentation of β-carotene rich sweet potato (Ipomoea batatas L.) into lacto-juice. Plant Foods Hum. Nutr. 2007, 62, 65–70. [Google Scholar] [CrossRef]
- Do, T.V.T.; Fan, L. Probiotic viability, qualitative characteristics, and sensory acceptability of vegetable juice mixture fermented with lactobacillus strains. Food Nutr. Sci. 2019, 10, 412–427. [Google Scholar] [CrossRef]
- Bouayed, J.; Hoffmann, L.; Bohn, T. Total phenolics, flavonoids, anthocyanins and antioxidant activity following simulated gastro-intestinal digestion and dialysis of apple varieties: Bioaccessibility and potential uptake. Food Chem. 2011, 128, 14–21. [Google Scholar] [CrossRef]
- Tagliazucchi, D.; Verzelloni, E.; Bertolini, D.; Conte, A. In vitro bio-accessibility and antioxidant activity of grape polyphenols. Food Chem. 2010, 120, 599–606. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and Polyphenolics in foods, beverages and spices: Antioxidant activity and health effects–A review. J. Food. Funct. 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Ketnawa, S.; Reginio, F.C., Jr.; Thuengtung, S.; Ogawa, Y. Changes in bioactive compounds and antioxidant activity of plant-based foods by gastrointestinal digestion: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 4684–4705. [Google Scholar] [CrossRef]
- Klip, A.; McGraw, T.E.; James, D.E. Thirty sweet years of GLUT4. J. Biol. Chem 2019, 294, 11369–11381. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Deguchi, A.; Hara, Y.; Moriwaki, H.; Weinstein, I.B. EGCG inhibits activation of the insulin-like growth factor-1 receptor in human colon cancer cells. Biochem. Biophys. Res. Commun. 2005, 334, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Park, K.S.; Kim, M.J.; Kim, S.K.; Cho, Y.W.; Park, S.W. Type 2 diabetes is associated with low muscle mass in older adults. Geriatr. Gerontol. Int. 2014, 14 (Suppl. 1), 115–121. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Galleano, M.; Verstraeten, S.V.; Oteiza, P.I. Basic biochemical mechanisms behind the health benefits of polyphenols. Mol. Asp. Med. 2010, 31, 435–445. [Google Scholar] [CrossRef]
- Aryaeian, N.; Sedehi, S.K.; Arablou, T. Polyphenols and their effects on diabetes management: A review. Med. J. Islam. Republi. Iran 2017, 31, 134. [Google Scholar] [CrossRef]
- Fraisse, D.; Bred, A.; Felgines, C.; Senejoux, F. Impact of simulated gastrointestinal conditions on Antiglycoxidant and α-glucosidase inhibition capacities of cyanidin-3-O-glucoside. Antioxidants 2021, 10, 1670. [Google Scholar] [CrossRef]
- Les, F.; Arbonés-Mainar, J.M.; Valero, M.S.; López, V. Pomegranate polyphenols and urolithin A inhibit α-glucosidase, dipeptidyl peptidase-4, lipase, triglyceride accumulation and adipogenesis related genes in 3T3-L1 adipocyte-like cells. J. Ethnopharm. 2018, 220, 67–74. [Google Scholar] [CrossRef]
- Rusak, G.; Šola, I.; Vujčić Bok, V. Matcha and Sencha green tea extracts with regard to their phenolics pattern and antioxidant and antidiabetic activity during in vitro digestion. J. Food Sci. Technol. 2021, 58, 3568–3578. [Google Scholar] [CrossRef]
- Alqahtani, M.S.; Alqahtani, A.; Al-Thabit, A.; Roni, M.; Syed, R. Novel lignin nanoparticles for oral drug delivery. J. Mater. Chem. B 2019, 7, 4461–4473. [Google Scholar] [CrossRef]
- Simsek, S.; El, S.N.; Kancabas Kilinc, A.K.; Karakaya, S. Vegetable and fermented vegetable juices containing germinated seeds and sprouts of lentil and cowpea. Food Chem. 2014, 156, 289–295. [Google Scholar] [CrossRef]
- Koh, W.Y.; Uthumporn, U.; Rosma, A.; Irfan, A.R.; Park, Y.H. Optimization of a fermented pumpkin-based beverage to improve Lactobacillus mali survival and α-glucosidase inhibitory activity: A response surface methodology approach. Food Sci. Hum. Wellness 2018, 7, 57–70. [Google Scholar] [CrossRef]
- De Vries, M.C.; Vaughan, E.E.; Kleerebezem, M.; de Vos, W.M. Lactobacillus plantarum—Survival, functional and potential probiotic properties in the human intestinal tract. Int. Dairy J. 2006, 16, 1018–1028. [Google Scholar] [CrossRef]
- Elizaquível, P.; Sánchez, G.; Salvador, A.; Fiszman, S.; Dueñas, M.T.; López, P.; de Palencia, P.F.; Aznar, R. Evaluation of yogurt and various beverages as carriers of lactic acid bacteria producing 2-branched (1, 3)-β-D-glucan. J. Dairy. Sci. 2011, 94, 3271–3278. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Hsieh, Y.M.; Huang, C.C.; Tsai, C.C. Inhibitory effects of probiotic Lactobacillus on the growth of human colonic carcinoma cell line HT-29. Molecules 2017, 22, 107. [Google Scholar] [CrossRef]
- Mesquita, M.C.; dos Santos Leandro, E.; Rodrigues de Alencar, E.; Botelho, R.B.A. Survival of Lactobacillus paracasei subsp. paracasei LBC 81 in fermented beverage from chickpeas and coconut in a static in vitro digestion model. Fermentation 2021, 7, 135. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, J.; Chen, W.; Wang, M.; Du, G.; Chen, J. A combined physiological and proteomic approach to reveal lactic-acid-induced alterations in Lactobacillus casei Zhang and its mutant with enhanced lactic acid tolerance. Appl. Microbiol. Biotech. 2012, 93, 707–722. [Google Scholar] [CrossRef]
| Cowpea Cultivars | Treatment | h | pH | TTA (mg/mL) | TSS (° BRIX) | TS (30 mg/100 g) |
|---|---|---|---|---|---|---|
| VOP 1 | Unfermented | 0 | 6.28 ± 0.21 a | 0.78 ± 0.05 c | 1.30 ± 0.28 c | 1.58 ± 0.16 b |
| VOP 3 | Unfermented | 0 | 6.57 ± 0.44 a | 0.69 ± 0.02 c | 1.35 ± 0.15 c | 1.35 ± 0.11 b |
| VOP 4 | Unfermented | 0 | 6.51 ± 0.12 a | 0.75 ± 0.01 c | 1.43 ± 0.47 c | 1.96 ± 0.23 a |
| VOP 1 | LAB 75 | 2 | 6.18 ± 0.33 a | 1.05 ± 0.04 c | 1.70 ± 0.08 b | 1.01 ± 0.27 b |
| VOP 3 | LAB 75 | 2 | 6.4 ± 0.41 a | 1.11 ± 0.08 c | 1.51 ± 0.11 c | 0.96 ± 0.13 c |
| VOP 4 | LAB 75 | 2 | 6.4 ± 0.31 a | 1.12 ± 0.05 c | 1.53 ± 0.17 a | 1.66 ± 0.27 b |
| VOP 1 | LAB 75 | 24 | 5.66 ± 0.82 b | 1.29 ± 0.08 b | 1.01 ± 0.11 d | 0.62 ± 0.02 c |
| VOP 3 | LAB 75 | 24 | 5.33 ± 0.10 b | 1.50 ± 0.10 b | 1.16 ± 0.05 d | 0.37 ± 0.01 d |
| VOP 4 | LAB 75 | 24 | 5.21 ± 0.61 b | 1.80 ± 0.10 b | 1.00 ± 0.08 d | 0.12 ± 0.01 e |
| VOP 1 | LAB 75 | 48 | 5.15 ± 0.03 c | 1.98 ± 0.10 a | 0.82 ± 0.11 e | 0.33 ± 0.04 d |
| VOP 3 | LAB 75 | 48 | 5.12 ± 0.01 c | 2.07 ± 0.19 a | 0.63 ± 0.15 e | 0.22 ± 0.01 d |
| VOP 4 | LAB 75 | 48 | 5.05 ± 0.02 c | 2.22 ± 0.29 a | 0.61 ± 0.05 e | 0.09 ± 0.01 e |
| LSD * | 0.63 ** | 0.80 ** | 0.37 *** | 0.30 ** |
| Cowpea Cultivar | h | Treatment | AA (mg/100 g) |
|---|---|---|---|
| VOP 1 | 0 | Unfermented | 6.02 ± 0.01 e |
| VOP 3 | 0 | Unfermented | 4.30 ± 0.48 g |
| VOP 4 | 0 | Unfermented | 5.20 ± 0.35 f |
| VOP 1 | 2 | LAB 75 | 6.33 ± 0.50 e |
| VOP 3 | 2 | LAB 75 | 4.32 ± 0.01 g |
| VOP 4 | 2 | LAB 75 | 5.52 ± 0.57 f |
| VOP 1 | 24 | LAB 75 | 16.10 ± 0.01 b |
| VOP 3 | 24 | LAB 75 | 12.38 ± 0.01 d |
| VOP 4 | 24 | LAB 75 | 15.33 ± 5.48 c |
| VOP 1 | 48 | LAB 75 | 17.67 ± 0.48 a |
| VOP 3 | 48 | LAB 75 | 15.67 ± 1.96 c |
| VOP 4 | 48 | LAB 75 | 16.34 ± 0.48 b |
| LSD * | 1.65 *** |
| Accession | h | Treatment | L* | a* | b* | ∆E |
|---|---|---|---|---|---|---|
| VOP 1 | 0 | Unfermented | 17.80 ± 0.24 i | −5.81 ± 0.13 b | 11.66 ± 0.02 c | |
| VOP 3 | 0 | Unfermented | 22.16 ± 0.92 e | −6.25 ± 0.29 a | 12.15 ± 0.10 b | |
| VOP 4 | 0 | Unfermented | 23.97 ± 0.19 e | −6.91 ± 0.32 c | 10.88 ± 0.21 d | |
| VOP 1 | 2 | LAB 75 | 20.33 ± 0.37 b | −5.55 ± 0.06 e | 10.03 ± 0.21 c | 1.03 ± 0.62 d |
| VOP 3 | 2 | LAB 75 | 23.86 ± 0.23 f | −6.09 ± 0.30 a | 12.03 ± 0.35 b | 1.05 ± 0.58 d |
| VOP 4 | 2 | LAB 75 | 25.14 ± 0.05 a | −6.88 ± 0.29 d | 10.11 ± 0.23 d | 1.00 ± 0.10 d |
| VOP 1 | 24 | LAB 75 | 21.90 ± 0.08 g | −4.07 ± 0.18 c | 12.69 ± 0.05 b | 3.83 ± 0.24 b |
| VOP 3 | 24 | LAB 75 | 25.73 ± 0.50 c | −5.98 ± 0.30 c | 10.92 ± 0.26 d | 2.44 ± 0.85 c |
| VOP 4 | 24 | LAB 75 | 27.64 ± 0.22 b | −5.70 ± 0.31 b | 11.78 ± 0.58 b | 5.75 ± 0.65 a |
| VOP 1 | 48 | LAB 75 | 21.70 ± 0.90 g | −4.62 ± 0.23 a | 9.96 ± 0.36 e | 1.32 ± 0.08 d |
| VOP 3 | 48 | LAB 75 | 24.57 ± 0.44 d | −3.68 ± 0.24 a | 12.55 ± 0.68 b | 2.67 ± 0.29 c |
| VOP 4 | 48 | LAB 75 | 27.61 ± 0.14 b | −3.90 ± 0.08 a | 12.88 ± 0.09 a | 3.89 ± 0.48 b |
| LSD * | 1.26 ** | 2.71 *** | 1.31 *** | 0.22 ** |
| Cultivars | h | Treatment | Total Phenols | Loss | FRAP | DPPH | ABTS |
|---|---|---|---|---|---|---|---|
| (mg/100 g DW) | (%) | (mmol TEAC/100 g DW) | (IC50 μg/mL) | (IC50 μg/mL) | |||
| VOP 1 | 0 | Unfermented | 249.80 ± 68.26 a | 171.79 ± 30.25 b | 1.14 ± 0.06 b | 30.41 ± 3.05 a | |
| VOP 3 | 0 | Unfermented | 214.49 ± 62.12 b | 167.32 ± 30.08 b | 1.50 ± 0.05 a | 24.52 ± 2.85 b | |
| VOP 4 | 0 | Unfermented | 205.17 ± 52.32 b | 163.38 ± 40.55 b | 1.38 ± 0.08 a | 15.47 ± 1.25 d | |
| VOP 1 | 2 | LAB 75 | 249.90 ± 61.59 a | 0.04 | 170.54 ± 35.26 b | 1.15 ± 0.02 b | 20.20 ± 2.56 c |
| VOP 3 | 2 | LAB 75 | 211.78 ± 58.12 b | 1.26 | 160.54 ± 52.32 b | 1.48 ± 0.05 a | 2.96 ± 0.59 g |
| VOP 4 | 2 | LAB 75 | 205.45 ± 45.28 b | 0.13 | 160.25 ± 45.36 b | 1.39 ± 0.05 a | 10.95 ± 2.45 f |
| VOP 1 | 24 | LAB 75 | 223.97 ± 43.92 b | 10.34 | 315.59 ± 45.13 a | 1.03 ± 0.08 b | 13.78 ± 4.25 e |
| VOP 3 | 24 | LAB 75 | 201.94 ± 64.88 b | 5.85 | 165.59 ± 33.32 b | 1.20 ± 0.04 b | 10.33 ± 1.56 f |
| VOP 4 | 24 | LAB 75 | 183.29 ± 45.97 c | 10.66 | 124.00 ± 24.25 c | 0.87 ± 0.01 c | 9.89 ± 1.28 f |
| VOP 1 | 48 | LAB 75 | 222.03 ± 34.98 b | 11.11 | 300.41 ± 45.05 a | 0.07 ± 0.01 e | 0.53 ± 0.01 i |
| VOP 3 | 48 | LAB 75 | 172.23 ± 48.10 c | 19.70 | 81.61 ± 14.24 d | 0.44 ± 0.01 d | 1.69 ± 0.21 h |
| VOP 4 | 48 | LAB 75 | 173.87 ± 43.80 c | 15.25 | 83.69 ± 9.05 d | 0.39 ± 0.01 d | 2.80 ± 0.18 g |
| LSD * | 25.89 *** | 41.88 ** | 0.18 * | 4.20 *** |
| Cultivars | Treatment | Hours | Lutein | Zeaxanthin | α-Carotene | 9-cis-β-Carotene | All-Trans β-Carotene | Total (Carotenoids) |
|---|---|---|---|---|---|---|---|---|
| VOP 1 | Unfermented | 0 | 99.88 ± 12.36 a | 2.19 ± 0.15 a | 4.63 ± 1.05 a | 3.76 ± 0.78 a | 38.27 ± 4.25 a | 148.71 ± 40.01 a |
| VOP 3 | Unfermented | 0 | 85.04 ± 9.28 b | 2.22 ± 0.25 a | 4.55 ± 1.85 a | 3.64 ± 0.65 a | 27.84 ± 3.95 b | 123.29 ± 23.58 b |
| VOP 4 | Unfermented | 0 | 70.38 ± 7.68 c | 2.29 ± 0.29 a | 5.07 ± 1.90 a | 3.12 ± 0.72 ab | 26.23 ± 2.86 b | 107.09 ± 19.08 c |
| VOP 1 | LAB 75 | 24 | 66.46 ± 8.01 d | 2.17 ± 0.26 a | 3.03 ± 1.02 b | 2.96 ± 0.52 b | 21.75 ± 3.58 b | 96.39 ± 14.02 d |
| VOP 3 | LAB 75 | 24 | 52.32 ± 4.32 e | 2.12 ± 0.30 a | 2.21 ± 1.26 c | 2.48 ± 0.48 b | 12.43 ± 2.96 c | 71.56 ± 10.58 e |
| VOP 4 | LAB 75 | 24 | 41.50 ± 5.09 e | 2.04 ± 0.21 a | 3.13 ± 0.98 b | 2.23 ± 0.59 b | 10.48 ± 2.54 c | 59.38 ± 8.36 f |
| VOP 1 | LAB 75 | 48 | 57.23 ± 4.18 e | 1.64 ± 0.15 b | 2.19 ± 0.19 b | 2.66 ± 0.60 b | 19.47 ± 1.95 b | 82.91 ± 11.25 e |
| VOP 3 | LAB 75 | 48 | 45.27 ± 5.00 e | 1.84 ± 0.17 b | 1.53 ± 0.20 c | 2.24 ± 0.54 b | 10.44 ± 2.47 c | 61.32 ± 8.96 f |
| VOP 4 | LAB 75 | 48 | 37.66 ± 4.89 f | 1.90 ± 0.18 b | 2.45 ± 1.28 b | 1.80 ± 0.48 c | 9.75 ± 1.05 c | 54.2 ± 7.49 f |
| LSD * | 14.28 ** | 0.25 * | 1.35 ** | 0.44 ** | 9.80 *** | 10.20 ** |
| Total Phenols | Bioaccessibility | FRAP | DPPH | ABTS | |
|---|---|---|---|---|---|
| (mg/100 g DW) | % | (μmol TEAC/100 g) | (IC50 μg/mL) | (IC50 μg/mL) | |
| Undigested | 223.97 ± 43.92 b | 320.78 ± 39.14 b | 1.03 ± 0.08 c | 30.78 ± 4.25 c | |
| Gastric | 192.78 ± 35.68 c | 86.07 ± 11.86 b | 304.89 ± 48.21 c | 5.49 ± 0.44 b | 32.28 ± 11.48 b |
| Intestinal | 335.25 ± 65.32 a | 149.57 ± 28.21 a | 345.46 ± 36.76 a | 0.94 ± 0.01 c | 31.09 ± 9.23 c |
| Dialysis | 68.70 ± 5.90 d | 30.68 ± 1.67 c | 156.13 ± 24.92 d | 16.97 ± 3.85 a | 46.69 ± 10.58 a |
| LSD * | 30.87 *** | 28.58 *** | 15.68 *** | 4.95 ** | 1.25 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moloto, M.R.; Akinola, S.A.; Seke, F.; Shoko, T.; Sultanbawa, Y.; Shai, J.L.; Remize, F.; Sivakumar, D. Influence of Fermentation on Functional Properties and Bioactivities of Different Cowpea Leaf Smoothies during In Vitro Digestion. Foods 2023, 12, 1701. https://doi.org/10.3390/foods12081701
Moloto MR, Akinola SA, Seke F, Shoko T, Sultanbawa Y, Shai JL, Remize F, Sivakumar D. Influence of Fermentation on Functional Properties and Bioactivities of Different Cowpea Leaf Smoothies during In Vitro Digestion. Foods. 2023; 12(8):1701. https://doi.org/10.3390/foods12081701
Chicago/Turabian StyleMoloto, Mapula R., Stephen A. Akinola, Faith Seke, Tinotenda Shoko, Yasmina Sultanbawa, Jerry L. Shai, Fabienne Remize, and Dharini Sivakumar. 2023. "Influence of Fermentation on Functional Properties and Bioactivities of Different Cowpea Leaf Smoothies during In Vitro Digestion" Foods 12, no. 8: 1701. https://doi.org/10.3390/foods12081701
APA StyleMoloto, M. R., Akinola, S. A., Seke, F., Shoko, T., Sultanbawa, Y., Shai, J. L., Remize, F., & Sivakumar, D. (2023). Influence of Fermentation on Functional Properties and Bioactivities of Different Cowpea Leaf Smoothies during In Vitro Digestion. Foods, 12(8), 1701. https://doi.org/10.3390/foods12081701








