Preparation, Multispectroscopic Characterization, and Stability Analysis of Monascus Red Pigments—Whey Protein Isolate Complex
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of WPH
2.3. Surface Hydrophobicity
2.4. Maximum Binding Constants
2.5. Maximum Binding Capacity
2.6. Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis (SDS–PAGE)
2.7. Structural Characterization of the Complexes
2.7.1. Preparation of the Complexes
2.7.2. Fourier Transform Infrared (FTIR) Spectroscopy
2.7.3. UV–Vis Absorption Spectrum
2.7.4. Circular Dichroism (CD) Spectrum
2.7.5. Scanning Electron Microscopy (SEM)
2.8. Stability Evaluation and Mechanism
2.8.1. Thermal Stability
2.8.2. Light Stability
2.8.3. pH Stability
2.8.4. Storage Stability
2.8.5. Oxidant and Reducing Agent Stability
2.8.6. Stability under Simulated Gastric and Intestinal Fluid Conditions
2.9. Statistical Analysis
3. Results and Discussion
3.1. Surface Hydrophobicity
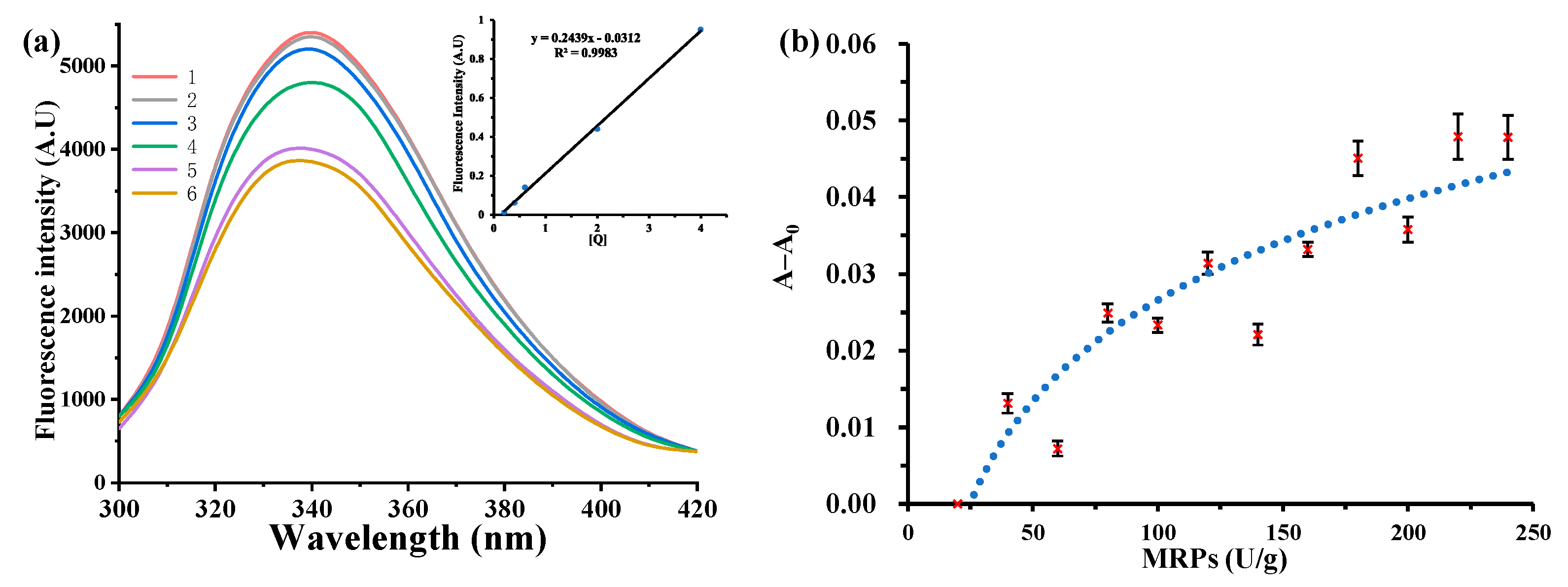
3.2. Maximum Binding Constants
3.3. Maximum Binding Capacity
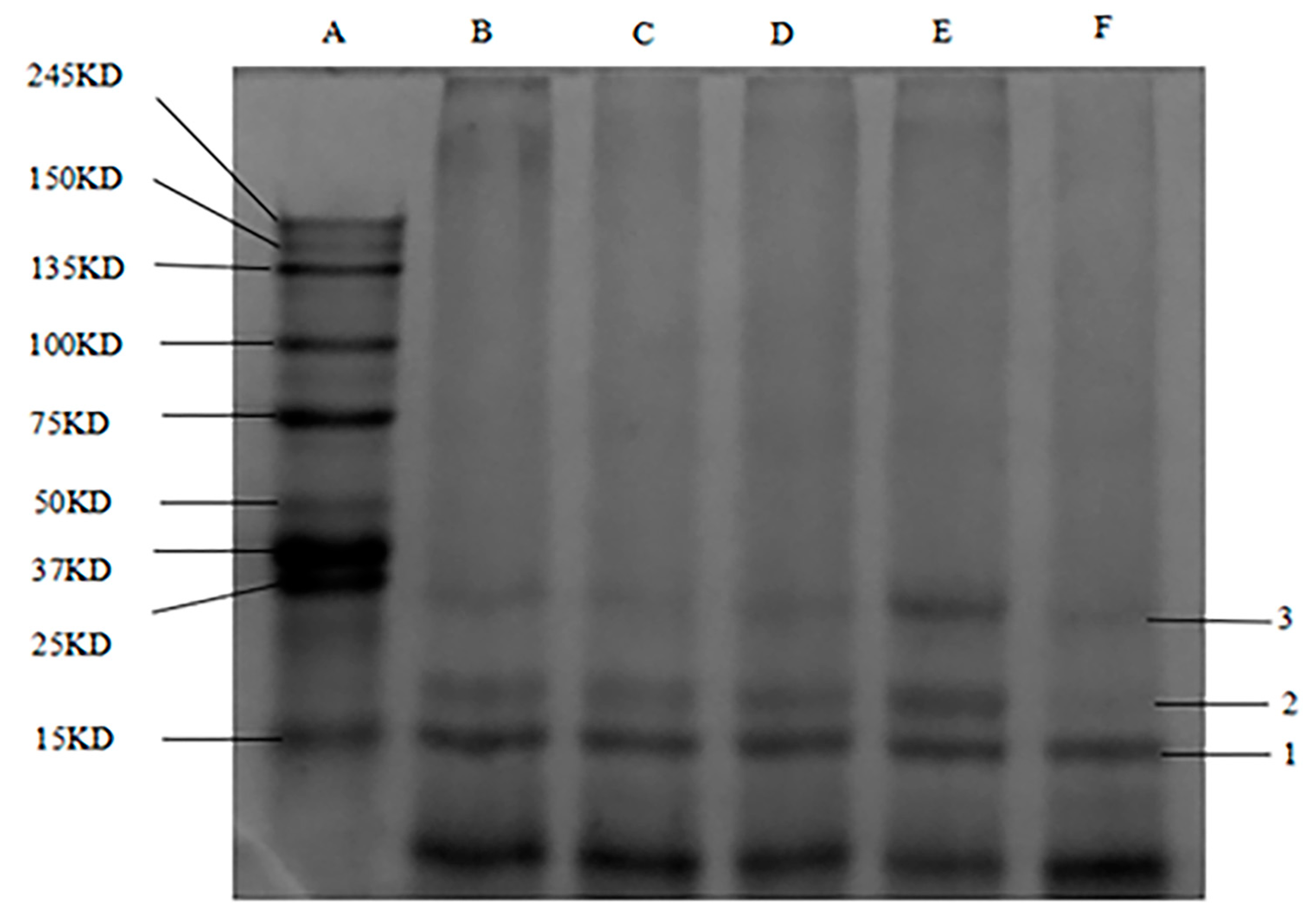
3.4. SDS–PAGE
3.5. Structural Characterization of WPI (DH = 0.5%) and Complex (DH = 0.5%)
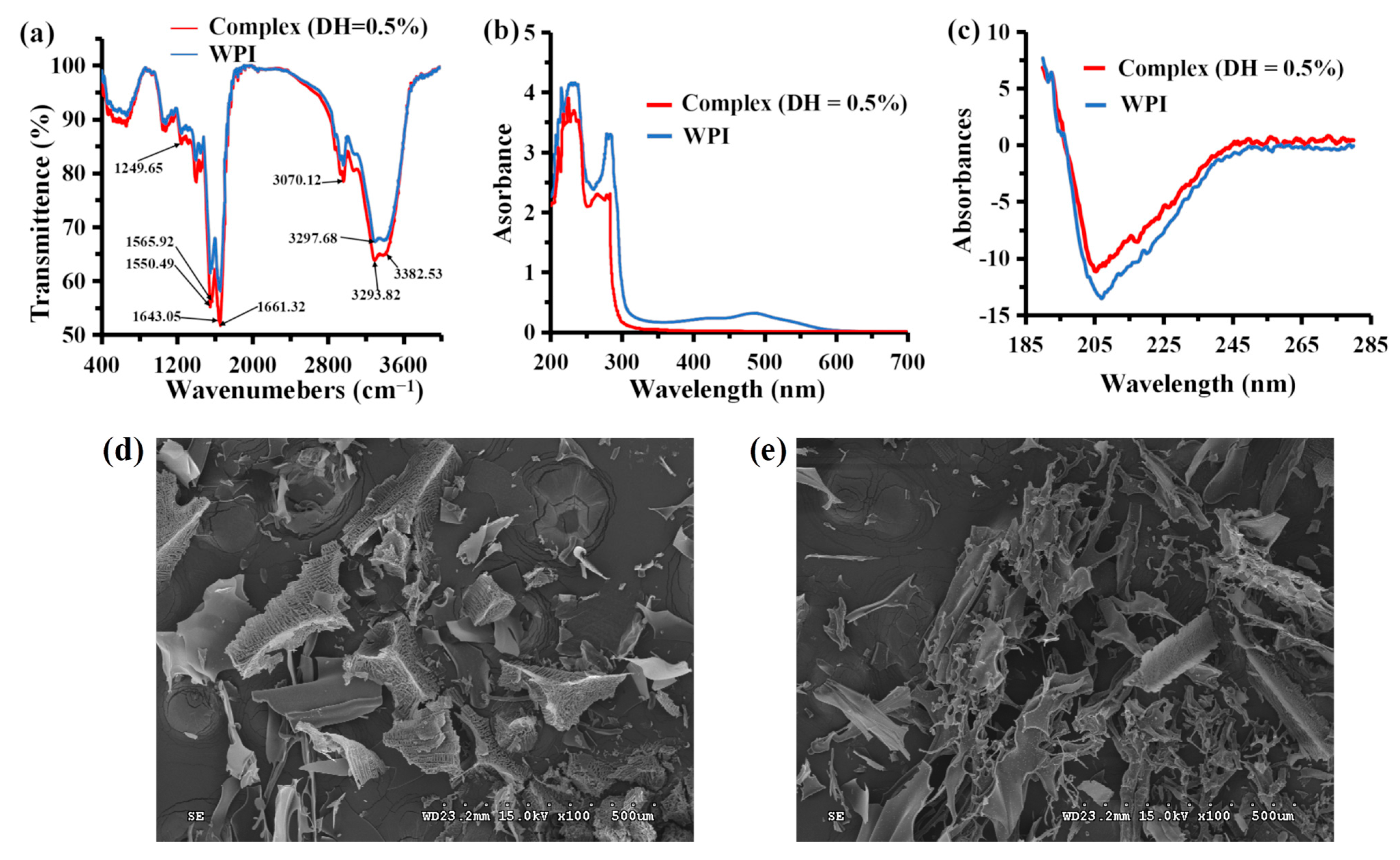
3.5.1. FTIR Spectroscopy
3.5.2. UV–Vis Spectroscopy
3.5.3. CD Spectroscopy
3.5.4. Scanning Electron Microscopy (SEM)
3.6. Stability of Complex (DH = 0.0%), Complex (DH = 0.5%), and MRPs
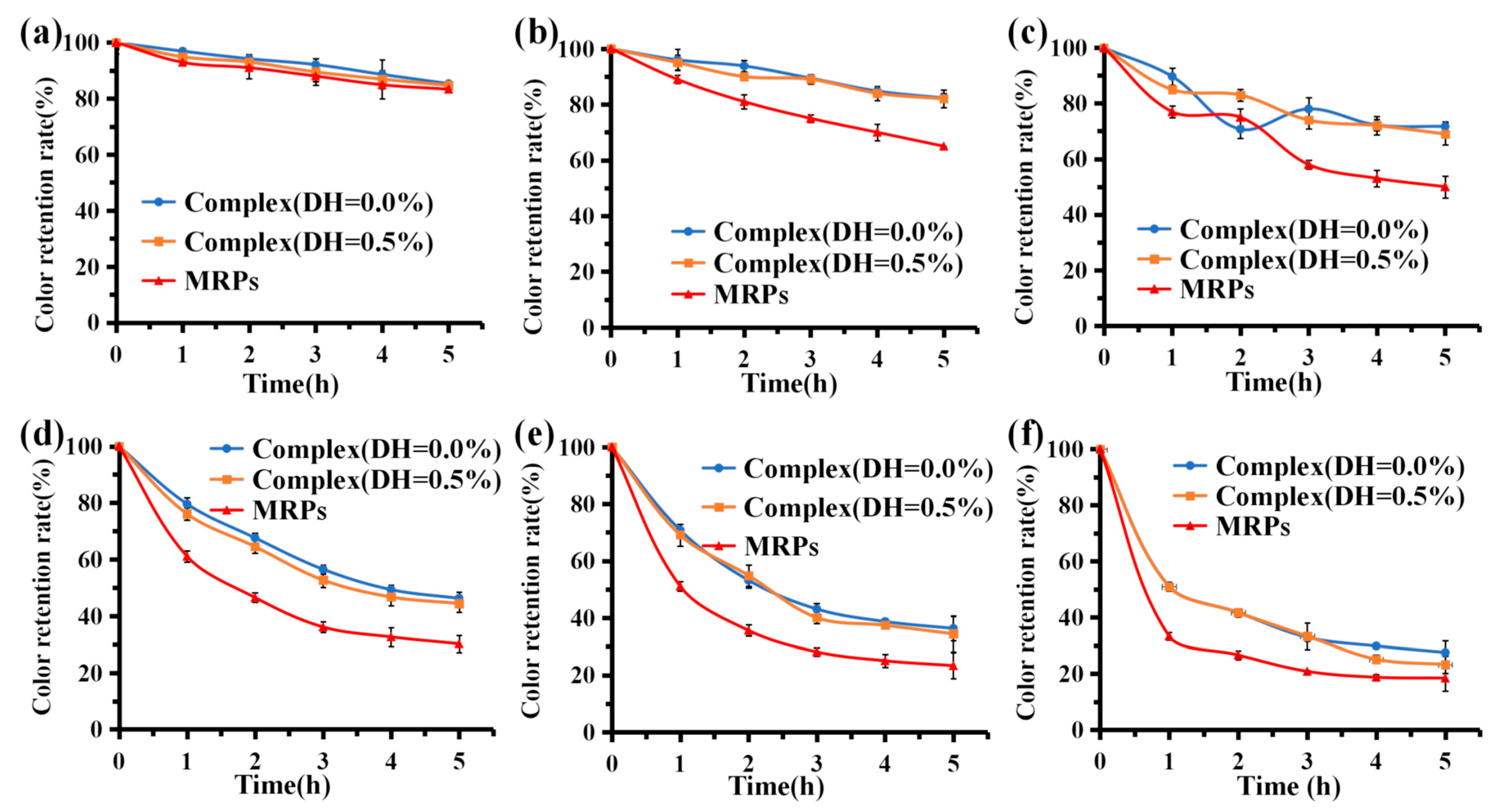
3.6.1. Temperature Stability
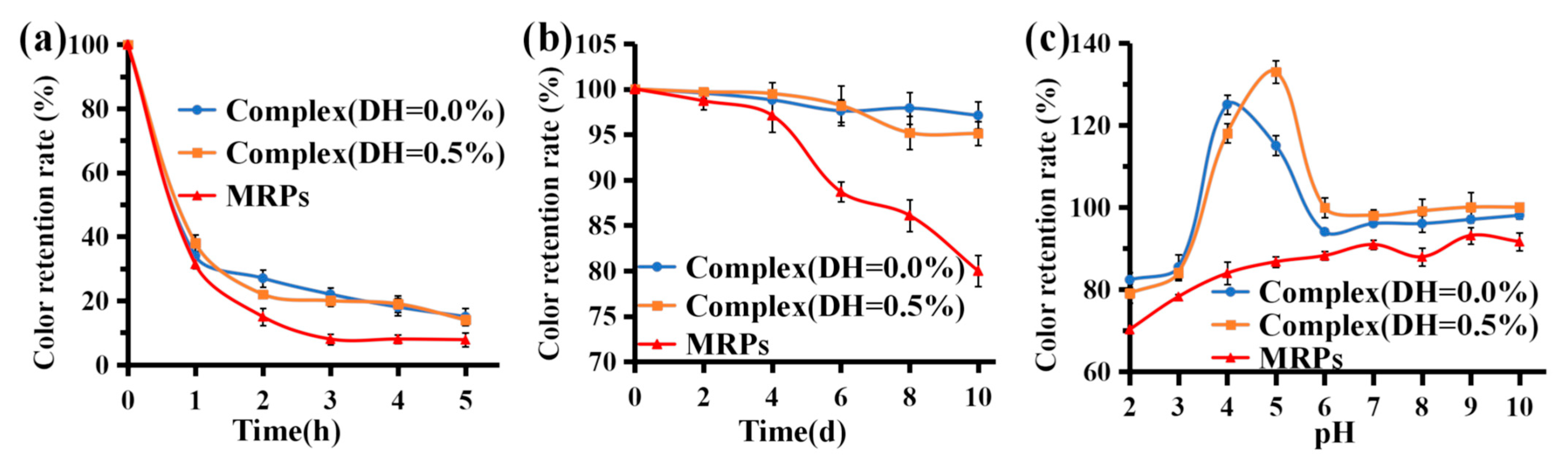
3.6.2. Light Stability
3.6.3. Storage Stability
3.6.4. pH Stability
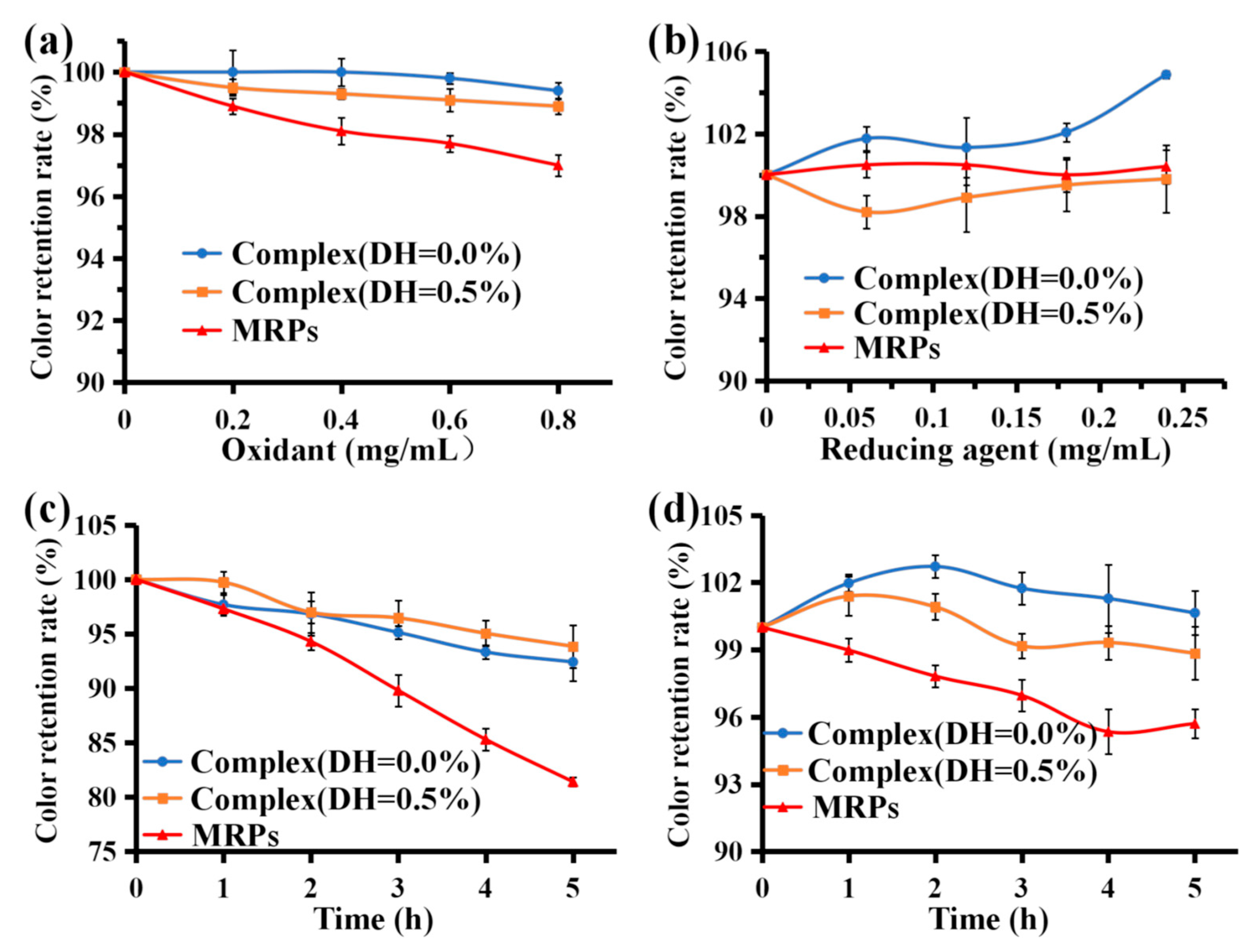
3.6.5. Stability of Oxidizing and Reducing Agents
3.6.6. Stability under Artificial Gastric and Intestinal Fluid Conditions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, S.; Wang, X.; Bao, Y.; Zhang, C.; Liu, H.; Li, Z.; Chen, M.; Wang, C.; Guo, Q.; Peng, X. Molecular insight on the binding of monascin to bovine serum albumin (BSA) and its effect on antioxidant characteristics of monascin. Food Chem. 2020, 315, 126228. [Google Scholar] [CrossRef]
- Bai, J.; Gong, Z.; Shu, M.; Zhao, H.; Ye, F.; Tang, C.; Zhang, S.; Zhou, B.; Lu, D.; Zhou, X.; et al. Increased Water-Soluble Yellow Monascus Pigment Productivity via Dual Mutagenesis and Submerged Repeated-Batch Fermentation of Monascus purpureus. Front. Microbiol. 2022, 13, 914828. [Google Scholar] [CrossRef]
- Abdollahi, F.; Jahadi, M.; Ghavami, M. Thermal stability of natural pigments produced by Monascus purpureus in submerged fermentation. Food Sci. Nutr. 2021, 9, 4855–4862. [Google Scholar] [CrossRef]
- Jian, W.; Sun, Y.; Wu, J.Y. Improving the water solubility of Monascus pigments under acidic conditions with gum arabic. J. Sci. Food Agric. 2017, 97, 2926–2933. [Google Scholar] [CrossRef]
- Chen, W.; Chen, R.; Liu, Q.; He, Y.; He, K.; Ding, X.; Kang, L.; Guo, X.; Xie, N.; Zhou, Y.; et al. Orange, red, yellow: Biosynthesis of azaphilone pigments in Monascus fungi. Chem. Sci. 2017, 8, 4917–4925. [Google Scholar] [CrossRef]
- Agboyibor, C.; Kong, W.-B.; Chen, D.; Zhang, A.-M.; Niu, S.-Q. Monascus pigments production, composition, bioactivity and its application: A review. Biocatal. Agric. Biotechnol. 2018, 16, 433–447. [Google Scholar] [CrossRef]
- Adin, S.N.; Gupta, I.; Panda, B.P.; Mujeeb, M. Monascin and ankaflavin-Biosynthesis from Monascus purpureus, production methods, pharmacological properties: A review. Biotechnol. Appl. Biochem. 2023, 70, 137–147. [Google Scholar] [CrossRef]
- Li, W.L.; Hong, J.L.; Lu, J.Q.; Tong, S.G.; Ni, L.; Liu, B.; Lv, X.C. Comparative Transcriptomic and Metabolomic Analyses Reveal the Regulatory Effect and Mechanism of Tea Extracts on the Biosynthesis of Monascus Pigments. Foods 2022, 11, 3159. [Google Scholar] [CrossRef]
- Feng, S.-S.; Li, W.; Hu, Y.-J.; Feng, J.-X.; Deng, J. The biological activity and application of Monascus pigments: A mini review. Int. J. Food Eng. 2022, 18, 253–266. [Google Scholar] [CrossRef]
- Adjonu, R.; Doran, G.; Torley, P.; Agboola, S. Formation of whey protein isolate hydrolysate stabilised nanoemulsion. Food Hydrocoll. 2014, 41, 169–177. [Google Scholar] [CrossRef]
- Bryant, C.M.; McClements, D.J. Influence of xanthan gum on physical characteristics of heat-denatured whey protein solutions and gels. Food Hydrocoll. 2000, 14, 383–390. [Google Scholar] [CrossRef]
- Larki, M.; Enayati, M.H.; Rostamabadi, H. Basil seed gum promotes the electrospinnability of WPI for co-encapsulation of ZnO nanoparticles and curcumin. Carbohydr. Polym. 2022, 296, 119966. [Google Scholar] [CrossRef]
- Chanet, A.; Verlaan, S.; Salles, J.; Giraudet, C.; Patrac, V.; Pidou, V.; Pouyet, C.; Hafnaoui, N.; Blot, A.; Cano, N.; et al. Supplementing Breakfast with a Vitamin D and Leucine-Enriched Whey Protein Medical Nutrition Drink Enhances Postprandial Muscle Protein Synthesis and Muscle Mass in Healthy Older Men. J. Nutr. 2017, 147, 2262–2271. [Google Scholar] [CrossRef] [PubMed]
- Martin-Rincon, M.; Perez-Suarez, I.; Pérez-López, A.; Ponce-González, J.G.; Morales-Alamo, D.; De Pablos-Velasco, P.; Holmberg, H.-C.; Calbet, J.A.L. Protein synthesis signaling in skeletal muscle is refractory to whey protein ingestion during a severe energy deficit evoked by prolonged exercise and caloric restriction. Int. J. Obes. 2019, 43, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-Q.; Zhang, L.-W.; Han, X.; Lu, Y. Cheese whey protein recovery by ultrafiltration through transglutaminase (TG) catalysis whey protein cross-linking. Food Chem. 2017, 215, 31–40. [Google Scholar] [CrossRef]
- Evans, J.; Zulewska, J.; Newbold, M.; Drake, M.A.; Barbano, D.M. Comparison of composition and sensory properties of 80% whey protein and milk serum protein concentrates1. J. Dairy Sci. 2010, 93, 1824–1843. [Google Scholar] [CrossRef]
- Yousefi, N.; Abbasi, S.; Azarikia, F.; Azizi, M.-H. Solubilization of concentrated protein dispersion: Effect of hydrogen peroxide (H2O2) and sodium hexametaphosphate (SHMP). Food Chem. 2023, 400, 133980. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, Z. Interaction between lactoferrin and whey proteins and its influence on the heat-induced gelation of whey proteins. Food Chem. 2018, 252, 92–98. [Google Scholar] [CrossRef]
- Wang, C.; Li, T.; Ma, L.; Li, T.; Yu, H.; Hou, J.; Jiang, Z. Consequences of superfine grinding treatment on structure, physicochemical and rheological properties of transglutaminase-crosslinked whey protein isolate. Food Chem. 2020, 309, 125757. [Google Scholar] [CrossRef]
- Chen, L.; Ma, R.R.; Zhang, Z.P.; Huang, M.G.; Cai, C.X.; Zhang, R.J.; McClements, D.J.; Tian, Y.Q.; Jin, Z.Y. Comprehensive investigation and comparison of surface microstructure of fractionated potato starches. Food Hydrocoll. 2019, 89, 11–19. [Google Scholar] [CrossRef]
- Zang, X.; Yue, C.; Wang, Y.; Shao, M.; Yu, G. Effect of limited enzymatic hydrolysis on the structure and emulsifying properties of rice bran protein. J. Cereal Sci. 2019, 85, 168–174. [Google Scholar] [CrossRef]
- Pan, Y.; Xie, Q.T.; Zhu, J.; Li, X.M.; Meng, R.; Zhang, B.; Chen, H.Q.; Jin, Z.Y. Study on the fabrication and in vitro digestion behavior of curcumin-loaded emulsions stabilized by succinylated whey protein hydrolysates. Food Chem. 2019, 287, 76–84. [Google Scholar] [CrossRef]
- Li, T.; Wang, C.; Li, T.; Ma, L.; Sun, D.; Hou, J.; Jiang, Z. Surface Hydrophobicity and Functional Properties of Citric Acid Cross-Linked Whey Protein Isolate: The Impact of pH and Concentration of Citric Acid. Molecules 2018, 23, 2383. [Google Scholar] [CrossRef]
- Asemi-Esfahani, Z.; Shareghi, B.; Farhadian, S.; Momeni, L. Food additive dye–lysozyme complexation: Determination of binding constants and binding sites by fluorescence spectroscopy and modeling methods. J. Mol. Liq. 2022, 363, 119749. [Google Scholar] [CrossRef]
- Yang, X.-Q.; Yan, J.-Y.; Hou, J.-J. Effect of Enzymatic Modification on Binding Capacity between Pigment and Glycinin. Mod. Food Sci. Technol. 2013, 29, 2566–2571. [Google Scholar] [CrossRef]
- Sarkar, T.; Bharadwaj, K.K.; Salauddin, M.; Pati, S.; Chakraborty, R. Phytochemical Characterization, Antioxidant, Anti-inflammatory, Anti-diabetic properties, Molecular Docking, Pharmacokinetic Profiling, and Network Pharmacology Analysis of the Major Phytoconstituents of Raw and Differently Dried Mangifera indica (Himsagar cultivar): An In Vitro and In Silico Investigations. Appl. Biochem. Biotechnol. 2022, 194, 950–987. [Google Scholar] [CrossRef]
- Lianguzov, A.Y.; Malygina, N.M.; Ivanov, A.M.; Petrova, T.A. On particular Regimens of Derivative UV Spectrophotometry for Comparative Analysis of Proteins. Appl. Biochem. Microbiol. 2023, 58, S153–S159. [Google Scholar] [CrossRef]
- Allahdad, Z.; Varidi, M.; Zadmard, R.; Saboury, A.A. Spectroscopic and docking studies on the interaction between caseins and β-carotene. Food Chem. 2018, 255, 187–196. [Google Scholar] [CrossRef]
- Hatziantoniou, S.; Deli, G.; Nikas, Y.; Demetzos, C.; Papaioannou, G.T. Scanning electron microscopy study on nanoemulsions and solid lipid nanoparticles containing high amounts of ceramides. Micron 2007, 38, 819–823. [Google Scholar] [CrossRef]
- Li, K.; Guo, Z.; Li, H.; Ren, X.; Sun, C.; Feng, Q.; Kou, S.; Li, Q. Nanoemulsion containing Yellow Monascus pigment: Fabrication, characterization, storage stability, and lipase hydrolytic activity in vitro digestion. Colloids Surf. B Biointerfaces 2023, 224, 113199. [Google Scholar] [CrossRef]
- Vendruscolo, F.; Luise Müller, B.; Esteves Moritz, D.; de Oliveira, D.; Schmidell, W.; Luiz Ninow, J. Thermal stability of natural pigments produced by Monascus ruber in submerged fermentation. Biocatal. Agric. Biotechnol. 2013, 2, 278–284. [Google Scholar] [CrossRef]
- Long, P.; Zhu, L.; Lai, H.; Xu, S.; Dong, X.; Shao, Y.; Wang, L.; Cheng, S.; Liu, G.; He, J.; et al. Monascus Red Pigment Liposomes: Microstructural Characteristics, Stability, and Anticancer Activity. Foods 2023, 12, 447. [Google Scholar] [CrossRef]
- Kayın, N.; Atalay, D.; Türken Akçay, T.; Erge, H.S. Color stability and change in bioactive compounds of red beet juice concentrate stored at different temperatures. J. Food Sci. Technol. 2019, 56, 5097–5106. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Zhang, J.; Cheng, L.; Liu, X.; Jiang, Y.; Zhang, T. Isolation and characterization of melanin from palm fiber. J. Nat. Fibers 2019, 16, 755–767. [Google Scholar] [CrossRef]
- Mun, S.; Kim, J.; McClements, D.J.; Kim, Y.R.; Choi, Y. Fluorescence imaging of spatial location of lipids and proteins during digestion of protein-stabilized oil-in-water emulsions: A simulated gastrointestinal tract study. Food Chem. 2017, 219, 297–303. [Google Scholar] [CrossRef]
- Farup, J.; Rahbek, S.K.; Storm, A.C.; Klitgaard, S.; Jørgensen, H.; Bibby, B.M.; Serena, A.; Vissing, K. Effect of degree of hydrolysis of whey protein on in vivo plasma amino acid appearance in humans. Springerplus 2016, 5, 382. [Google Scholar] [CrossRef]
- Panyam, D.; Kilara, A. Enhancing the functionality of food proteins by enzymatic modification. Trends Food Sci. Technol. 1996, 7, 120–125. [Google Scholar] [CrossRef]
- Huang, L.; Liu, Q.; Xia, X.; Kong, B.; Xiong, Y.L. Oxidative changes and weakened gelling ability of salt-extracted protein are responsible for textural losses in dumpling meat fillings during frozen storage. Food Chem. 2015, 185, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-Q.; Yuan, P.-P.; Zhou, J.-Y.; Gu, Z.-H. Effect of temperature and pH on the gelation, rheology, texture, and structural properties of whey protein and sugar gels based on Maillard reaction. J. Food Sci. 2021, 86, 1228–1242. [Google Scholar] [CrossRef]
- Dudarev, A.N.; Gorodetskay, A.Y.; Tkachenko, T.A.; Usynin, I.F. Effects of Cortisol and Tetrahydrocortisol on the Secondary Structure of Apolipoprotein A-I as Measured by Fourier Transform Infrared Spectroscopy. Russ. J. Bioorg. Chem. 2022, 48, 96–104. [Google Scholar] [CrossRef]
- He, J.W.; Wu, D.; Zhai, Y.M.; Wang, Q.; Ma, X.L.; Yang, H.Q.; Li, H. Interaction of inosine with human serum albumin as determined by NMR relaxation data and fluorescence methodology. J. Mol. Liq. 2016, 219, 547–553. [Google Scholar] [CrossRef]
- Chanphai, P.; Vesper, A.R.; Bariyanga, J.; Bérubé, G.; Tajmir-Riahi, H.A. Review on the delivery of steroids by carrier proteins. J. Photochem. Photobiol. B Biol. 2016, 161, 184–191. [Google Scholar] [CrossRef]
- Ali, I.; Al-Dalali, S.; Hao, J.; Ikram, A.; Zhang, J.; Xu, D.; Cao, Y. The stabilization of Monascus pigment by formation of Monascus pigment-sodium caseinate complex. Food Chem. 2022, 384, 132480. [Google Scholar] [CrossRef] [PubMed]
- Louhasakul, Y.; Wado, H.; Lateh, R.; Cheirsilp, B. Solid-state fermentation of Saba banana peel for pigment production by Monascus purpureus. Braz. J. Microbiol. 2022, 54, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Rovers, T.A.M.; Sala, G.; van der Linden, E.; Meinders, M.B.J. Temperature is key to yield and stability of BSA stabilized microbubbles. Food Hydrocoll. 2016, 52, 106–115. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, M.; Hurren, C.; Zhou, L.; Wu, J.; Sun, L. Study on photofading of two natural dyes sodium copper chlorophyllin and gardenia yellow on cotton. Cellulose 2020, 27, 431–439. [Google Scholar] [CrossRef]
- Khan, A.Z.; Jackson, C.J.; Utheim, T.P.; Reppe, S.; Sapkota, D.; Olstad, O.K.; Thiede, B.; Eidet, J.R. Sericin-Induced Melanogenesis in Cultured Retinal Pigment Epithelial Cells Is Associated with Elevated Levels of Hydrogen Peroxide and Inflammatory Proteins. Molecules 2020, 25, 4395. [Google Scholar] [CrossRef]
- Berardi, A.; Castells-Graells, R.; Lomonossoff, G.P. High stability of plant-expressed virus-like particles of an insect virus in artificial gastric and intestinal fluids. Eur. J. Pharm. Biopharm. 2020, 155, 103–111. [Google Scholar] [CrossRef]
| Samples | PSH | R2 |
|---|---|---|
| WPI (DH 0.0%) | 2344.03 ± 159.50 a | 0.9932 |
| WPI (DH 0.5%) | 2370.00 ± 65.04 a | 0.9920 |
| WPI (DH 1.0%) | 2474.13 ± 234.66 a | 0.9931 |
| WPI (DH 1.38%) | 2592.18 ± 243.67 a | 0.9941 |
| Samples | KA/U−1 | R2 | n |
|---|---|---|---|
| WPI (DH 0.00%) | 0.445 ± 0.02 b | 0.9938 | 0.88 ± 0.05 |
| WPI (DH 0.50%) | 0.670 ± 0.06 a | 0.9821 | 1.02 ± 0.26 |
| WPI (DH 1.00%) | 0.590 ± 0.08 a | 0.9902 | 0.95 ± 0.06 |
| WPI (DH 1.38%) | 0.472 ± 0.01 b | 0.9870 | 0.85 ± 0.08 |
| Treatment | Samples | k (h−1) | t1/2 (h) | R2 |
|---|---|---|---|---|
| 50 °C | complex (DH = 0.0%) | 0.031 ± 0.007 a | 22.22 ± 1.68 a | 0.9928 |
| complex (DH = 0.5%) | 0.032 ± 0.005 a | 21.39 ± 0.90 a | 0.9865 | |
| MRPs | 0.035 ± 0.018 a | 19.64 ± 1.10 a | 0.9576 | |
| 60 °C | complex (DH = 0.0%) | 0.040 ± 0.007 b | 17.42 ± 0.47 a | 0.9901 |
| complex (DH = 0.5%) | 0.039 ± 0.007 b | 17.68 ± 0.49 a | 0.9772 | |
| MRPs | 0.084 ± 0.004 a | 8.22 ± 0.21 b | 0.9895 | |
| 70 °C | complex (DH = 0.0%) | 0.068 ± 0.006 b | 10.18 ± 0.31 a | 0.9665 |
| complex (DH = 0.5%) | 0.073 ± 0.009 b | 9.50 ± 0.77 a | 0.9820 | |
| MRPs | 0.147 ± 0.013 a | 4.72 ± 0.05 b | 0.9862 | |
| 80 °C | complex (DH = 0.0%) | 0.157 ± 0.011 b | 4.43 ± 0.16 a | 0.9751 |
| complex (DH = 0.5%) | 0.164 ± 0.010 b | 4.24 ± 0.23 a | 0.9566 | |
| MRPs | 0.248 ± 0.007 a | 2.79 ± 0.24 b | 0.9850 | |
| 90 °C | complex (DH = 0.0%) | 0.199 ± 0.016 b | 3.48 ± 0.11 a | 0.9822 |
| complex (DH = 0.5%) | 0.211 ± 0.021 b | 3.28 ± 0.16 a | 0.9722 | |
| MRPs | 0.279 ± 0.014 a | 2.49 ± 0.16 b | 0.9709 | |
| 100 °C | complex (DH = 0.0%) | 0.322 ± 0.035 a | 2.15 ± 0.21 a | 0.9648 |
| complex (DH = 0.5%) | 0.323 ± 0.024 a | 2.14 ± 0.26 a | 0.9657 | |
| MRPs | 0.380 ± 0.028 a | 1.82 ± 0.27 a | 0.9543 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, H.; Wang, J.; Liao, S.; Liu, G.; Wang, L.; He, Y.; Gao, C. Preparation, Multispectroscopic Characterization, and Stability Analysis of Monascus Red Pigments—Whey Protein Isolate Complex. Foods 2023, 12, 1745. https://doi.org/10.3390/foods12091745
Lai H, Wang J, Liao S, Liu G, Wang L, He Y, Gao C. Preparation, Multispectroscopic Characterization, and Stability Analysis of Monascus Red Pigments—Whey Protein Isolate Complex. Foods. 2023; 12(9):1745. https://doi.org/10.3390/foods12091745
Chicago/Turabian StyleLai, Huafa, Jiahao Wang, Shengjia Liao, Gang Liu, Liling Wang, Yi He, and Chao Gao. 2023. "Preparation, Multispectroscopic Characterization, and Stability Analysis of Monascus Red Pigments—Whey Protein Isolate Complex" Foods 12, no. 9: 1745. https://doi.org/10.3390/foods12091745





