The Stability of Phenolic Compounds in Fruit, Berry, and Vegetable Purees Based on Accelerated Shelf-Life Testing Methodology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Purees and Packaging
2.2. Reagents and Standards
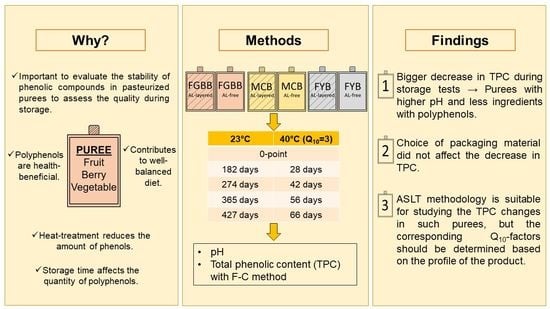
2.3. Design of Shelf-Life Tests
2.4. pH Analysis
2.5. Analysis of Total Phenolic Content
2.6. Statistical Analysis
3. Results and Discussion
3.1. pH
3.2. Effect of Packaging Material on the Changes in TPC
3.3. Comparison of Changes in TPC of Three Purees
3.4. Effect of Storage Temperature and Following Linear Regression Models of TPC Changes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Slavin, J.L.; Lloyd, B. Health Benefits of Fruits and Vegetables. Adv. Nutr. Int. Rev. J. 2012, 3, 506–516. [Google Scholar] [CrossRef]
- Friedman, M. Effects of Food Processing. Available online: http://www.wheatfoods.org (accessed on 18 November 2022).
- Cano-Lamadrid, M.; Artés-Hernández, F. Thermal and Non-Thermal Treatments to Preserve and Encourage Bioactive Compounds in Fruit- and Vegetable-Based Products. Foods 2022, 11, 3400. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Orbea, G.L.; García-Villalba, R.; Bernal, M.J.; Hernández, A.; Tomás-Barberán, F.A.; Sánchez-Siles, L.M. Stability of phenolic compounds in apple and strawberry: Effect of different processing techniques in industrial set up. Food Chem. 2023, 401, 134099. [Google Scholar] [CrossRef]
- Zhang, Y.; Truzzi, F.; D’amen, E.; Dinelli, G. Effect of Storage Conditions and Time on the Polyphenol Content of Wheat Flours. Processes 2021, 9, 248. [Google Scholar] [CrossRef]
- Govers, C.; Kasikci, M.B.; Van Der Sluis, A.A.; Mes, J.J. Review of the health effects of berries and their phytochemicals on the digestive and immune systems. Nutr. Rev. 2018, 76, 29–46. [Google Scholar] [CrossRef] [PubMed]
- Michalska, A.; Łysiak, G. Bioactive Compounds of Blueberries: Post-Harvest Factors Influencing the Nutritional Value of Products. Int. J. Mol. Sci. 2015, 16, 18642–18663. [Google Scholar] [CrossRef]
- Siracusa, L.; Ruberto, G. Plant polyphenol profiles as a tool for traceability and valuable support to biodiversity. In Polyphenols in Plants: Isolation, Purification and Extract Preparation; Elsevier: Amsterdam, The Netherlands, 2014; pp. 15–33. [Google Scholar] [CrossRef]
- Korekar, G.; Dolkar, P.; Singh, H.; Srivastava, R.B.; Stobdan, T. Variability and the genotypic effect on antioxidant activity, total phenolics, carotenoids and ascorbic acid content in seventeen natural population of Seabuckthorn (Hippophae rhamnoides L.) from trans-Himalaya. LWT 2014, 55, 157–162. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Bioactive compounds in banana and their associated health benefits—A review. Food Chem. 2016, 206, 1–11. [Google Scholar] [CrossRef]
- Frías-Moreno, M.N.; Parra-Quezada, R.A.; González-Aguilar, G.; Ruíz-Canizales, J.; Molina-Corral, F.J.; Sepulveda, D.R.; Salas-Salazar, N.; Olivas, G.I. Quality, Bioactive Compounds, Antioxidant Capacity, and Enzymes of Raspberries at Different Maturity Stages, Effects of Organic vs. Conventional Fertilization. Foods 2021, 10, 953. [Google Scholar] [CrossRef]
- Ren, R.; Li, N.; Su, C.; Wang, Y.; Zhao, X.; Yang, L.; Li, Y.; Zhang, B.; Chen, J.; Ma, X. The bioactive components as well as the nutritional and health effects of sea buckthorn. RSC Adv. 2020, 10, 44654–44671. [Google Scholar] [CrossRef] [PubMed]
- Martynenko, A.; Chen, Y. Degradation kinetics of total anthocyanins and formation of polymeric color in blueberry hydrothermodynamic (HTD) processing. J. Food Eng. 2016, 171, 44–51. [Google Scholar] [CrossRef]
- Chaaban, H.; Ioannou, I.; Chebil, L.; Slimane, M.; Gérardin, C.; Paris, C.; Charbonnel, C.; Chekir, L.; Ghoul, M. Effect of heat processing on thermal stability and antioxidant activity of six flavonoids. J. Food Process. Preserv. 2017, 41, e13203. [Google Scholar] [CrossRef]
- Esparza, I.; Cimminelli, M.J.; Moler, J.A.; Jiménez-Moreno, N.; Ancín-Azpilicueta, C. Stability of Phenolic Compounds in Grape Stem Extracts. Antioxidants 2020, 9, 720. [Google Scholar] [CrossRef]
- Friedman, M.; Jürgens, H.S. Effect of pH on the Stability of Plant Phenolic Compounds. J. Agric. Food Chem. 2000, 48, 2101–2110. [Google Scholar] [CrossRef] [PubMed]
- Sójka, M.; Janowski, M.; Grzelak-Błaszczyk, K. Stability and transformations of raspberry (Rubus idaeus L.) ellagitannins in aqueous solutions. Eur. Food Res. Technol. 2019, 245, 1113–1122. [Google Scholar] [CrossRef]
- Kim, H.; Castellon-Chicas, M.J.; Arbizu, S.; Talcott, S.T.; Drury, N.L.; Smith, S.; Mertens-Talcott, S.U. Mango (Mangifera indica L.) Polyphenols: Anti-Inflammatory Intestinal Microbial Health Benefits, and Associated Mechanisms of Actions. Molecules 2021, 26, 2732. [Google Scholar] [CrossRef] [PubMed]
- Masibo, M.; He, Q. Major Mango Polyphenols and Their Potential Significance to Human Health. Compr. Rev. Food Sci. Food Saf. 2008, 7, 309–319. [Google Scholar] [CrossRef]
- Arscott, S.A.; Tanumihardjo, S.A. Carrots of Many Colors Provide Basic Nutrition and Bioavailable Phytochemicals Acting as a Functional Food. Compr. Rev. Food Sci. Food Saf. 2010, 9, 223–239. [Google Scholar] [CrossRef]
- Ragaee, S.; Seetharaman, K.; Abdel-Aal, E.-S.M. The Impact of Milling and Thermal Processing on Phenolic Compounds in Cereal Grains. Crit. Rev. Food Sci. Nutr. 2014, 54, 837–849. [Google Scholar] [CrossRef]
- ilić, S. Phenolic Compounds of Wheat. Their Content, Antioxidant Capacity and Bioaccessibility. MOJ Food Process. Technol. 2016, 2, 00037. [Google Scholar] [CrossRef]
- Bridi, R.; Troncoso, M.J.; Folch-Cano, C.; Fuentes, J.; Speisky, H.; López-Alarcón, C. A Polyvinylpolypyrrolidone (PVPP)-Assisted Folin–Ciocalteu Assay to Assess Total Phenol Content of Commercial Beverages. Food Anal. Methods 2014, 7, 2075–2083. [Google Scholar] [CrossRef]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B. Thorough study of reactivity of various compound classes toward the Folin−Ciocalteu reagent. J. Agric. Food Chem. 2010, 58, 8139–8144. [Google Scholar] [CrossRef]
- Ma, S.; Kim, C.; Neilson, A.P.; Griffin, L.E.; Peck, G.M.; O’Keefe, S.F.; Stewart, A.C. Comparison of Common Analytical Methods for the Quantification of Total Polyphenols and Flavanols in Fruit Juices and Ciders. J. Food Sci. 2019, 84, 2147–2158. [Google Scholar] [CrossRef]
- Grand View Research, “Fruit Puree Market Size, Share & Trends Analysis Report by Product (Tropical & Exotic, Citrus, Berries), by Application (Beverages, Bakery & Snacks, Baby Food), by Region, and Segment Forecasts, 2020–2027,” 2019. Available online: https://www.grandviewresearch.com/industry-analysis/fruit-puree-market (accessed on 10 March 2023).
- Bauer, A.-S.; Tacker, M.; Uysal-Unalan, I.; Cruz, R.M.S.; Varzakas, T.; Krauter, V. Recyclability and Redesign Challenges in Multilayer Flexible Food Packaging—A Review. Foods 2021, 10, 2702. [Google Scholar] [CrossRef] [PubMed]
- Sonar, C.R.; Rasco, B.; Tang, J.; Sablani, S.S. Natural color pigments: Oxidative stability and degradation kinetics during storage in thermally pasteurized vegetable purees. J. Sci. Food Agric. 2019, 99, 5934–5945. [Google Scholar] [CrossRef] [PubMed]
- Franco, R.R.; Ojeda, G.A.; Rompato, K.M.; Sgroppo, S.C. Effects of short-wave ultraviolet light, ultrasonic and microwave treatments on banana puree during refrigerated storage. Food Sci. Technol. Int. 2021, 29, 50–61. [Google Scholar] [CrossRef]
- Bayus, J.; Ge, C.; Thorn, B. A preliminary environmental assessment of foil and metallized film centered laminates. Resour. Conserv. Recycl. 2016, 115, 31–41. [Google Scholar] [CrossRef]
- Tamburini, E.; Costa, S.; Summa, D.; Battistella, L.; Fano, E.A.; Castaldelli, G. Plastic (PET) vs bioplastic (PLA) or refillable aluminium bottles—What is the most sustainable choice for drinking water? A life-cycle (LCA) analysis. Environ. Res. 2021, 196, 110974. [Google Scholar] [CrossRef]
- Schmidt, J.; Grau, L.; Auer, M.; Maletz, R.; Woidasky, J. Multilayer Packaging in a Circular Economy. Polymers 2022, 14, 1825. [Google Scholar] [CrossRef]
- Robertson, G.L. Food Packaging: Principles and Practice, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Haouet, M.N.; Tommasino, M.; Mercuri, M.L.; Benedetti, F.; Di Bella, S.; Framboas, M.; Pelli, S.; Altissimi, M.S. Experimental accelerated shelf life determination of a ready-to-eat processed food. Ital. J. Food Saf. 2018, 7, 6919. [Google Scholar] [CrossRef]
- Mizrahi, S. Accelerated shelf-life tests. Understanding and Measuring the Shelf-Life of Food; Woodhead Publishing: Sawston, UK, 2004; pp. 317–339. [Google Scholar] [CrossRef]
- Corradini, M.G. Shelf Life of Food Products: From Open Labeling to Real-Time Measurements. Annu. Rev. Food Sci. Technol. 2018, 9, 251–269. [Google Scholar] [CrossRef]
- Calligaris, S.; Manzocco, L.; Anese, M.; Nicoli, M.C. Accelerated shelf life testing. In Food Quality and Shelf Life; Academic Press: Cambridge, MA, USA, 2019; pp. 359–392. [Google Scholar] [CrossRef]
- Kilcast, D.; Subramanian, P. Introduction. In The Stability and Shelf-Life of Food; Elsevier: Amsterdam, The Netherlands, 2000; pp. 1–22. [Google Scholar] [CrossRef]
- Taoukis, P.; Giannakourou, M. Temperature and food stability: Analysis and control. In Understanding and Measuring the Shelf-Life of Food; Elsevier: Amsterdam, The Netherlands, 2004; pp. 42–68. [Google Scholar] [CrossRef]
- Toledo, R.T. Kinetics of Chemical Reactions in Foods. In Fundamentals of Food Process Engineering; Springer: Berlin/Heidelberg, Germany, 2007; pp. 285–299. [Google Scholar] [CrossRef]
- Bravi, E.; Sileoni, V.; Perretti, G.; Marconi, O. Accelerated shelf-life model of gluten-free rusks by using oxidation indices. Food Chem. 2020, 326, 126971. [Google Scholar] [CrossRef]
- Fu, B.; Labuza, T.P. Shelf-Life Testing: Procedures and Prediction Methods. In Quality in Frozen Foods; Academic Press: Cambridge, MA, USA, 1997; pp. 377–415. [Google Scholar] [CrossRef]
- Srivastava, A.; Akoh, C.C.; Yi, W.; Fischer, J.; Krewer, G. Effect of Storage Conditions on the Biological Activity of Phenolic Compounds of Blueberry Extract Packed in Glass Bottles. J. Agric. Food Chem. 2007, 55, 2705–2713. [Google Scholar] [CrossRef] [PubMed]
- Castrejón, A.D.R.; Eichholz, I.; Rohn, S.; Kroh, L.W.; Huyskens-Keil, S. Phenolic profile and antioxidant activity of highbush blueberry (Vaccinium corymbosum L.) during fruit maturation and ripening. Food Chem. 2008, 109, 564–572. [Google Scholar] [CrossRef]
- Patras, A.; Brunton, N.; Tiwari, B.K.; Butler, F. Stability and Degradation Kinetics of Bioactive Compounds and Colour in Strawberry Jam during Storage. Food Bioprocess Technol. 2011, 4, 1245–1252. [Google Scholar] [CrossRef]
- Celli, G.B.; Dibazar, R.; Ghanem, A.; Brooks, M.S.-L. Degradation kinetics of anthocyanins in freeze-dried microencapsulates from lowbush blueberries (Vaccinium angustifolium Aiton) and prediction of shelf-life. Dry. Technol. 2016, 34, 1175–1184. [Google Scholar] [CrossRef]
- Andersson, S.C.; Ekholm, A.; Johansson, E.; Olsson, M.E.; Sjöholm, I.; Nyberg, L.; Nilsson, A.; Rumpunen, K. Effect of storage time and temperature on stability of bioactive compounds in aseptically packed beverages prepared from rose hips and sea buckthorn berries. Agric. Food Sci. 2015, 24, 273–288. [Google Scholar] [CrossRef]
- ASTM International. Standard Guide for Accelerated Aging of Sterile Barrier Systems for Medical Devices (ASTM F1980-16). Available online: https://www.astm.org/f1980-16.html (accessed on 14 April 2023).
- Choi, J.-Y.; Lee, H.-J.; Cho, J.-S.; Lee, Y.-M.; Woo, J.-H.; Moon, K.-D. Prediction of shelf-life and changes in the quality characteristics of semidried persimmons stored at different temperatures. Food Sci. Biotechnol. 2017, 26, 1255–1262. [Google Scholar] [CrossRef]
- Yap, S.K.; Chin, N.L.; Yusof, Y.A.; Chong, K.Y. Quality characteristics of dehydrated raw Kelulut honey. Int. J. Food Prop. 2019, 22, 556–571. [Google Scholar] [CrossRef]
- Sulaiman, S.F.; Sajak, A.A.B.; Ooi, K.L.; Supriatno; Seow, E.M. Effect of solvents in extracting polyphenols and antioxidants of selected raw vegetables. J. Food Compos. Anal. 2011, 24, 506–515. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Yu, L.; Wu, Y.; Liu, D.; Sheng, Z.; Liu, J.; Chen, H.; Feng, W. The kinetic behavior of antioxidant activity and the stability of aqueous and organic polyphenol extracts from navel orange peel. Food Sci. Technol. 2022, 42, e90621. [Google Scholar] [CrossRef]
- Canadanovic-Brunet, J.; Vulic, J.; Cebovic, T.; Cetkovic, G.; Canadanovic, V.; Djilas, S.; Saponjac, V.T. Phenolic profile, antiradical and antitumour evaluation of raspberries pomace extract from Serbia. Iran. J. Pharm. Res. 2017, 16, 142–152. [Google Scholar] [CrossRef]
- Maldonado-Celis, M.E.; Yahia, E.M.; Bedoya, R.; Landázuri, P.; Loango, N.; Aguillón, J.; Restrepo, B.; Ospina, J.C.G. Chemical Composition of Mango (Mangifera indica L.) Fruit: Nutritional and Phytochemical Compounds. Front. Plant Sci. 2019, 10, 1073. [Google Scholar] [CrossRef]
- Criste, A.; Urcan, A.C.; Bunea, A.; Furtuna, F.R.P.; Olah, N.K.; Madden, R.H.; Corcionivoschi, N. Phytochemical Composition and Biological Activity of Berries and Leaves from Four Romanian Sea Buckthorn (Hippophae Rhamnoides L.) Varieties. Molecules 2020, 25, 1170. [Google Scholar] [CrossRef] [PubMed]
- Leja, M.; Kamińska, I.; Kramer, M.; Maksylewicz-Kaul, A.; Kammerer, D.; Carle, R.; Baranski, R. The Content of Phenolic Compounds and Radical Scavenging Activity Varies with Carrot Origin and Root Color. Plant Foods Hum. Nutr. 2013, 68, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Narita, Y.; Inouye, K. Degradation Kinetics of Chlorogenic Acid at Various pH Values and Effects of Ascorbic Acid and Epigallocatechin Gallate on Its Stability under Alkaline Conditions. J. Agric. Food Chem. 2013, 61, 966–972. [Google Scholar] [CrossRef]
- Cheng, Y.; Xu, Q.; Liu, J.; Zhao, C.; Xue, F.; Zhao, Y. Decomposition of Five Phenolic Compounds in High Temperature Water. J. Braz. Chem. Soc. 2014, 25, 2102–2107. [Google Scholar] [CrossRef]
- Jankovská, P.; Čopíková, J.; Sinitsya, A. The determination of ferulic acid in sugar beet pulp. Czech J. Food Sci. 2001, 19, 143–147. [Google Scholar] [CrossRef]
- van Lith, R.; Ameer, G.A. Antioxidant Polymers as Biomaterial. In Oxidative Stress and Biomaterials; Dziubla, T., Butterfield, D.A., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 251–296. [Google Scholar] [CrossRef]
- Lavelli, V.; Kerr, W. Moisture properties and stability of novel bioactive ingredients. In Food Quality and Shelf Life; Elsevier: Amsterdam, The Netherlands, 2019; pp. 33–54. [Google Scholar] [CrossRef]
- Escobar, M.A.M.; Jaramillo, F. Thermally and UV Stable Natural Dyes with Potential Use in Efficient Photoelectrochemical Devices. J. Renew. Mater. 2015, 3, 302–317. [Google Scholar] [CrossRef]
- Mäkilä, L.; Laaksonen, O.; Kallio, H.; Yang, B. Effect of processing technologies and storage conditions on stability of black currant juices with special focus on phenolic compounds and sensory properties. Food Chem. 2017, 221, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Veronique, T.; Wing, Y.M.; Christian, D. Chapter Seabuckthorn Polyphenols: Characterization, Bioactivities and Associated Health Benefits. Available online: www.intechopen.com (accessed on 30 November 2022).
- Zadernowski, R.; Naczk, M.; Czaplicki, S.; Rubinskiene, M.; Szałkiewicz, M. Composition of phenolic acids in sea buckthorn (Hippophae rhamnoides L.) berries. J. Am. Oil Chem. Soc. 2005, 82, 175–179. [Google Scholar] [CrossRef]


| Nutritional Composition | FGBB | MCB | FYB |
|---|---|---|---|
| Ingredients | Water, banana puree (30%), blueberry puree (8%), four-grain cereals (rye, oat, wheat, barley) (7%), and rapeseed oil | Mango puree (52%), carrot puree (40%), and sea buckthorn puree (8%) | Banana puree (37%), mango puree (36%), yogurt (15%), raspberry puree (10%), and biscuit (2%) including wheat flour, butter, and water |
| Energy (kcal/kJ) | 62/262 | 62/262 | 88/368 |
| Fat (g/100 g) | 1.4 | 0.0 | 1.3 |
| Unsaturated Fatty Acids (g/100 g) | 0.0 | 0.0 | 0.8 |
| Carbohydrates (g/100 g) | 11.0 | 13.0 | 16.0 |
| Sugars (g/100 g) | 3.8 | 11.0 | 11.0 |
| Protein (g/100 g) | 1.2 | 0.6 | 1.3 |
| Salt (g/100 g) | 0.0 | 0.0 | 0.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saarniit, K.; Lang, H.; Kuldjärv, R.; Laaksonen, O.; Rosenvald, S. The Stability of Phenolic Compounds in Fruit, Berry, and Vegetable Purees Based on Accelerated Shelf-Life Testing Methodology. Foods 2023, 12, 1777. https://doi.org/10.3390/foods12091777
Saarniit K, Lang H, Kuldjärv R, Laaksonen O, Rosenvald S. The Stability of Phenolic Compounds in Fruit, Berry, and Vegetable Purees Based on Accelerated Shelf-Life Testing Methodology. Foods. 2023; 12(9):1777. https://doi.org/10.3390/foods12091777
Chicago/Turabian StyleSaarniit, Kärt, Hanna Lang, Rain Kuldjärv, Oskar Laaksonen, and Sirli Rosenvald. 2023. "The Stability of Phenolic Compounds in Fruit, Berry, and Vegetable Purees Based on Accelerated Shelf-Life Testing Methodology" Foods 12, no. 9: 1777. https://doi.org/10.3390/foods12091777
APA StyleSaarniit, K., Lang, H., Kuldjärv, R., Laaksonen, O., & Rosenvald, S. (2023). The Stability of Phenolic Compounds in Fruit, Berry, and Vegetable Purees Based on Accelerated Shelf-Life Testing Methodology. Foods, 12(9), 1777. https://doi.org/10.3390/foods12091777