Anthocyanins and Vascular Health: A Matter of Metabolites
Abstract
1. Introduction
2. Method and Literature Search
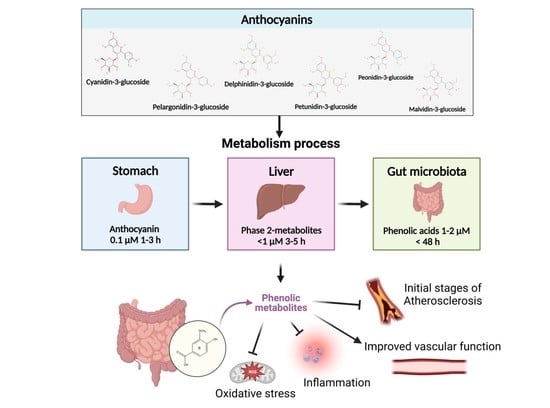
3. Metabolism of Anthocyanins
4. Anthocyanin Metabolites and Endothelial Function In Vivo
5. Anthocyanin Metabolites, Nitric Oxide Production via Akt-eNOS Pathway
6. Anthocyanin Metabolites and the Adhesion of Monocytes
7. Anthocyanin Metabolites and NF-κB Pathway
8. Anthocyanin Metabolites and Nrf2 Pathway
9. Implications and Future Directions
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Endemann, D.H.; Schiffrin, E.L. Endothelial Dysfunction. J. Am. Soc. Nephrol. 2004, 15, 1983–1992. [Google Scholar] [CrossRef] [PubMed]
- Kimble, R.; Keane, K.M.; Lodge, J.K.; Howatson, G. Dietary intake of anthocyanins and risk of cardiovascular disease: A systematic review and meta-analysis of prospective cohort studies. Crit. Rev. Food Sci. Nutr. 2019, 59, 3032–3043. [Google Scholar] [CrossRef]
- Wang, Y.; Gallegos, J.L.; Haskell-Ramsay, C.; Lodge, J.K. Effects of chronic consumption of specific fruit (berries, citrus and cherries) on CVD risk factors: A systematic review and meta-analysis of randomised controlled trials. Eur. J. Nutr. 2020, 60, 615–639. [Google Scholar] [CrossRef] [PubMed]
- Heneghan, C.; Kiely, M.; Lyons, J.; Lucey, A. The Effect of Berry-Based Food Interventions on Markers of Cardiovascular and Metabolic Health: A Systematic Review of Randomized Controlled Trials. Mol. Nutr. Food Res. 2018, 62, 1700645. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef]
- Kuntz, S.; Rudloff, S.; Asseburg, H.; Borsch, C.; Fröhling, B.; Unger, F.; Dold, S.; Spengler, B.; Römpp, A.; Kunz, C. Uptake and bioavailability of anthocyanins and phenolic acids from grape/blueberry juice and smoothie in vitro and in vivo. Br. J. Nutr. 2015, 113, 1044–1055. [Google Scholar] [CrossRef]
- Czank, C.; Cassidy, A.; Zhang, Q.; Morrison, D.J.; Preston, T.; Kroon, P.A.; Botting, N.P.; Kay, C.D. Human metabolism and elimination of the anthocyanin, cyanidin-3-glucoside: A 13C-tracer study. Am. J. Clin. Nutr. 2013, 97, 995–1003. [Google Scholar] [CrossRef]
- Rodriguez-Mateos, A.; Rendeiro, C.; Bergillos-Meca, T.; Tabatabaee, S.; George, T.W.; Heiss, C.; Spencer, J.P. Intake and time dependence of blueberry flavonoid–induced improvements in vascular function: A randomized, controlled, double-blind, crossover intervention study with mechanistic insights into biological activity. Am. J. Clin. Nutr. 2013, 98, 1179–1191. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 136–146. [Google Scholar] [CrossRef]
- Blesso, C.N. Dietary Anthocyanins and Human Health. Nutrients 2019, 11, 2107. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Kamonpatana, K.; Giusti, M.M.; Chitchumroonchokchai, C.; MorenoCruz, M.; Riedl, K.M.; Kumar, P.; Failla, M.L. Susceptibility of anthocyanins to ex vivo degradation in human saliva. Food Chem. 2012, 135, 738–747. [Google Scholar] [CrossRef]
- Milbury, P.E.; Vita, J.A.; Blumberg, J.B. Anthocyanins are Bioavailable in Humans following an Acute Dose of Cranberry Juice. J. Nutr. 2010, 140, 1099–1104. [Google Scholar] [CrossRef] [PubMed]
- Kay, C.D.; Mazza, G.J.; Holub, B.J. Anthocyanins Exist in the Circulation Primarily as Metabolites in Adult Men. J. Nutr. 2005, 135, 2582–2588. [Google Scholar] [CrossRef] [PubMed]
- Kay, C.D.; Kroon, P.A.; Cassidy, A. The bioactivity of dietary anthocyanins is likely to be mediated by their degradation products. Mol. Nutr. Food Res. 2009, 53 (Suppl. S1), S92–S101. [Google Scholar] [CrossRef] [PubMed]
- Fang, J. Some Anthocyanins Could Be Efficiently Absorbed across the Gastrointestinal Mucosa: Extensive Presystemic Metabolism Reduces Apparent Bioavailability. J. Agric. Food Chem. 2014, 62, 3904–3911. [Google Scholar] [CrossRef]
- de Ferrars, R.M.; Czank, C.; Zhang, Q.; Botting, N.P.; Kroon, P.; Cassidy, A.; Kay, C. The pharmacokinetics of anthocyanins and their metabolites in humans. Br. J. Pharmacol. 2014, 171, 3268–3282. [Google Scholar] [CrossRef] [PubMed]
- Eker, M.E.; Aaby, K.; Budic-Leto, I.; Rimac Brnčić, S.; El, S.N.; Karakaya, S.; Simsek, S.; Manach, C.; Wiczkowski, W.; De Pascual-Teresa, S. A Review of Factors Affecting Anthocyanin Bioavailability: Possible Implications for the Inter-Individual Variability. Foods 2019, 9, 2. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Capanoglu, E.; Grootaert, C.; Van Camp, J. Anthocyanin Absorption and Metabolism by Human Intestinal Caco-2 Cells—A Review. Int. J. Mol. Sci. 2015, 16, 21555–21574. [Google Scholar] [CrossRef]
- Chandra, P.; Rathore, A.S.; Kay, K.L.; Everhart, J.L.; Curtis, P.; Burton-Freeman, B.; Cassidy, A.; Kay, C.D. Contribution of Berry Polyphenols to the Human Metabolome. Molecules 2019, 24, 4220. [Google Scholar] [CrossRef]
- Edwards, M.; Czank, C.; Woodward, G.M.; Cassidy, A.; Kay, C.D. Phenolic Metabolites of Anthocyanins Modulate Mechanisms of Endothelial Function. J. Agric. Food Chem. 2015, 63, 2423–2431. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, H.; Meroño, T.; Miñarro, A.; Sánchez-Pla, A.; Lanuza, F.; Zamora-Ros, R.; Rostgaard-Hansen, A.L.; Estanyol-Torres, N.; Cubedo-Culleré, M.; Tjønneland, A.; et al. Dietary Sources of Anthocyanins and Their Association with Metabolome Biomarkers and Cardiometabolic Risk Factors in an Observational Study. Nutrients 2023, 15, 1208. [Google Scholar] [CrossRef]
- Huang, L.; Xiao, D.; Zhang, X.; Sandhu, A.K.; Chandra, P.; Kay, C.; Edirisinghe, I.; Burton-Freeman, B. Strawberry Consumption, Cardiometabolic Risk Factors, and Vascular Function: A Randomized Controlled Trial in Adults with Moderate Hypercholesterolemia. J. Nutr. 2021, 151, 1517–1526. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mateos, A.; Istas, G.; Boschek, L.; Feliciano, R.P.; Mills, C.E.; Boby, C.; Gómez-Alonso, S.; Milenkovic, D.; Heiss, C. Circulating Anthocyanin Metabolites Mediate Vascular Benefits of Blueberries: Insights from Randomized Controlled Trials, Metabolomics, and Nutrigenomics. J. Gerontol. Ser. A 2019, 74, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Heiss, C.; Istas, G.; Feliciano, R.P.; Weber, T.; Wang, B.; Favari, C.; Mena, P.; Del Rio, D.; Rodriguez-Mateos, A. Daily consumption of cranberry improves endothelial function in healthy adults: A double blind randomized controlled trial. Food Funct. 2022, 13, 3812–3824. [Google Scholar] [CrossRef]
- Curtis, P.J.; van der Velpen, V.; Berends, L.; Jennings, A.; Feelisch, M.; Umpleby, A.M.; Evans, M.; Fernandez, B.O.; Meiss, M.S.; Minnion, M.; et al. Blueberries improve biomarkers of cardiometabolic function in participants with metabolic syndrome—Results from a 6-month, double-blind, randomized controlled trial. Am. J. Clin. Nutr. 2019, 109, 1535–1545. [Google Scholar] [CrossRef]
- Woolf, E.K.; Terwoord, J.D.; Litwin, N.S.; Vazquez, A.R.; Lee, S.Y.; Ghanem, N.; Michell, K.A.; Smith, B.T.; Grabos, L.E.; Ketelhut, N.B.; et al. Daily Blueberry Consumption for 12 Weeks Improves Endothelial Function in Postmenopausal Women with Above-Normal Blood Pressure through Reductions in Oxidative Stress: A Randomized Controlled Trial. Food Funct. 2023, 14, 2621–2641. [Google Scholar] [CrossRef]
- Curtis, P.J.; Berends, L.; van der Velpen, V.; Jennings, A.; Haag, L.; Chandra, P.; Kay, C.D.; Rimm, E.B.; Cassidy, A. Blueberry anthocyanin intake attenuates the postprandial cardiometabolic effect of an energy-dense food challenge: Results from a double blind, randomized controlled trial in metabolic syndrome participants. Clin. Nutr. 2021, 41, 165–176. [Google Scholar] [CrossRef]
- Le Sayec, M.; Xu, Y.; Laiola, M.; Gallego, F.A.; Katsikioti, D.; Durbidge, C.; Kivisild, U.; Armes, S.; Lecomte, M.; Fança-Berthon, P.; et al. The effects of Aronia berry (poly)phenol supplementation on arterial function and the gut microbiome in middle aged men and women: Results from a randomized controlled trial. Clin. Nutr. 2022, 41, 2549–2561. [Google Scholar] [CrossRef]
- Ellis, L.R.; Zulfiqar, S.; Holmes, M.; Marshall, L.; Dye, L.; Boesch, C. A systematic review and meta-analysis of the effects of Hibiscus sabdariffa on blood pressure and cardiometabolic markers. Nutr. Rev. 2022, 80, 1723–1737. [Google Scholar] [CrossRef]
- Joven, J.; March, I.; Espinel, E.; Fernández-Arroyo, S.; Rodríguez-Gallego, E.; Aragonès, G.; Beltrán-Debón, R.; Alonso-Villaverde, C.; Rios, L.; Martin-Paredero, V.; et al. Hibiscus sabdariffa extract lowers blood pressure and improves endothelial function. Mol. Nutr. Food Res. 2014, 58, 1374–1378. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, S.M.; Ukeyima, M.T.; Spencer, J.P.E.; Lovegrove, J.A. Acute Effects of Hibiscus sabdariffa Calyces on Postprandial Blood Pressure, Vascular Function, Blood Lipids, Biomarkers of Insulin Resistance and Inflammation in Humans. Nutrients 2019, 11, 341. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Aroor, A.R.; Jia, C.; Sowers, J.R. Endothelial cell senescence in aging-related vascular dysfunction. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2019, 1865, 1802–1809. [Google Scholar] [CrossRef] [PubMed]
- McCarron, J.G.; Wilson, C.; Heathcote, H.R.; Zhang, X.; Buckley, C.; Lee, M.D. Heterogeneity and emergent behaviour in the vascular endothelium. Curr. Opin. Pharmacol. 2019, 45, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Gullo, A.L.; Aragona, C.O.; Scuruchi, M.; Versace, A.G.; Saitta, A.; Imbalzano, E.; Loddo, S.; Campo, G.M.; Mandraffino, G. Endothelial progenitor cells and rheumatic disease modifying therapy. Vasc. Pharmacol. 2018, 108, 8–14. [Google Scholar] [CrossRef]
- Schulz, E.; Gori, T.; Münzel, T. Oxidative stress and endothelial dysfunction in hypertension. Hypertens. Res. 2011, 34, 665–673. [Google Scholar] [CrossRef]
- Rohlenova, K.; Veys, K.; Miranda-Santos, I.; De Bock, K.; Carmeliet, P. Endothelial Cell Metabolism in Health and Disease. Trends Cell Biol. 2018, 28, 224–236. [Google Scholar] [CrossRef]
- Münzel, T.; Daiber, A. Inorganic nitrite and nitrate in cardiovascular therapy: A better alternative to organic nitrates as nitric oxide donors? Vasc. Pharmacol. 2018, 102, 1–10. [Google Scholar] [CrossRef]
- Beckman, J.S.; Koppenol, W.H. Nitric oxide, superoxide, and peroxynitrite: The good, the bad, and ugly. Am. J. Physiol. Cell Physiol. 1996, 271, C1424–C1437. [Google Scholar] [CrossRef]
- Münzel, T.; Daiber, A.; Ullrich, V.; Mülsch, A. Vascular Consequences of Endothelial Nitric Oxide Synthase Uncoupling for the Activity and Expression of the Soluble Guanylyl Cyclase and the cGMP-Dependent Protein Kinase. Arter. Thromb. Vasc. Biol. 2005, 25, 1551–1557. [Google Scholar] [CrossRef]
- Selemidis, S.; Dusting, G.J.; Peshavariya, H.; Kemp-Harper, B.K.; Drummond, G.R. Nitric oxide suppresses NADPH oxidase-dependent superoxide production by S-nitrosylation in human endothelial cells. Cardiovasc. Res. 2007, 75, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Dudzinski, D.M.; Michel, T. Life history of eNOS: Partners and pathways. Cardiovasc. Res. 2007, 75, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Festa, J.; Da Boit, M.; Hussain, A.; Singh, H. Potential Benefits of Berry Anthocyanins on Vascular Function. Mol. Nutr. Food Res. 2021, 65, 2100170. [Google Scholar] [CrossRef]
- Festa, J.; Singh, H.; Hussain, A.; Da Boit, M. Elderberries as a potential supplement to improve vascular function in a SARS-CoV-2 environment. J. Food Biochem. 2022, 46, e14091. [Google Scholar] [CrossRef] [PubMed]
- Spigoni, V.; Mena, P.; Cito, M.; Fantuzzi, F.; Bonadonna, R.C.; Brighenti, F.; Cas, A.D.; Del Rio, D. Effects on Nitric Oxide Production of Urolithins, Gut-Derived Ellagitannin Metabolites, in Human Aortic Endothelial Cells. Molecules 2016, 21, 1009. [Google Scholar] [CrossRef]
- Toaldo, I.M.; Van Camp, J.; Gonzales, G.B.; Kamiloglu, S.; Bordignon-Luiz, M.T.; Smagghe, G.; Raes, K.; Capanoglu, E.; Grootaert, C. Resveratrol improves TNF-α-induced endothelial dysfunction in a coculture model of a Caco-2 with an endothelial cell line. J. Nutr. Biochem. 2016, 36, 21–30. [Google Scholar] [CrossRef]
- Ladurner, A.; Schachner, D.; Schueller, K.; Pignitter, M.; Heiss, E.H.; Somoza, V.; Dirsch, V.M. Impact of Trans-Resveratrol-Sulfates and -Glucuronides on Endothelial Nitric Oxide Synthase Activity, Nitric Oxide Release and Intracellular Reactive Oxygen Species. Molecules 2014, 19, 16724–16736. [Google Scholar] [CrossRef]
- Liu, C.; Wang, W.; Lin, W.; Ling, W.; Wang, D. Established atherosclerosis might be a prerequisite for chicory and its constituent protocatechuic acid to promote endothelium-dependent vasodilation in mice. Mol. Nutr. Food Res. 2016, 60, 2141–2150. [Google Scholar] [CrossRef]
- Kang, Z.; Zhu, H.; Jiang, W.; Zhang, S. Protocatechuic Acid Induces Angiogenesis through PI3K-Akt-eNOS-VEGF Signalling Pathway. Basic Clin. Pharmacol. Toxicol. 2013, 113, 221–227. [Google Scholar] [CrossRef]
- Xue, X.-Y.; Liao, M.-J.; Lin, L.-F.; Zhang, Z.; Zhou, X.-W.; Zhou, X.; Luo, H.-M. Phosphorylation of Akt is involved in protocatechuic acid-induced neurotrophic activity. Neurol. Res. 2012, 34, 901–907. [Google Scholar] [CrossRef]
- Chen, X.; Yao, F.; Song, J.; Fu, B.; Sun, G.; Song, X.; Fu, C.; Jiang, R.; Sun, L. Protective effects of phenolic acid extract from ginseng on vascular endothelial cell injury induced by palmitate via activation of PI3K/Akt/eNOS pathway. J. Food Sci. 2020, 85, 576–581. [Google Scholar] [CrossRef]
- Yu, M.; Kim, H.J.; Heo, H.; Kim, M.; Jeon, Y.; Lee, H.; Lee, J. Comparison of the Antihypertensive Activity of Phenolic Acids. Molecules 2022, 27, 6185. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Prahalathan, P.; Saravanakumar, M.; Raja, B. Vanillic acid prevents the deregulation of lipid metabolism, endothelin 1 and up regulation of endothelial nitric oxide synthase in nitric oxide deficient hypertensive rats. Eur. J. Pharmacol. 2014, 743, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Zhang, Q.-Y.; Zhang, Y.-L.; Han, X.; Guo, S.-B.; Li, H.-H. Gallic Acid Attenuates Angiotensin II-Induced Hypertension and Vascular Dysfunction by Inhibiting the Degradation of Endothelial Nitric Oxide Synthase. Front. Pharmacol. 2020, 11, 1121. [Google Scholar] [CrossRef]
- Tang, J.S.; Bozonet, S.M.; McKenzie, J.L.; Anderson, R.F.; Melton, L.D.; Vissers, M.C.M. Physiological Concentrations of Blueberry-Derived Phenolic Acids Reduce Monocyte Adhesion to Human Endothelial Cells. Mol. Nutr. Food Res. 2019, 63, e1900478. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Yi, L.; Jin, X.; Zhang, T.; Fu, Y.-J.; Zhu, J.-D.; Mi, M.-T.; Zhang, Q.-Y.; Ling, W.-H.; Yu, B. Inhibitory Effect of Delphinidin on Monocyte–Endothelial Cell Adhesion Induced by Oxidized Low-Density Lipoprotein via ROS/p38MAPK/NF-κB Pathway. Cell Biochem. Biophys. 2011, 61, 337–348. [Google Scholar] [CrossRef]
- Fratantonio, D.; Speciale, A.; Ferrari, D.; Cristani, M.; Saija, A.; Cimino, F. Palmitate-induced endothelial dysfunction is attenuated by cyanidin-3-O-glucoside through modulation of Nrf2/Bach1 and NF-κB pathways. Toxicol. Lett. 2015, 239, 152–160. [Google Scholar] [CrossRef]
- Chao, P.-Y.; Lin, K.-H.; Chiu, C.-C.; Yang, Y.-Y.; Huang, M.-Y.; Yang, C.-M. Inhibitive Effects of Mulberry Leaf-Related Extracts on Cell Adhesion and Inflammatory Response in Human Aortic Endothelial Cells. Evid.-Based Complement. Altern. Med. 2013, 2013, 267217. [Google Scholar] [CrossRef]
- Krga, I.; Monfoulet, L.-E.; Konic-Ristic, A.; Mercier, S.; Glibetic, M.; Morand, C.; Milenkovic, D. Anthocyanins and their gut metabolites reduce the adhesion of monocyte to TNFα-activated endothelial cells at physiologically relevant concentrations. Arch. Biochem. Biophys. 2016, 599, 51–59. [Google Scholar] [CrossRef]
- Del Bo, C.; Marino, M.; Riso, P.; Møller, P.; Porrini, M. Anthocyanins and metabolites resolve TNF-α-mediated production of E-selectin and adhesion of monocytes to endothelial cells. Chem.-Biol. Interact. 2019, 300, 49–55. [Google Scholar] [CrossRef]
- Mestas, J.; Ley, K. Monocyte-Endothelial Cell Interactions in the Development of Atherosclerosis. Trends Cardiovasc. Med. 2008, 18, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-Y.; Li, C.-J.; Hou, M.-F.; Chu, P.-Y. New Insights into the Role of Inflammation in the Pathogenesis of Atherosclerosis. Int. J. Mol. Sci. 2017, 18, 2034. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-Y.; Wang, J.; Liu, Y.-M.; Zheng, Q.-S.; Li, C.-Y. Inhibitory effect of Malvidin on TNF-α-induced inflammatory response in endothelial cells. Eur. J. Pharmacol. 2014, 723, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Kuntz, S.; Asseburg, H.; Dold, S.; Römpp, A.; Fröhling, B.; Kunz, C.; Rudloff, S. Inhibition of low-grade inflammation by anthocyanins from grape extract in an in vitro epithelial-endothelial co-culture model. Food Funct. 2015, 6, 1136–1149. [Google Scholar] [CrossRef] [PubMed]
- Warner, E.F.; Zhang, Q.; Raheem, K.S.; O’Hagan, D.; O’Connell, M.A.; Kay, C.D. Common phenolic metabolites of flavonoids, but not their unmetabolized precursors, reduce the secretion of vascular cellular adhesion molecules by human endothelial cells. J. Nutr. 2016, 146, 465–473. [Google Scholar] [CrossRef]
- Vitaglione, P.; Donnarumma, G.; Napolitano, A.; Galvano, F.; Gallo, A.; Scalfi, L.; Fogliano, V. Protocatechuic Acid Is the Major Human Metabolite of Cyanidin-Glucosides. J. Nutr. 2007, 137, 2043–2048. [Google Scholar] [CrossRef]
- Amin, H.P.; Czank, C.; Raheem, S.; Zhang, Q.; Botting, N.P.; Cassidy, A.; Kay, C.D. Anthocyanins and their physiologically relevant metabolites alter the expression of IL-6 and VCAM-1 in CD40L and oxidized LDL challenged vascular endothelial cells. Mol. Nutr. Food Res. 2015, 59, 1095–1106. [Google Scholar] [CrossRef]
- Lusis, A.J. Atherosclerosis. Nature 2000, 407, 233–241. [Google Scholar] [CrossRef]
- Bharat, D.; Cavalcanti, R.R.M.; Petersen, C.; Begaye, N.; Cutler, B.R.; Costa, M.M.A.; Ramos, R.K.L.G.; Ferreira, M.R.; Li, Y.; Bharath, L.P.; et al. Blueberry Metabolites Attenuate Lipotoxicity-Induced Endothelial Dysfunction. Mol. Nutr. Food Res. 2018, 62, 1700601. [Google Scholar] [CrossRef]
- Danese, S.; Sans, M.; Scaldaferri, F.; Sgambato, A.; Rutella, S.; Cittadini, A.; Piqué, J.M.; Panes, J.; Katz, J.A.; Gasbarrini, A.; et al. TNF-α Blockade Down-Regulates the CD40/CD40L Pathway in the Mucosal Microcirculation: A Novel Anti-Inflammatory Mechanism of Infliximab in Crohn’s Disease. J. Immunol. 2006, 176, 2617–2624. [Google Scholar] [CrossRef]
- di Gesso, J.L.; Kerr, J.S.; Zhang, Q.; Raheem, S.; Yalamanchili, S.K.; O’Hagan, D.; Kay, C.D.; O’Connell, M.A. Flavonoid metabolites reduce tumor necrosis factor-α secretion to a greater extent than their precursor compounds in human THP-1 monocytes. Mol. Nutr. Food Res. 2015, 59, 1143–1154. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mateos, A.; Del Pino-García, R.; George, T.W.; Vidal-Diez, A.; Heiss, C.; Spencer, J.P.E. Impact of processing on the bioavailability and vascular effects of blueberry (poly)phenols. Mol. Nutr. Food Res. 2014, 58, 1952–1961. [Google Scholar] [CrossRef] [PubMed]
- Mussbacher, M.; Salzmann, M.; Brostjan, C.; Hoesel, B.; Schoergenhofer, C.; Datler, H.; Hohensinner, P.; Basílio, J.; Petzelbauer, P.; Assinger, A.; et al. Cell Type-Specific Roles of NF-κB Linking Inflammation and Thrombosis. Front. Immunol. 2019, 10, 85. [Google Scholar] [CrossRef]
- Li, Q.; Verma, I.M. NF-κB regulation in the immune system. Nat. Rev. Immunol. 2002, 2, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Virdis, A.; Dell’agnello, U.; Taddei, S. Impact of inflammation on vascular disease in hypertension. Maturitas 2014, 78, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Mauray, A.; Felgines, C.; Morand, C.; Mazur, A.; Scalbert, A.; Milenkovic, D. Bilberry anthocyanin-rich extract alters expression of genes related to atherosclerosis development in aorta of apo E-deficient mice. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 72–80. [Google Scholar] [CrossRef]
- Natarelli, L.; Ranaldi, G.; Leoni, G.; Roselli, M.; Guantario, B.; Comitato, R.; Ambra, R.; Cimino, F.; Speciale, A.; Virgili, F.; et al. Nanomolar Caffeic Acid Decreases Glucose Uptake and the Effects of High Glucose in Endothelial Cells. PLoS ONE 2015, 10, e0142421. [Google Scholar] [CrossRef]
- Fratantonio, D.; Speciale, A.; Canali, R.; Natarelli, L.; Ferrari, D.; Saija, A.; Virgili, F.; Cimino, F. Low nanomolar caffeic acid attenuates high glucose-induced endothelial dysfunction in primary human umbilical-vein endothelial cells by affecting NF-κB and Nrf2 pathways. BioFactors 2017, 43, 54–62. [Google Scholar] [CrossRef]
- Mostafa, H.; Behrendt, I.; Meroño, T.; González-Domínguez, R.; Fasshauer, M.; Rudloff, S.; Andres-Lacueva, C.; Kuntz, S. Plasma anthocyanins and their metabolites reduce in vitro migration of pancreatic cancer cells, PANC-1, in a FAK- and NF-kB dependent manner: Results from the ATTACH-study a randomized, controlled, crossover trial in healthy subjects. Biomed. Pharmacother. 2023, 158, 114076. [Google Scholar] [CrossRef]
- Wang, D.; Wei, X.; Yan, X.; Jin, T.; Ling, W. Protocatechuic acid, a metabolite of anthocyanins, inhibits monocyte adhesion and reduces atherosclerosis in apolipoprotein E-deficient mice. J Agric Food Chem. 2010, 58, 12722–12728. [Google Scholar] [CrossRef]
- Claude, S.; Boby, C.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Gérard, N.; Morand, C.; Milenkovic, D. Flavanol metabolites reduce monocyte adhesion to endothelial cells through modulation of expression of genes via p38-MAPK and p65-Nf-kB pathways. Mol. Nutr. Food Res. 2014, 58, 1016–1027. [Google Scholar] [CrossRef] [PubMed]
- Wongwichai, T.; Teeyakasem, P.; Pruksakorn, D.; Kongtawelert, P.; Pothacharoen, P. Anthocyanins and metabolites from purple rice inhibit IL-1β-induced matrix metalloproteinases expression in human articular chondrocytes through the NF-κB and ERK/MAPK pathway. Biomed. Pharmacother. 2019, 112, 108610. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-C.; Kim, S.-J.; Kim, D.-S.; Jeon, Y.-D.; Park, S.J.; Lee, H.S.; Um, J.-Y.; Hong, S.-H. Vanillic acid inhibits inflammatory mediators by suppressing NF-κB in lipopolysaccharide-stimulated mouse peritoneal macrophages. Immunopharmacol. Immunotoxicol. 2011, 33, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Kumar, A.; Singh, H.; Kaur, S.; Arora, S.; Singh, B. Protective effect of vanillic acid against diabetes and diabetic nephropathy by attenuating oxidative stress and upregulation of NF-κB, TNF-α and COX-2 proteins in rats. Phytotherapy Res. 2022, 36, 1338–1352. [Google Scholar] [CrossRef] [PubMed]
- Aboonabi, A.; Aboonabi, A. Anthocyanins reduce inflammation and improve glucose and lipid metabolism associated with inhibiting nuclear factor-kappaB activation and increasing PPAR-γ gene expression in metabolic syndrome subjects. Free. Radic. Biol. Med. 2020, 150, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huo, Y.; Zhao, L.; Lu, F.; Wang, O.; Yang, X.; Ji, B.; Zhou, F. Cyanidin-3-glucoside and its phenolic acid metabolites attenuate visible light-induced retinal degeneration in vivo via activation of Nrf2/HO-1 pathway and NF-κB suppression. Mol. Nutr. Food Res. 2016, 60, 1564–1577. [Google Scholar] [CrossRef]
- Baird, L.; Yamamoto, M. The molecular mechanisms regulating the KEAP1-NRF2 pathway. Mol. Cell. Biol. 2020, 40, e00099-20. [Google Scholar] [CrossRef]
- Cimino, F.; Speciale, A.; Anwar, S.; Canali, R.; Ricciardi, E.; Virgili, F.; Trombetta, D.; Saija, A. Anthocyanins protect human endothelial cells from mild hyperoxia damage through modulation of Nrf2 pathway. Genes Nutr. 2013, 8, 391–399. [Google Scholar] [CrossRef]
- Vinothiya, K.; Ashokkumar, N. Modulatory effect of vanillic acid on antioxidant status in high fat diet-induced changes in diabetic hypertensive rats. Biomed. Pharmacother. 2017, 87, 640–652. [Google Scholar] [CrossRef]
- Ma, W.-F.; Duan, X.-C.; Han, L.; Zhang, L.-L.; Meng, X.-M.; Li, Y.-L.; Wang, M. Vanillic acid alleviates palmitic acid-induced oxidative stress in human umbilical vein endothelial cells via Adenosine Monophosphate-Activated Protein Kinase signaling pathway. J. Food Biochem. 2019, 43, e12893. [Google Scholar] [CrossRef]
- Speciale, A.; Anwar, S.; Canali, R.; Chirafisi, J.; Saija, A.; Virgili, F.; Cimino, F. Cyanidin-3-O-glucoside counters the response to TNF-alpha of endothelial cells by activating Nrf2 pathway. Mol. Nutr. Food Res. 2013, 57, 1979–1987. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Lu, Y.; Chen, Y.; Cheng, J. The role of Nrf2 in oxidative stress-induced endothelial injuries. J. Endocrinol. 2015, 225, R83–R99. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.S.; Vissers, M.C.M.; Anderson, R.F.; Sreebhavan, S.; Bozonet, S.M.; Scheepens, A.; Melton, L.D. Bioavailable Blueberry-Derived Phenolic Acids at Physiological Concentrations Enhance Nrf2-Regulated Antioxidant Responses in Human Vascular Endothelial Cells. Mol. Nutr. Food Res. 2018, 62, 1700647. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Yang, Q.; Ma, W.; Li, J.; Qu, L.-Z.; Wang, M. Protocatechuic Acid Ameliorated Palmitic-Acid-Induced Oxidative Damage in Endothelial Cells through Activating Endogenous Antioxidant Enzymes via an Adenosine-Monophosphate-Activated-Protein-Kinase-Dependent Pathway. J. Agric. Food Chem. 2018, 66, 10400–10409. [Google Scholar] [CrossRef] [PubMed]
- Petry, A.; Djordjevic, T.; Weitnauer, M.; Kietzmann, T.; Hess, J.; Görlach, A. NOX2 and NOX4 Mediate Proliferative Response in Endothelial Cells. Antioxid. Redox Signal. 2006, 8, 1473–1484. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Yan, Z.; Li, D.; Ma, Y.; Zhou, J.; Sui, Z. Antioxidant and Anti-Inflammatory Effects of Blueberry Anthocyanins on High Glucose-Induced Human Retinal Capillary Endothelial Cells. Oxidative Med. Cell. Longev. 2018, 2018, 1862462. [Google Scholar] [CrossRef]
- Fukumoto, L.R.; Mazza, G. Assessing Antioxidant and Prooxidant Activities of Phenolic Compounds. J. Agric. Food Chem. 2000, 48, 3597–3604. [Google Scholar] [CrossRef]
- Huang, W.; Zhu, Y.; Li, C.; Sui, Z.; Min, W. Effect of Blueberry Anthocyanins Malvidin and Glycosides on the Antioxidant Properties in Endothelial Cells. Oxidative Med. Cell. Longev. 2016, 2016, 1591803. [Google Scholar] [CrossRef]
- Ferrari, D.; Speciale, A.; Cristani, M.; Fratantonio, D.; Molonia, M.S.; Ranaldi, G.; Saija, A.; Cimino, F. Cyanidin-3-O-glucoside inhibits NF-kB signalling in intestinal epithelial cells exposed to TNF-α and exerts protective effects via Nrf2 pathway activation. Toxicol. Lett. 2016, 264, 51–58. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X. Anthocyanins: Structural characteristics that result in unique metabolic patterns and biological activities. Free. Radic. Res. 2006, 40, 1014–1028. [Google Scholar] [CrossRef]
- Warner, E.F.; Smith, M.J.; Zhang, Q.; Raheem, K.S.; O’Hagan, D.; O’Connell, M.A.; Kay, C.D. Signatures of anthocyanin metabolites identified in humans inhibit biomarkers of vascular inflammation in human endothelial cells. Mol. Nutr. Food Res. 2017, 61, 1700053. [Google Scholar] [CrossRef] [PubMed]
- Canali, R.; Comitato, R.; Ambra, R.; Virgili, F. Red wine metabolites modulate NF-κB, activator protein-1 and cAMP response element-binding proteins in human endothelial cells. Br. J. Nutr. 2010, 103, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Goers, L.; Freemont, P.; Polizzi, K.M. Co-culture systems and technologies: Taking synthetic biology to the next level. J. R. Soc. Interface 2014, 11, 20140065. [Google Scholar] [CrossRef]
- Riva, A.; Ronchi, M.; Petrangolini, G.; Bosisio, S.; Allegrini, P. Improved Oral Absorption of Quercetin from Quercetin Phytosome®, a New Delivery System Based on Food Grade Lecithin. Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Chi, J.; Ge, J.; Yue, X.; Liang, J.; Sun, Y.; Gao, X.; Yue, P. Preparation of nanoliposomal carriers to improve the stability of anthocyanins. LWT 2019, 109, 101–107. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.M.; Cuadrado, C.; Redondo, I.B.; Giampieri, F.; González-Paramás, A.M.; Santos-Buelga, C. Novel approaches in anthocyanin research—Plant fortification and bioavailability issues. Trends Food Sci. Technol. 2021, 117, 92–105. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Festa, J.; Hussain, A.; Al-Hareth, Z.; Singh, H.; Da Boit, M. Anthocyanins and Vascular Health: A Matter of Metabolites. Foods 2023, 12, 1796. https://doi.org/10.3390/foods12091796
Festa J, Hussain A, Al-Hareth Z, Singh H, Da Boit M. Anthocyanins and Vascular Health: A Matter of Metabolites. Foods. 2023; 12(9):1796. https://doi.org/10.3390/foods12091796
Chicago/Turabian StyleFesta, Joseph, Aamir Hussain, Zakia Al-Hareth, Harprit Singh, and Mariasole Da Boit. 2023. "Anthocyanins and Vascular Health: A Matter of Metabolites" Foods 12, no. 9: 1796. https://doi.org/10.3390/foods12091796
APA StyleFesta, J., Hussain, A., Al-Hareth, Z., Singh, H., & Da Boit, M. (2023). Anthocyanins and Vascular Health: A Matter of Metabolites. Foods, 12(9), 1796. https://doi.org/10.3390/foods12091796