Meat Irradiation: A Comprehensive Review of Its Impact on Food Quality and Safety
Abstract
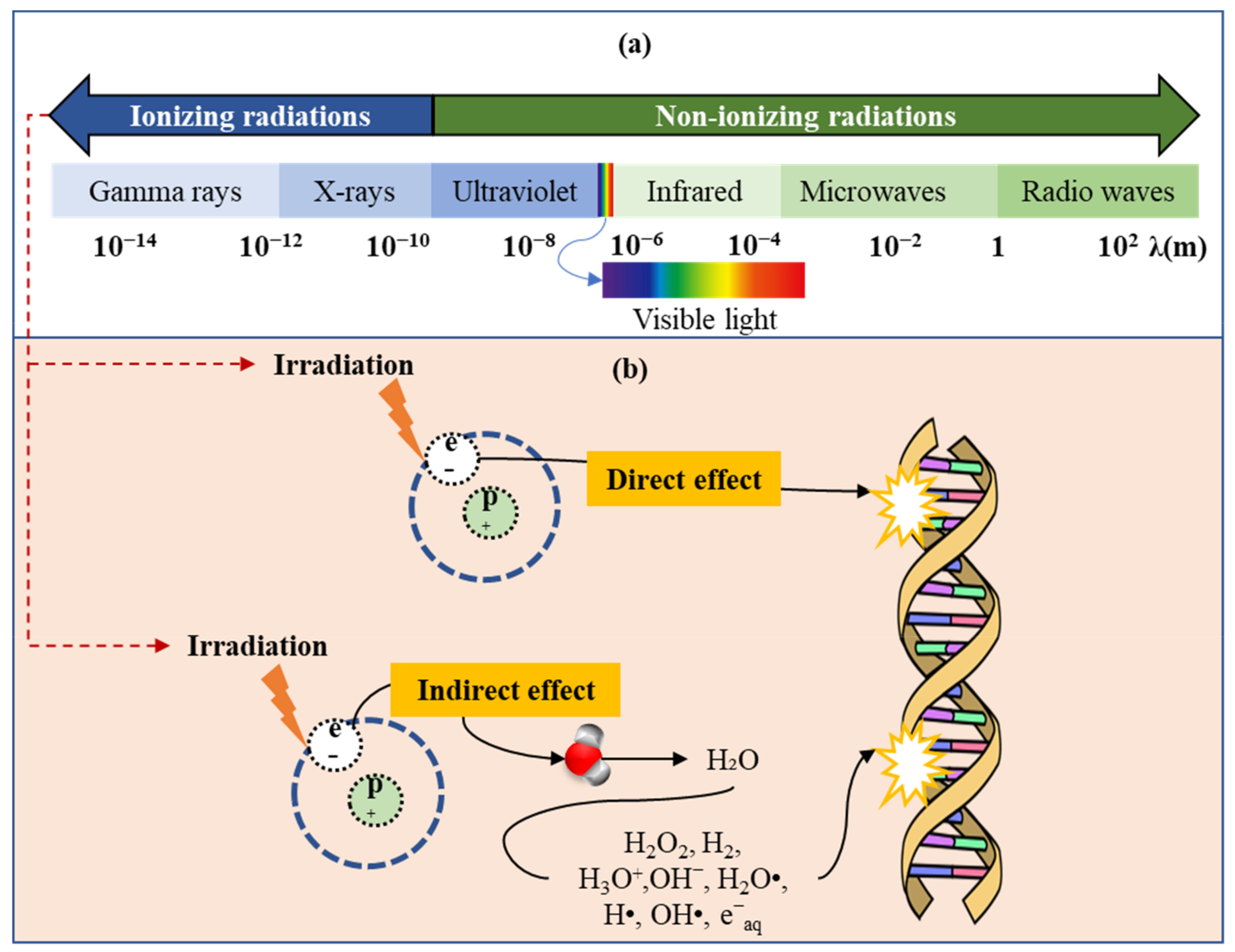
:1. Introduction
2. Sources and Principles of Food Irradiation
3. The Effects of Irradiation on Meat
3.1. Microbial Safety
3.2. Chemical Properties
3.3. Physical Properties
3.4. Shelf Life Extension
3.5. Nutritional Quality
3.6. Sensory Properties
4. Regulatory Framework for Food Irradiation
5. Research Needs and Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Pereira, P.M.d.C.C.; Vicente, A.F.d.R.B. Meat Nutritional Composition and Nutritive Role in the Human Diet. Meat Sci. 2013, 93, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Klurfeld, D.M. What Is the Role of Meat in a Healthy Diet? Anim. Front. 2018, 8, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Saucier, L. Microbial Spoilage, Quality and Safety within the Context of Meat Sustainability. Meat Sci. 2016, 120, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Bantawa, K.; Rai, K.; Subba Limbu, D.; Khanal, H. Food-Borne Bacterial Pathogens in Marketed Raw Meat of Dharan, Eastern Nepal. BMC Res. Notes 2018, 11, 618. [Google Scholar] [CrossRef] [PubMed]
- Madoroba, E.; Magwedere, K.; Chaora, N.S.; Matle, I.; Muchadeyi, F.; Mathole, M.A.; Pierneef, R. Microbial Communities of Meat and Meat Products: An Exploratory Analysis of the Product Quality and Safety at Selected Enterprises in South Africa. Microorganisms 2021, 9, 507. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Singh, A. Food Irradiation: An Established Food Processing Technology for Food Safety and Security. Def. Life Sci. J. 2019, 4, 206–213. [Google Scholar] [CrossRef]
- Farkas, J. Irradiation for Better Foods. Trends Food Sci. Technol. 2006, 17, 148–152. [Google Scholar] [CrossRef]
- Munir, M.T.; Federighi, M. Control of Foodborne Biological Hazards by Ionizing Radiations. Foods 2020, 9, 878. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Richa; Kumar, A.; Tyagi, M.B.; Sinha, R.P. Molecular Mechanisms of Ultraviolet Radiation-Induced DNA Damage and Repair. J. Nucleic Acids 2010, 2010, 592980. [Google Scholar] [CrossRef]
- Borrego-Soto, G.; Ortiz-López, R.; Rojas-Martínez, A. Ionizing Radiation-Induced DNA Injury and Damage Detection in Patients with Breast Cancer. Genet. Mol. Biol. 2015, 38, 420–432. [Google Scholar] [CrossRef]
- Lima, F.; Vieira, K.; Santos, M.; de Souza, P.M. Effects of Radiation Technologies on Food Nutritional Quality; IntechOpen: London, UK, 2018; pp. 137–146. [Google Scholar]
- Gómez, I.; Janardhanan, R.; Ibañez, F.C.; Beriain, M.J. The Effects of Processing and Preservation Technologies on Meat Quality: Sensory and Nutritional Aspects. Foods 2020, 9, 1416. [Google Scholar] [CrossRef] [PubMed]
- Da Vinha, A.C.M.F.; Sousa e Silva, C.A.d.A. Overview of Irradiation: Advantages to Foods of Plant Origin. South Florida J. Health 2022, 3, 248–262. [Google Scholar] [CrossRef]
- Gunes, G.; Deniz Tekin, M. Consumer Awareness and Acceptance of Irradiated Foods: Results of a Survey Conducted on Turkish Consumers. LWT 2006, 39, 444–448. [Google Scholar] [CrossRef]
- Food and Drug Administration; HHS. Irradiation in the Production, Processing and Handling of Food. Final Rule. Fed. Regist. 2012, 77, 71316–71320. [Google Scholar]
- Bonomo, L. A Critical Analysis Risk Assessment: Food Irradiation: Pro or Con? ESSAI 2006, 4, 8. Available online: https://dc.cod.edu/essai/vol4/iss1/8 (accessed on 30 March 2023).
- Monteiro, M.L.G.; Mársico, E.T.; Mano, S.B.; Teixeira, C.E.; Canto, A.C.V.d.C.S.; Carvalho Vital, H.; Conte-Júnior, C.A. Influence of Good Manufacturing Practices on the Shelf Life of Refrigerated Fillets of Tilapia (Oreochromis Niloticus) Packed in Modified Atmosphere and Gamma-irradiated. Food Sci. Nutr. 2013, 1, 298–306. [Google Scholar] [CrossRef]
- European Food Safety Authority. Scientific Opinion on the Efficacy and Microbiological Safety of Irradiation of Food. EFSA J. 2011, 9, 2103. [Google Scholar] [CrossRef]
- Castell-Perez, M.E.; Moreira, R.G. Irradiation and Consumers Acceptance. Innov. Food Process. Technol. A Compr. Rev. 2021, 2, 122–135. [Google Scholar] [CrossRef]
- D’Souza, C.; Apaolaza, V.; Hartmann, P.; Brouwer, A.R.; Nguyen, N. Consumer Acceptance of Irradiated Food and Information Disclosure—A Retail Imperative. J. Retail. Consum. Serv. 2021, 63, 102699. [Google Scholar] [CrossRef]
- Ehlermann, D.A.E. Safety of Food and Beverages: Safety of Irradiated Foods. In Encyclopedia of Food Safety; Motarjemi, Y.B.T., Ed.; Academic Press: Waltham, MA, USA, 2014; Volume 3, pp. 447–452. ISBN 9780123786128. [Google Scholar]
- Arvanitoyannis, I.S. Consumer Behavior toward Irradiated Food. In Irradiation of Food Commodities: Techniques, Applications, Detection, Legislation, Safety and Consumer Opinion; Arvanitoyannis, I.S.B.T.-I., Ed.; Academic Press: Boston, MA, USA, 2010; pp. 673–698. ISBN 9780123747181. [Google Scholar]
- Erkmen, O.; Bozoglu, T.F. Food Preservation by Irradiation. In Food Microbiology: Principles into Practice; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016; pp. 106–126. ISBN 9781119237860. [Google Scholar]
- Farkas, J.; Mohácsi-Farkas, C. History and Future of Food Irradiation. Trends Food Sci. Technol. 2011, 22, 121–126. [Google Scholar] [CrossRef]
- Maherani, B.; Hossain, F.; Criado, P.; Ben-Fadhel, Y.; Salmieri, S.; Lacroix, M. World Market Development and Consumer Acceptance of Irradiation Technology. Foods 2016, 5, 79. [Google Scholar] [CrossRef] [PubMed]
- Indiarto, R.; Pratama, A.W.; Sari, T.I.; Theodora, H.C. Food Irradiation Technology: A Review of the Uses and Their Capabilities. SSRG Int. J. Eng. Trends Technol. 2020, 68, 91–98. [Google Scholar] [CrossRef]
- Indiarto, R.; Qonit, M.A.H. A Review of Irradiation Technologies on Food and Agricultural Products. Int. J. Sci. Technol. Res. 2020, 9, 4411–4414. [Google Scholar]
- Morrison, R.M. Economics of Food Irradiation: Comparison between Electron Accelerators and Cobalt-60. Int. J. Radiat. Appl. Instrum. Part 1990, 35, 673–679. [Google Scholar] [CrossRef]
- Shahi, S.; Khorvash, R.; Goli, M.; Ranjbaran, S.M.; Najarian, A.; Mohammadi Nafchi, A. Review of Proposed Different Irradiation Methods to Inactivate Food-Processing Viruses and Microorganisms. Food Sci. Nutr. 2021, 9, 5883–5896. [Google Scholar] [CrossRef]
- Ehlermann, D.A.E. Particular Applications of Food Irradiation: Meat, Fish and Others. Radiat. Phys. Chem. 2016, 129, 53–57. [Google Scholar] [CrossRef]
- Lianou, A.; Panagou, E.Z.; Nychas, G.J.E. Meat Safety-I Foodborne Pathogens and Other Biological Issues. In Lawrie’s Meat Science: Eighth Edition; Toldra´, F., Ed.; Woodhead Publishing: Cambridge, UK, 2017; pp. 521–552. ISBN 9780081006979. [Google Scholar]
- Bintsis, T. Foodborne Pathogens. AIMS Microbiol. 2017, 3, 529–563. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.; Yoon, Y.; Yoon, J.W.; Oh, S.W.; Lee, S.; Lee, H. Salmonella Risk Assessment in Poultry Meat from Farm to Consumer in Korea. Foods 2023, 12, 649. [Google Scholar] [CrossRef]
- Fajardo-Guerrero, M.; Rojas-Quintero, C.; Chamorro-Tobar, I.; Zambrano, C.; Sampedro, F.; Carrascal-Camacho, A.K. Exposure Assessment of Salmonella Spp. in Fresh Pork Meat from Two Abattoirs in Colombia. Food Sci. Technol. Int. 2020, 26, 21–27. [Google Scholar] [CrossRef]
- Marin, C.; Cerdà-Cuéllar, M.; González-Bodi, S.; Lorenzo-Rebenaque, L.; Vega, S. Research Note: Persistent Salmonella Problems in Slaughterhouses Related to Clones Linked to Poultry Companies. Poult. Sci. 2022, 101, 101968. [Google Scholar] [CrossRef]
- Mkhungo, M.C.; Oyedeji, A.B.; Ijabadeniyi, O.A. Food Safety Knowledge and Microbiological Hygiene of Households in Selected Areas of Kwa-Zulu Natal, South Africa. Ital. J. Food Saf. 2018, 7, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.C.; Jo, C.; Ahn, D.U. Irradiation of Meat and Meat Products. In Emerging Technologies in Meat Processing: Production, Processing and Technology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016; pp. 7–36. ISBN 9781118350676. [Google Scholar]
- Ahn, D.U.; Kim, I.S.; Lee, E.J. Irradiation and Additive Combinations on the Pathogen Reduction and Quality of Poultry Meat. Poult. Sci. 2013, 92, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Chun, H.H.; Kim, J.Y.; Lee, B.D.; Yu, D.J.; Song, K.B. Effect of UV-C Irradiation on the Inactivation of Inoculated Pathogens and Quality of Chicken Breasts during Storage. Food Control 2010, 21, 276–280. [Google Scholar] [CrossRef]
- Yemmireddy, V.; Adhikari, A.; Moreira, J. Effect of Ultraviolet Light Treatment on Microbiological Safety and Quality of Fresh Produce: An Overview. Front. Nutr. 2022, 9, 871243. [Google Scholar] [CrossRef]
- Amiri, A.; Zandi, H.; Khosravi, H.M. Effect of Electron Beam Irradiation on Survival of Escherichia Coli O157:H7 and Salmonella Enterica Serovar Thyphimurium in Minced Camel Meat during Refrigerated Storage. J. Food Qual. Hazards Control 2019, 6, 174–178. [Google Scholar] [CrossRef]
- Park, J.G.; Yoon, Y.; Park, J.N.; Han, I.J.; Song, B.S.; Kim, J.H.; Kim, W.G.; Hwang, H.J.; Han, S.B.; Lee, J.W. Effects of Gamma Irradiation and Electron Beam Irradiation on Quality, Sensory, and Bacterial Populations in Beef Sausage Patties. Meat Sci. 2010, 85, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.; de Moura, F.H.; Van Den Broek, K.; de Mello, A.S. Effect of Ultraviolet Light, Organic Acids, and Bacteriophage on Salmonella Populations in Ground Beef. Meat Sci. 2018, 139, 44–48. [Google Scholar] [CrossRef]
- Hassanzadeh, P.; Tajik, H.; Rohani, S.M.R.; Moradi, M.; Hashemi, M.; Aliakbarlu, J. Effect of Functional Chitosan Coating and Gamma Irradiation on the Shelf-Life of Chicken Meat during Refrigerated Storage. Radiat. Phys. Chem. 2017, 141, 103–109. [Google Scholar] [CrossRef]
- Sedeh, F.M.; Arbabi, K.; Fatolahi, H.; Abhari, M. Using Gamma Irradiation and Low Temperature on Microbial Decontamination of Red Meat in Iran. Indian J. Microbiol. 2007, 47, 72–76. [Google Scholar] [CrossRef]
- Schevey, C.T.; Toshkov, S.; Brewer, M.S. Effect of Natural Antioxidants, Irradiation, and Cooking on Lipid Oxidation in Refrigerated, Salted Ground Beef Patties. J. Food Sci. 2013, 78, S1793–S1799. [Google Scholar] [CrossRef]
- C Reygaert, W. An Overview of the Antimicrobial Resistance Mechanisms of Bacteria. AIMS Microbiol. 2018, 4, 482–501. [Google Scholar] [CrossRef] [PubMed]
- Putri, M.S.; Susanna, D. Food Safety Knowledge, Attitudes, and Practices of Food Handlers at Kitchen Premises in the Port ‘X’ Area, North Jakarta, Indonesia 2018. Ital. J. Food Saf. 2021, 10, 9215. [Google Scholar] [CrossRef] [PubMed]
- Ham, Y.K.; Kim, H.W.; Hwang, K.E.; Song, D.H.; Kim, Y.J.; Choi, Y.S.; Song, B.S.; Park, J.H.; Kim, C.J. Effects of Irradiation Source and Dose Level on Quality Characteristics of Processed Meat Products. Radiat. Phys. Chem. 2017, 130, 259–264. [Google Scholar] [CrossRef]
- Song, B.S.; Lee, Y.; Park, J.H.; Kim, J.K.; Park, H.Y.; Kim, D.H.; Kim, C.J.; Kang, I.J. Toxicological and Radiological Safety of Chicken Meat Irradiated with 7.5 MeV X-rays. Radiat. Phys. Chem. 2018, 144, 211–217. [Google Scholar] [CrossRef]
- Otoo, E.A.; Ocloo, F.C.K.; Appiah, V. Effect of Gamma Irradiation on Shelf Life of Smoked Guinea Fowl (Numida Meleagris) Meat Stored at Refrigeration Temperature. Radiat. Phys. Chem. 2022, 194, 110041. [Google Scholar] [CrossRef]
- Reichel, J.; Kehrenberg, C.; Krischek, C. UV-C Irradiation of Rolled Fillets of Ham Inoculated with Yersinia Enterocolitica and Brochothrix Thermosphacta. Foods 2020, 9, 552. [Google Scholar] [CrossRef]
- Irmanita, V.; Wardani, A.K. Harsojo Pengaruh Iradiasi Gamma Terhadap Kadar Protein Dan Mikrobiologis Daging Ayam Broiler Pasar Tradisional Dan Pasar Modern Jakarta Selatan. J. Pangan Dan Agroindustri 2016, 4, 428–435. [Google Scholar]
- Damdam, A.N.; Alzahrani, A.; Salah, L.; Salama, K.N. Effects of UV-C Irradiation and Vacuum Sealing on the Shelf-Life of Beef, Chicken and Salmon Fillets. Foods 2023, 12, 606. [Google Scholar] [CrossRef]
- Calle, A.; Fernandez, M.; Montoya, B.; Schmidt, M.; Thompson, J. UV-C LED Irradiation Reduces Salmonella on Chicken and Food Contact Surfaces. Foods 2021, 10, 1459. [Google Scholar] [CrossRef]
- Feng, X.; Jo, C.; Nam, K.C.; Ahn, D.U. Impact of Electron-Beam Irradiation on the Quality Characteristics of Raw Ground Beef. Innov. Food Sci. Emerg. Technol. 2019, 54, 87–92. [Google Scholar] [CrossRef]
- Cap, M.; Lires, C.; Cingolani, C.; Mozgovoj, M.; Soteras, T.; Gentiluomo, J.; Principe, F.; Sucari, A.; Horak, C.; Signorini, M.; et al. Identification of the Gamma Irradiation Dose Applied to Ground Beef That Reduces Shiga Toxin Producing Escherichia Coli but Has No Impact on Consumer Acceptance. Meat Sci. 2021, 174, 108414. [Google Scholar] [CrossRef] [PubMed]
- Al-Bachir, M.; Zeinou, R. Effect of Gamma Irradiation on Microbial Load and Quality Characteristics of Minced Camel Meat. Meat Sci. 2009, 82, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Tolentino, M.; Diano, G.; Abrera, G.; Montefalcon, D.R.; Cobar, M.L.; Deocaris, C.; Baule, A.; Asaad, C. Electron Beam Irradiation of Raw Ground Beef Patties in the Philippines: Microbial Quality, Sensory Characteristics, and Cost-Analysis. Radiat. Phys. Chem. 2021, 186, 109536. [Google Scholar] [CrossRef]
- Xavier, M.d.l.P.; Dauber, C.; Mussio, P.; Delgado, E.; Maquieira, A.; Soria, A.; Curuchet, A.; Márquez, R.; Méndez, C.; López, T. Use of Mild Irradiation Doses to Control Pathogenic Bacteria on Meat Trimmings for Production of Patties Aiming at Provoking Minimal Changes in Quality Attributes. Meat Sci. 2014, 98, 383–391. [Google Scholar] [CrossRef]
- Isohanni, P.M.I.; Lyhs, U. Use of Ultraviolet Irradiation to Reduce Campylobacter Jejuni on Broiler Meat. Poult. Sci. 2009, 88, 661–668. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Y.; Guo, S.; Xiong, W.; Xia, H.; Liu, W.; Pan, Z.; Venkitasamy, C. Effect of Irradiation on Quality of Vacuum-Packed Spicy Beef Chops. J. Food Qual. 2017, 2017, 1054523. [Google Scholar] [CrossRef]
- Possas, A.; Valero, A.; García-Gimeno, R.M.; Pérez-Rodríguez, F.; Mendes de Souza, P. Combining UV-C Technology and Caffeine Application to Inactivate Escherichia Coli on Chicken Breast Fillets. Food Control 2021, 129, 108206. [Google Scholar] [CrossRef]
- Corrêa, T.Q.; Blanco, K.C.; Garcia, É.B.; Perez, S.M.L.; Chianfrone, D.J.; Morais, V.S.; Bagnato, V.S. Effects of Ultraviolet Light and Curcumin-Mediated Photodynamic Inactivation on Microbiological Food Safety: A Study in Meat and Fruit. Photodiagnosis Photodyn. Ther. 2020, 30, 101678. [Google Scholar] [CrossRef]
- Lázaro, C.A.; Conte, C.A.; Monteiro, M.L.G.; Canto, A.C.V.S.; Costa-Lima, B.R.C.; Mano, S.B.; Franco, R.M. Effects of Ultraviolet Light on Biogenic Amines and Other Quality Indicators of Chicken Meat during Refrigerated Storage. Poult. Sci. 2014, 93, 2304–2313. [Google Scholar] [CrossRef]
- Suklim, K.; Flick, G.J.; Vichitphan, K. Effects of Gamma Irradiation on the Physical and Sensory Quality and Inactivation of Listeria Monocytogenes in Blue Swimming Crab Meat (Portunas Pelagicus). Radiat. Phys. Chem. 2014, 103, 22–26. [Google Scholar] [CrossRef]
- Fregonesi, R.P.; Portes, R.G.; Aguiar, A.M.M.; Figueira, L.C.; Gonçalves, C.B.; Arthur, V.; Lima, C.G.; Fernandes, A.M.; Trindade, M.A. Irradiated Vacuum-Packed Lamb Meat Stored under Refrigeration: Microbiology, Physicochemical Stability and Sensory Acceptance. Meat Sci. 2014, 97, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Jouki, M. Evaluation of Gamma Irradiation and Frozen Storage on Microbial Load and Physico-Chemical Quality of Turkey Breast Meat. Radiat. Phys. Chem. 2013, 85, 243–245. [Google Scholar] [CrossRef]
- Badr, H.M. Use of Irradiation to Control Foodborne Pathogens and Extend the Refrigerated Market Life of Rabbit Meat. Meat Sci. 2004, 67, 541–548. [Google Scholar] [CrossRef]
- Khalafalla, G.M.; Nasr, N.F.; Gaafar, A.M.; Abo-Zaid, R.M. Effect of Gamma Irradiation on Microbial Load, Physicochemical Characteristics and Shelf-Life of Raw Minced Beef Meat. Middle East J. Appl. Sci. 2018, 8, 625–634. [Google Scholar]
- Brugnini, G.; Rodríguez, S.; Rodríguez, J.; Rufo, C. Effect of UV-C Irradiation and Lactic Acid Application on the Inactivation of Listeria Monocytogenes and Lactic Acid Bacteria in Vacuum-Packaged Beef. Foods 2021, 10, 1217. [Google Scholar] [CrossRef] [PubMed]
- Dini, H.; Fallah, A.A.; Bonyadian, M.; Abbasvali, M.; Soleimani, M. Effect of Edible Composite Film Based on Chitosan and Cumin Essential Oil-Loaded Nanoemulsion Combined with Low-Dose Gamma Irradiation on Microbiological Safety and Quality of Beef Loins during Refrigerated Storage. Int. J. Biol. Macromol. 2020, 164, 1501–1509. [Google Scholar] [CrossRef] [PubMed]
- Modi, V.K.; Sakhare, P.Z.; Sachindra, N.M.; Mahendrakar, N.S. Changes in Quality of Minced Meat from Goat Due to Gamma Irradiation. J. Muscle Foods 2008, 19, 430–442. [Google Scholar] [CrossRef]
- Gunes, G.; Yilmaz, N.; Ozturk, A. Effects of Irradiation Dose and O2 and CO2 Concentrations in Packages on Foodborne Pathogenic Bacteria and Quality of Ready-to-Cook Seasoned Ground Beef Product (Meatball) during Refrigerated Storage. Sci. World J. 2012, 2012, 274219. [Google Scholar] [CrossRef]
- Derakhshan, Z.; Oliveri Conti, G.; Heydari, A.; Hosseini, M.S.; Mohajeri, F.A.; Gheisari, H.; Kargar, S.; Karimi, E.; Ferrante, M. Survey on the Effects of Electron Beam Irradiation on Chemical Quality and Sensory Properties on Quail Meat. Food Chem. Toxicol. 2018, 112, 416–420. [Google Scholar] [CrossRef]
- Xiong, Y.L.; Guo, A. Animal and Plant Protein Oxidation: Chemical and Functional Property Significance. Foods 2021, 10, 40. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A Comprehensive Review on Lipid Oxidation in Meat and Meat Products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef] [PubMed]
- Indiarto, R.; Nurhadi, B.; Tensiska; Subroto, E.; Istiqamah, Y.J. Effect of Liquid Smoke on Microbiological and Physico-Chemical Properties of Beef Meatballs during Storage. Food Res. 2020, 4, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Indiarto, R.; Rezaharsamto, B. The Physical, Chemical, and Microbiological Properties of Peanuts during Storage: A Review. Int. J. Sci. Technol. Res. 2020, 9, 1909–1913. [Google Scholar]
- Momchilova, S.; Kazakova, A.; Taneva, S.; Aleksieva, K.; Mladenova, R.; Karakirova, Y.; Petkova, Z.; Kamenova-Nacheva, M.; Teneva, D.; Denev, P. Effect of Gamma Irradiation on Fat Content, Fatty Acids, Antioxidants and Oxidative Stability of Almonds, and Electron Paramagnetic Resonance (EPR) Study of Treated Nuts. Molecules 2023, 28, 1439. [Google Scholar] [CrossRef]
- Jo, Y.; An, K.A.; Arshad, M.S.; Kwon, J.H. Effects of E-Beam Irradiation on Amino Acids, Fatty Acids, and Volatiles of Smoked Duck Meat during Storage. Innov. Food Sci. Emerg. Technol. 2018, 47, 101–109. [Google Scholar] [CrossRef]
- Feng, X.; Moon, S.H.; Lee, H.Y.; Ahn, D.U. Effect of Irradiation on the Parameters That Influence Quality Characteristics of Raw Turkey Breast Meat. Radiat. Phys. Chem. 2017, 130, 40–46. [Google Scholar] [CrossRef]
- Cieśla, K.; Roos, Y.; Głuszewski, W. Denaturation Processes in Gamma Irradiated Proteins Studied by Differential Scanning Calorimetry. Radiat. Phys. Chem. 2000, 58, 233–243. [Google Scholar] [CrossRef]
- Rezaharsamto, B.; Subroto, E. A Review on Bioactive Peptides Derived from Various Sources of Meat and Meat By-Products. Int. J. Sci. Technol. Res. 2019, 8, 3151–3156. [Google Scholar]
- Li, Z.; Chu, S.; Wang, P.; Gao, S.; Li, S.; Yu, X. Effects of Irradiation Treatment on Protein Structure and Digestion Characteristics of Seed-Watermelon (Citrullus lanatus Var.) Kernel Protein. Food Sci. Biotechnol. 2020, 29, 1201–1211. [Google Scholar] [CrossRef]
- Taofiq, O.; Fernandes, Â.; Barros, L.; Barreiro, M.F.; Ferreira, I.C.F.R. UV-Irradiated Mushrooms as a Source of Vitamin D2: A Review. Trends Food Sci. Technol. 2017, 70, 82–94. [Google Scholar] [CrossRef]
- Dionísio, A.P.; Gomes, R.T.; Oetterer, M. Ionizing Radiation Effects on Food Vitamins—A Review. Braz. Arch. Biol. Technol. 2009, 52, 1267–1278. [Google Scholar] [CrossRef]
- Louria, D.B. Food Irradiation: Unresolved Issues. Clin. Infect. Dis. 2001, 33, 378–380. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Nam, K.C.; Ahn, D.U. Color, Oxidation-Reduction Potential, and Gas Production of Irradiated Meats from Different Animal Species. J. Food Sci. 2002, 67, 1692–1695. [Google Scholar] [CrossRef]
- Indiarto, R.; Pranoto, Y.; Santoso, U.S. Evaluation of Physicochemical Properties and Antioxidant Activity of Polyphenol-Rich Cacao Bean Extract through Water Blanching. Pak. J. Nutr. 2019, 18, 278–287. [Google Scholar] [CrossRef]
- Indiarto, R.; Pranoto, Y.; Santoso, U. Supriyanto In Vitro Antioxidant Activity and Profile of Polyphenol Compounds Extracts and Their Fractions on Cacao Beans. Pak. J. Biol. Sci. 2019, 22, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.E.; Abd El-Rahman, A.A.; Farag, M.D.E.D.H.; Abd El-Salam, Z.A.; Shehata, F.M. Impact of Ultraviolet Irradiation Processing on Quality of Fresh Beef Meat during Cold Storage. Agric. Eng. Int. CIGR J. 2015, 17, 130–139. [Google Scholar]
- Hwang, K.-E.; Kim, H.-W.; Song, D.-H.; Kim, Y.-J.; Ham, Y.-K.; Lee, J.-W.; Choi, Y.-S.; Kim, C.-J. Effects of Antioxidant Combinations on Shelf Stability of Irradiated Chicken Sausage during Storage. Radiat. Phys. Chem. 2015, 106, 315–319. [Google Scholar] [CrossRef]
- Khalid, W.; Arshad, M.S.; Nayik, G.A.; Alfarraj, S.; Ansari, M.J.; Guiné, R.P.F. Impact of Gamma Irradiation and Kale Leaf Powder on Amino Acid and Fatty Acid Profiles of Chicken Meat under Different Storage Intervals. Molecules 2022, 27, 8201. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, Y.J.; Eun, J.B. Effects of Ultraviolet Radiation on the Physicochemical Characteristics of Korean Native Cattle (Hanwoo) Beef. J. Korean Soc. Appl. Biol. Chem. 2015, 58, 149–156. [Google Scholar] [CrossRef]
- Bhoir, S.A.; Jhaveri, M.; Chawla, S.P. Evaluation and Predictive Modeling of the Effect of Chitosan and Gamma Irradiation on Quality of Stored Chilled Chicken Meat. J. Food Process Eng. 2019, 42, e13254. [Google Scholar] [CrossRef]
- Chouliara, E.; Badeka, A.; Savvaidis, I.; Kontominas, M.G. Combined Effect of Irradiation and Modified Atmosphere Packaging on Shelf-Life Extension of Chicken Breast Meat: Microbiological, Chemical and Sensory Changes. Eur. Food Res. Technol. 2008, 226, 877–888. [Google Scholar] [CrossRef]
- Movileanu, I.; Núñez De González, M.T.; Hafley, B.; Miller, R.K.; Keeton, J.T. Comparison of Dried Plum Puree, Rosemary Extract, and BHA/BHT as Antioxidants in Irradiated Ground Beef Patties. Int. J. Food Sci. 2013, 2013, 360732. [Google Scholar] [CrossRef] [PubMed]
- Lasmawati, D.; Nurlidar, F.; Mustika Pratama, I.; Widyastuti, H.; Mukti Benita, A.; Panca Tanhindarto, R. Physical Quality of Gamma Rays-Irradiated Meatball Stored At Room Temperature. Food Sci. J. Food Sci. Technol. 2021, 1, 69–86. [Google Scholar] [CrossRef]
- Chiesa, L.M.; Di Cesare, F.; Mosconi, G.; Pavlovic, R.; Campaniello, M.; Tomaiuolo, M.; Mangiacotti, M.; Chiaravalle, E.; Panseri, S. Lipidomics Profile of Irradiated Ground Meat to Support Food Safety. Food Chem. 2022, 375, 131700. [Google Scholar] [CrossRef]
- Lv, M.; Mei, K.; Zhang, H.; Xu, D.; Yang, W. Effects of Electron Beam Irradiation on the Biochemical Properties and Structure of Myofibrillar Protein from Tegillarca Granosa Meat. Food Chem. 2018, 254, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Tomac, A.; Cova, M.C.; Narvaiz, P.; Yeannes, M.I. Texture, Color, Lipid Oxidation and Sensory Acceptability of Gamma-Irradiated Marinated Anchovy Fillets. Radiat. Phys. Chem. 2015, 106, 337–342. [Google Scholar] [CrossRef]
- Kim, S.Y.; Yong, H.I.; Nam, K.C.; Jung, S.; Yim, D.G.; Jo, C. Application of High Temperature (14 °C) Aging of Beef M. Semimembranosus with Low-Dose Electron Beam and X-Ray Irradiation. Meat Sci. 2018, 136, 85–92. [Google Scholar] [CrossRef]
- Brewer, S. Irradiation Effects on Meat Color—A Review. Meat Sci. 2004, 68, 1–17. [Google Scholar] [CrossRef]
- Nusairat, B.; Tellez-Isaias, G.; Qudsieh, R. An Overview of Poultry Meat Quality and Myopathies. In Broiler Industry; Tellez-Isaias, G., Latorre, J.D., Martínez-Aguilar, Y., Eds.; IntechOpen: Rijeka, Croatia, 2022. [Google Scholar]
- Millar, S.J.; Moss, B.W.; Stevenson, M.H. The Effect of Ionising Radiation on the Colour of Beef, Pork and Lamb. Meat Sci. 2000, 55, 349–360. [Google Scholar] [CrossRef]
- Xiao, S.; Zhang, W.G.; Lee, E.J.; Ma, C.W.; Ahn, D.U. Effects of Diet, Packaging, and Irradiation on Protein Oxidation, Lipid Oxidation, and Color of Raw Broiler Thigh Meat during Refrigerated Storage. Poult. Sci. 2011, 90, 1348–1357. [Google Scholar] [CrossRef]
- Nam, K.C.; Ahn, D.U. Carbon Monoxide-Heme Pigment Is Responsible for the Pink Color in Irradiated Raw Turkey Breast Meat. Meat Sci. 2002, 60, 25–33. [Google Scholar] [CrossRef]
- Ramamoorthi, L.; Toshkov, S.; Brewer, M.S. Effects of Irradiation on Color and Sensory Characteristics of Carbon Monoxide-Modified Atmosphere Packaged Beef. J. Food Process. Preserv. 2011, 35, 701–707. [Google Scholar] [CrossRef]
- Ismail, H.A.; Lee, E.J.; Ko, K.Y.; Ahn, D.U. Fat Content Influences the Color, Lipid Oxidation, and Volatiles of Irradiated Ground Beef. J. Food Sci. 2009, 74, C432–C440. [Google Scholar] [CrossRef] [PubMed]
- Maqsood, S.; Manheem, K.; Gani, A.; Abushelaibi, A. Degradation of Myofibrillar, Sarcoplasmic and Connective Tissue Proteins by Plant Proteolytic Enzymes and Their Impact on Camel Meat Tenderness. J. Food Sci. Technol. 2018, 55, 3427–3438. [Google Scholar] [CrossRef] [PubMed]
- Khalid, W.; Maggiolino, A.; Kour, J.; Arshad, M.S.; Aslam, N.; Afzal, M.F.; Meghwar, P.; Zafar, K.-W.; De Palo, P.; Korma, S.A. Dynamic Alterations in Protein, Sensory, Chemical, and Oxidative Properties Occurring in Meat during Thermal and Non-Thermal Processing Techniques: A Comprehensive Review. Front. Nutr. 2023, 9, 1057457. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, L.M.; Sales, L.A.; Fontes, P.R.; Torres Filho, R.d.A.; Andrade, M.P.D.; Ramos, A.d.L.S.; Ramos, E.M. Combined Effects of Gamma Irradiation and Aging on Tenderness and Quality of Beef from Nellore Cattle. Food Chem. 2020, 313, 126137. [Google Scholar] [CrossRef]
- Franqueira De Toledo, T.C.; Canniatti-Brazaca, S.G.; Spoto, M.H.F.; Arthur, V. Sensory Evaluation of Chicken Breast under Gamma Irradiation at Commercial Doses. J. Food Sci. 2005, 70, S8–S12. [Google Scholar] [CrossRef]
- Suwattitanun, W.; Wattanachant, S. Effect of Various Temperature and Storage Time during Process on Physical Quality and Water-Holding Capacity of Broiler Breast Meat. KKU Respository J. 2014, 19, 628–635. [Google Scholar]
- Isleroglu, H.; Kemerli, T.; Kaymak-Ertekin, F. Effect of Steam-Assisted Hybrid Cooking on Textural Quality Characteristics, Cooking Loss, and Free Moisture Content of Beef. Int. J. Food Prop. 2015, 18, 403–414. [Google Scholar] [CrossRef]
- Hernández-García, E.; Vargas, M.; Torres-Giner, S. Quality and Shelf-Life Stability of Pork Meat Fillets Packaged in Multilayer Polylactide Films. Foods 2022, 11, 426. [Google Scholar] [CrossRef]
- Indiarto, R.; Indriana, L.P.A.; Andoyo, R.; Subroto, E.; Nurhadi, B. Bottom–up Nanoparticle Synthesis: A Review of Techniques, Polyphenol-Based Core Materials, and Their Properties. Eur. Food Res. Technol. 2022, 248, 1–24. [Google Scholar] [CrossRef]
- Addis, M. Major Causes Of Meat Spoilage and Preservation Techniques: A Review. Food Sci. Qual. Manag. 2015, 41, 2224–6088. [Google Scholar]
- Soladoye, O.P.; Aalhus, J.; Dugan, M. Oxidative and Enzymatic Factors Affecting Meat Spoilage. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2022; ISBN 978-0-08-100596-5. [Google Scholar]
- Iulietto, M.F.; Sechi, P.; Borgogni, E.; Cenci-Goga, B.T. Meat Spoilage: A Critical Review of a Neglected Alteration Due to Ropy Slime Producing Bacteria. Ital. J. Anim. Sci. 2015, 14, 316–326. [Google Scholar] [CrossRef]
- Amit, S.K.; Uddin, M.M.; Rahman, R.; Islam, S.M.R.; Khan, M.S. A Review on Mechanisms and Commercial Aspects of Food Preservation and Processing. Agric. Food Secur. 2017, 6, 51. [Google Scholar] [CrossRef]
- Blackburn, C.D.W. Food Spoilage Microorganisms; Woodhead Publishing Limited: Cambridge, UK, 2006; ISBN 9781855739666. [Google Scholar]
- Lorenzo, J.M.; Munekata, P.E.; Dominguez, R.; Pateiro, M.; Saraiva, J.A.; Franco, D. Main Groups of Microorganisms of Relevance for Food Safety and Stability: General Aspects and Overall Description. In Innovative technologies for food preservation: Inactivation of spoilage and pathogenic microorganisms; Barba, F.J., Sant’Ana, A.S., Orlien, V., Koubaa, M., Eds.; Academic Press, Elsevier: Cambridge, MA, USA, 2018; pp. 53–107. ISBN 9780128110324. [Google Scholar]
- Panseri, S.; Arioli, F.; Pavlovic, R.; Di Cesare, F.; Nobile, M.; Mosconi, G.; Villa, R.; Chiesa, L.M.; Bonerba, E. Impact of Irradiation on Metabolomics Profile of Ground Meat and Its Implications toward Food Safety. LWT 2022, 161, 113305. [Google Scholar] [CrossRef]
- Rima, F.; Sadakuzzaman, M.; Hossain, M.; Ali, M.; Hashem, M. Effect of Gamma Irradiation on Shelf Life and Quality of Broiler Meat. SAARC J. Agric. 2019, 17, 149–159. [Google Scholar] [CrossRef]
- Arshad, M.S.; Amjad, Z.; Yasin, M.; Saeed, F.; Imran, A.; Sohaib, M.; Anjum, F.M.; Hussain, S. Quality and Stability Evaluation of Chicken Meat Treated with Gamma Irradiation and Turmeric Powder. Int. J. Food Prop. 2019, 22, 153–171. [Google Scholar] [CrossRef]
- Mrityunjoy, A.; Israt, I.; Rashed, N. Effects of Gamma Irradiation on the Propagation of Microbial Growth in Commonly Available Meat in Bangladesh. Int. Food Res. J. 2019, 26, 1211–1218. [Google Scholar]
- Verma, A.K.; Kumar, D.; Kumar, P.; Angad, G.; Veterinary, D. Non Thermal Preservation of Meat by Irradiation: A Review. J. Food Res. Technol. 2015, 3, 7–13. [Google Scholar]
- Bhatnagar, P.; Gururani, P.; Bisht, B.; Kumar, V.; Kumar, N.; Joshi, R.; Vlaskin, M.S. Impact of Irradiation on Physico-Chemical and Nutritional Properties of Fruits and Vegetables: A Mini Review. Heliyon 2022, 8, e10918. [Google Scholar] [CrossRef]
- Gabelko, S.V.; Sapozhnikov, A.N. Development of Combined Semi-Finished Products from Poultry and Vegetables with Prolonged Shelf Life. IOP Conf. Ser. Earth Environ. Sci. 2021, 640, 032028. [Google Scholar] [CrossRef]
- Roberts, W.T.; Weese, J.O. Shelf Life of Ground Beef Patties Treated by Gamma Radiation. J. Food Prot. 1998, 61, 1387–1389. [Google Scholar] [CrossRef] [PubMed]
- García-Márquez, I.; Ordóñez, J.A.; Cambero, M.I.; Cabeza, M.C. Use of E-Beam for Shelf-Life Extension and Sanitizing of Marinated Pork Loin. Int. J. Microbiol. 2012, 2012, 962846. [Google Scholar] [CrossRef] [PubMed]
- Jayathilakan, K.; Sultana, K.; Jalarama Reddy, K.; Pandey, M.C. Radiation Processing of Meat and Meat Products-An Overview. Int. J. New Technol. Res. 2015, 1, 5–12. [Google Scholar]
- Hrubša, M.; Siatka, T.; Nejmanová, I.; Vopršalová, M.; Krčmová, L.K.; Matoušová, K.; Javorská, L.; Macáková, K.; Mercolini, L.; Remião, F.; et al. Biological Properties of Vitamins of the B-Complex, Part 1: Vitamins B1, B2, B3, and B5. Nutrients 2022, 14, 484. [Google Scholar] [CrossRef] [PubMed]
- Thayer, D.W.; Shieh, J.J.; Jenkins, R.K.; Phillips, J.G.; Wierbicki, E.; Ackerman, S.A. Effect of Gamma Ray Irradiation and Frying on the Thiamine Content of Bacon. J. Food Qual. 1989, 12, 115–134. [Google Scholar] [CrossRef]
- Fox, J.B.; Thayer, D.W.; Jenkins, R.K.; Phillips, J.G.; Ackerman, S.A.; Beecher, G.R.; Holden, J.M.; Morrow, F.D.; Quirbach, D.M. Effect of Gamma Irradiation on the b Vitamins of Pork Chops and Chicken Breasts. Int. J. Radiat. Biol. 1989, 55, 689–703. [Google Scholar] [CrossRef]
- Costa Henry, F. Irradiation Effects on Meat: A Review. Rev. Ciências Agrárias 2009, 32, 255–262. [Google Scholar]
- Osman, A.M.A.; Hassan, A.B.; Osman, G.A.M.; Mohammed, N.; Rushdi, M.A.H.; Diab, E.E.; Babiker, E.E. Effects of Gamma Irradiation and/or Cooking on Nutritional Quality of Faba Bean (Vicia faba L.) Cultivars Seeds. J. Food Sci. Technol. 2014, 51, 1554–1560. [Google Scholar] [CrossRef]
- El-Niely, H.F.G. Effect of Radiation Processing on Antinutrients, in-Vitro Protein Digestibility and Protein Efficiency Ratio Bioassay of Legume Seeds. Radiat. Phys. Chem. 2007, 76, 1050–1057. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Y.; Pan, Z.; Venkitasamy, C.; Zhang, L.; Xiong, W.; Guo, S.; Xia, H.; Liu, W. Effect of Electron Beam Irradiation on Quality and Protein Nutrition Values of Spicy Yak Jerky. LWT 2018, 87, 1–7. [Google Scholar] [CrossRef]
- Stadtman, E.R. Protein Oxidation in Aging and Age-Related Diseases. Ann. N. Y. Acad. Sci. 2001, 928, 22–38. [Google Scholar] [CrossRef] [PubMed]
- Stadtman, E.R.; Levine, R.L. Free Radical-Mediated Oxidation of Free Amino Acids and Amino Acid Residues in Proteins. Amino Acids 2003, 25, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Ahn, D.U. Lipid Oxidation and Its Implications to Meat Quality and Human Health. Food Sci. Biotechnol. 2019, 28, 1275–1285. [Google Scholar] [CrossRef]
- Jia, W.; Wang, X.; Zhang, R.; Shi, Q.; Shi, L. Irradiation Role on Meat Quality Induced Dynamic Molecular Transformation: From Nutrition to Texture. Food Rev. Int. 2022, 1–23. [Google Scholar] [CrossRef]
- Huang, X.; You, Y.; Liu, Q.; Dong, H.; Bai, W.; Lan, B.; Wu, J. Effect of Gamma Irradiation Treatment on Microstructure, Water Mobility, Flavor, Sensory and Quality Properties of Smoked Chicken Breast. Food Chem. 2023, 421, 136174. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Q.; Fan, J.; Yu, J.; Li, K.; Bai, J. Lipidomics Reveals Alterations of Lipid Composition and Molecular Nutrition in Irradiated Marble Beef. Food Chem. X 2023, 17, 100617. [Google Scholar] [CrossRef]
- Amaral, A.B.; Solva, M.V.D.; Lannes, S.C.D.S. Lipid Oxidation in Meat: Mechanisms and Protective Factors—A Review. Food Sci. Technol. 2018, 38, 1–15. [Google Scholar] [CrossRef]
- Roche, M.; Neti, P.V.S.V.; Kemp, F.W.; Azzam, E.I.; Ferraris, R.P.; Howell, R.W. High Levels of Dietary Supplement Vitamins A, C and e Are Absorbed in the Small Intestine and Protect Nutrient Transport against Chronic Gamma Irradiation. Radiat. Res. 2015, 184, 470–481. [Google Scholar] [CrossRef]
- Yang, H.; Xu, L.L.; Hou, L.; Xu, T.C.; Ye, S.H. Stability of Vitamin A, E, C and Thiamine during Storage of Different Powdered Enteral Formulas. Heliyon 2022, 8, e11460. [Google Scholar] [CrossRef]
- Ramírez-Cahero, H.F.; Valdivia-López, M.A. Effect of Gamma Radiation on Sugars and Vitamin C: Radiolytic Pathways. Food Chem. 2018, 245, 1131–1140. [Google Scholar] [CrossRef] [PubMed]
- Cef, P.A. Scientific Opinion on the Chemical Safety of Irradiation of Food. EFSA J. 2011, 9, 1930. [Google Scholar] [CrossRef]
- O’Bryan, C.A.; Crandall, P.G.; Ricke, S.C.; Olson, D.G. Impact of Irradiation on the Safety and Quality of Poultry and Meat Products: A Review. Crit. Rev. Food Sci. Nutr. 2008, 48, 442–457. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Shi, Q.; Shi, L. Effect of Irradiation Treatment on the Lipid Composition and Nutritional Quality of Goat Meat. Food Chem. 2021, 351, 129295. [Google Scholar] [CrossRef] [PubMed]
- Starowicz, M.; Kubara Poznar, K.; Zieliński, H. What Are the Main Sensory Attributes That Determine the Acceptance of Meat Alternatives? Curr. Opin. Food Sci. 2022, 48, 100924. [Google Scholar] [CrossRef]
- Du, M.; Hur, S.J.; Ahn, D.U. Raw-Meat Packaging and Storage Affect the Color and Odor of Irradiated Broiler Breast Fillets after Cooking. Meat Sci. 2002, 61, 49–54. [Google Scholar] [CrossRef]
- Junqueira-Gonçalves, M.P.; Galotto, M.J.; Valenzuela, X.; Dinten, C.M.; Aguirre, P.; Miltz, J. Perception and View of Consumers on Food Irradiation and the Radura Symbol. Radiat. Phys. Chem. 2011, 80, 119–122. [Google Scholar] [CrossRef]
- Bearth, A.; Siegrist, M. “As Long as It Is Not Irradiated”—Influencing Factors of US Consumers’ Acceptance of Food Irradiation. Food Qual. Prefer. 2019, 71, 141–148. [Google Scholar] [CrossRef]
- Yim, D.G.; Ahn, D.U.; Nam, K.C. Effect of Packaging and Antioxidant Combinations on Physicochemical Properties of Irradiated Restructured Chicken Rolls. Korean J. Food Sci. Anim. Resour. 2015, 35, 248–257. [Google Scholar] [CrossRef]
- Ursachi, C.Ș.; Perța-Crișan, S.; Munteanu, F.-D. Strategies to Improve Meat Products’ Quality. Foods 2020, 9, 1883. [Google Scholar] [CrossRef]
- Koenari, Z.I.; Siagian, C.M.; Simanungkalit, B.; Nilatany, A.; Pratama, I.M.; Lasmawati, D.; Nurcahya, C.M. Potential Use of Gamma-Irradiated Ethnic Ready-to-Eat Foods to Improve the Nutritional Status of Landslide Victims. Foods 2016, 5, 53. [Google Scholar] [CrossRef]
- Caputo, V. Does Information on Food Safety Affect Consumers’ Acceptance of New Food Technologies? The Case of Irradiated Beef in South Korea under a New Labelling System and across Different Information Regimes. Aust. J. Agric. Resour. Econ. 2020, 64, 1003–1033. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.S.; Tserkezou, P. Legislation on Food Irradiation: European Union, United States, Canada, and Australia. In Irradiation of Food Commodities: Techniques, Applications, Detection, Legislation, Safety and Consumer Opinion; Academic Press, an imprint of Elsevier: London, UK, 2010; pp. 3–20. ISBN 9780123747181. [Google Scholar]
- Derr, D.D. International Regulatory Status and Harmonization of Food Irradiation. J. Food Prot. 1993, 56, 882–886. [Google Scholar] [CrossRef] [PubMed]
- Ji, A.; An, I. Irradiation: Utilization, Advances, Safety, Acceptance, Future Trends, and a Means to Enhance Food Security. Adv. Appl. Sci. Res. 2020, 11, 1. [Google Scholar]
- FDA, U.S. Irradiation in the Production, Processing, and Handling of Food, Final Rule. Fed. Regist. 2004, 69, 76844–76847. [Google Scholar]
- Mostafavi, H.A.; Fathollahi, H.; Motamedi, F.; Mirmajlessi, S.M. Food Irradiation: Applications, Public Acceptance and Global Trade. Afr. J. Biotechnol. 2010, 9, 2826–2833. [Google Scholar]
- Turgis, M.; Han, J.; Borsa, J.; Lacroix, M. Combined Effect of Natural Essential Oils, Modified Atmosphere Packaging, and Gamma Radiation on the Microbial Growth on Ground Beef. J. Food Prot. 2008, 71, 1237–1243. [Google Scholar] [CrossRef]
- Monique, L.; Samia, A.; Dominic, D.; Mélanie, T.; Stéphane, S.; Takala, P.; Dang Khanh, V. Irradiation in Combined Treatments and Food Safety. J. Radioanal. Nucl. Chem. 2013, 296, 1065–1069. [Google Scholar] [CrossRef]
- Turgis, M.; Borsa, J.; Millette, M.; Salmieri, S.; Lacroix, M. Effect of Selected Plant Essential Oils or Their Constituents and Modified Atmosphere Packaging on the Radiosensitivity of Escherichia Coli O157:H7 and Salmonella Typhi in Ground Beef. J. Food Prot. 2008, 71, 516–521. [Google Scholar] [CrossRef]
- Lacroix, M.; Ouattara, B. Combined Industrial Processes with Irradiation to Assure Innocuity and Preservation of Food Products–A Review. Food Res. Int. 2000, 33, 719–724. [Google Scholar] [CrossRef]
- Gimeno, D.; Gonzalez-Buesa, J.; Oria, R.; Venturini, M.E.; Arias, E. Effect of Modified Atmosphere Packaging (MAP) and UV-C Irradiation on Postharvest Quality of Red Raspberries. Agriculture 2022, 12, 29. [Google Scholar] [CrossRef]
- Szczawinska, M.E. Application of Ionizing Radiation for Control of Salmonella in Food. In Current Topics in Salmonella and Salmonellosis; Mares, M., Ed.; IntechOpen: London, UK, 2017; pp. 253–274. ISBN 978-953-51-3066-6. [Google Scholar]
- Xu, J.; Zhang, M.; Bhandari, B.; Cao, P. Microorganism Control and Product Quality Improvement of Twice-Cooked Pork Dish Using ZnO Nanoparticles Combined Radio Frequency Pasteurization. LWT 2018, 95, 65–71. [Google Scholar] [CrossRef]
| Sample | Treatment | Dose | Sample Storage Conditions | Microbiological Effect(s) | Ref. |
|---|---|---|---|---|---|
| Ground beef | UV irradiation + bacteriophage | 800 µW/cm2 | Refrigerator storage at 4 °C | The most effective approach to reduce Salmonella in ground beef samples was a combination of UV radiation and bacteriophage, resulting in a 99% reduction (2 log cycles) in levels of the pathogen. | [43] |
| Smoked guinea fowl meat | Gamma irradiation | 2.5, 5 and 7.5 kGy | Stored in the refrigerator (2–4 °C); storage period of 7 days | The amount of Staphylococcus sp., Escherichia coli, and Bacillus sp. decreased as the irradiation dose and storage period increased. | [51] |
| Bovine meat | Gamma irradiation | 0, 0.5, 1, 2 and 3 kGy | Commercial freezing bags; storage: 3 weeks in refrigerator (4–7 °C), and 8 months in freezer (−18 °C) | Mesophilic bacteria, coliforms, and Staphylococcus aureus content was reduced with 3 kGy irradiation. | [45] |
| Sliced ham | UV-C irradiation | 408, 2040, 4080, and 6120 mJ/cm2 | Refrigerated storage; 0, 7, and 14 days | Irradiation effectively reduced the growth of Yersinia enterocolitica and Brochothrix thermosphacta, as well as total microbial count, without any significant impact on the quality of the product. | [52] |
| Beef sausage patties | Gamma irradiation and electron-beam irradiation | 0–20 kGy | Vacuum-packed, stored at 30 °C; 10 days | After 5 kGy and 15 kGy irradiation, the bacterial count was less than 2 log CFU/g. | [42] |
| Broiler chicken | Gamma irradiation | 0–5 kGy | Packed in plastic + sealer, cold storage | Escherichia coli and Staphylococcus aureus were inhibited by 5 kGy irradiation. | [53] |
| Beef, Chicken and Salmon Fillets | UV-C irradiation | 360 J/m2 | Vacuum storage; 5 days | Vacuum packing combined with UV-C irradiation was very effective for slowing the growth of Pseudomonas sp., aerobic bacteria, lactic acid bacteria, Salmonella, and Escherichia coli during storage. | [54] |
| Chicken breast | UV-C irradiation | 0.12–3.6 J/cm2; 0–15 min | Placed on the tray; storage in the refrigerator; 30 min | The exposure of chicken breast to irradiation for a minimum of 1 min led to a reduction of over 90% in Salmonella. | [55] |
| Raw ground beef | Electron-beam irradiation | 0, 1.5, 3.0 and 4.5 kGy | Vacuum packed for 24 h at 4 °C | Dimethyl sulfoxide, a characteristic off-odor compound in irradiated meat, was detected in samples produced from the radiolysis of methionine when subjected to 4.5 kGy irradiation. | [56] |
| Ground beef | Gamma irradiation | First assay: 0.26, 0.44, 0.67 and 0.86 Second assay: 1 kGy Third assay: 2.5 kGy | Freezer storage at −18 °C | 1 kGy reduced Shiga toxin-producing Escherichia coli (STEC) by 5 log CFU/g in ground beef. | [57] |
| Minced camel meat | Gamma irradiation | 0, 2, 4, and 6 kGy | Refrigerator storage at 1–4 °C for 2, 4, and 6 weeks | Irradiation increased shelf life from 2 weeks to 6 weeks by reducing mesophilic aerobic plates and coliforms. | [58] |
| Raw ground beef patties | Electron beam irradiation | 2, 4, and 6 kGy | Packed with nylon polyethylene bag; stored at −18 °C; storage period: up to 7 months | Exposing the sample to electron beams for a dosage of 2 kGy had reduced the microbial population to a safe level. | [59] |
| Beef trimmings (20% fat) | Gamma irradiation | 2 to 5 kGy | Sterile bags; polyethylene bags; stored at −18 °C or 2 °C; up to 30 days | Pathogenic microorganisms like Listeria monocytogenes and Escherichia coli can be reduced by 2.5 kGy irradiation. | [60] |
| Broiler meat | UV irradiation, activated oxygen (AO) | 9.4, 18.8 dan 32.9 mW/s per cm2 | Up to 12 days in vacuum bags at 3–5 °C | UV irradiation alone or in combination with AO affected Campylobacter jejuni survival. Campylobacter jejuni was reduced most when irradiated at 32.9 mW/s per cm2. | [61] |
| Spicy beef jerky | Gamma irradiation | 0, 0.5, 1.5, 3, 4, 6, and 8 kGy | Vacuum packed; refrigerator storage (4–8 °C); 1 week | Irradiation at a minimum of 1.5 kGy resulted in Escherichia coli levels lower than 30 MPN/100g. A minimum irradiation dose of 6 kGy led to aerobic bacteria levels lower than 10 CFU/g. | [62] |
| Chicken breast fillets | UV-C irradiation; caffeine application (20 mM/g) | 0–15 J/cm2 | Styrofoam boxes; stored at −10 °C | The inactivation of Escherichia coli was found to increase with higher concentrations of caffeine and UV-C doses. | [63] |
| Chicken breast fillets | UV-C irradiation; caffeine application (20 mM/g) | 0–15 J/cm2 | Styrofoam boxes; stored at −10 °C | The combination of caffeine + UV-C can reduce Escherichia coli by more than 6 logs. | [63] |
| Meat (beef and chicken) | UV-C and curcumin-mediated photodynamic inactivation (PDI) | 0.8, 1.6, 2.3, 3.1, 3.9 and 7.8 J/cm², for 1, 2, 3, 4, 5 and 10 min | Placed on a stainless steel grid | Meats treated with UV-C radiation and PDI had significantly reduced levels of Escherichia coli and Staphylococcus aureus. | [64] |
| Chicken meat | UV-C irradiation | 0.62, 1.13, and 1.95 mW/cm² | Packaged in polyvinyl chloride film and kept at 4 °C for 9 days | Irradiation reduced pathogenic bacteria at 1.95 mW/cm2 without affecting chicken meat quality. The highest UV-C intensity reduced the initial bacterial load and extended the lag phase and shelf life of meat products. | [65] |
| Blue swimming crab meat (Portunas pelagicus) | Gamma irradiation | 2, 4, and 6 kGy | Stored at 0–4 °C in polypropylene bags for 28 days | Listeria monocytogenes were inactivated by 4 and 6 kGy gamma irradiation. | [66] |
| Lamb loin cuts (Longissimus dorsi) | Gamma irradiation | 1.5 and 3.0 kGy | Vacuum packed with EVA/PVDC plastic bags; stored at 1 ± 1 °C for 56 days. | Microorganisms were reduced in a 3.0 kGy extended lamb loin stored at 1 °C for 14 to 56 days without affecting physicochemical properties or consumer acceptance. | [67] |
| Turkey breast meat | Gamma irradiation | 0.0, 0.5, 2.0 and 4.0 kGy | 2 months at −18 °C | Mesophilic and coliform bacteria were reduced by over 5 log units with a 4 kGy dose. The shelf life was six months because salmonella was not present. | [68] |
| Rabbit meat | Gamma irradiation | 0, 1.5, and 3 kGy | Packed in polyethylene pouches; stored in the refrigerator 3–5 °C; 3 weeks | In meat samples, 3 kGy irradiation inhibited Staphylococcus aureus, Listeria monocytogenes, Salmonella, and Enterobacteriaceae growth. It extended meat shelf life without affecting sensory quality. | [69] |
| Raw minced beef meat | Gamma irradiation | 2, 4, 6, 8, and 10 kGy | Sterile bags; stored in refrigerator 4–5 °C for 28 days | The growth of Staphylococcus aureus, Salmonella sp., Shigella sp., and other dangerous microorganisms were significantly reduced, while beef shelf life was increased by over four weeks. | [70] |
| Beef | UV-C irradiation | 165, 330 and 398 mJ/cm2 | Vacuum-packed; stored at 4 °C for 8 weeks | In vacuum-packed meat, lactic acid and irradiation inhibited the growth of L. monocytogenes and delayed lactic acid bacteria growth for 2 weeks. This method preserved meat color for up to eight weeks at 4 °C. | [71] |
| Beef loins | Gamma irradiation; chitosan film containing cumin essential oil nanoemulsion | 2.5 kGy | Stored at 3 °C for 21 days in polyethylene pouches. | The combination of these treatments effectively controlled the growth of microbial flora and inoculated foodborne pathogens. | [72] |
| Goat meat | Gamma irradiation | 4 kGy | Packed in polyethylene bags without vacuum; refrigerator storage at 2–4 °C; 8 days | Irradiation reduced total aerobic bacteria, psychrotrophic, total staphylococci, yeast, and molds. Irradiated samples had no enterococci, Escherichia coli, or Staphylococcus aureus. | [73] |
| Meatball | Gamma irradiation | 2 and 4 kGy | Aerobic packaging and MAP; refrigerator storage (2–4 °C); 0–14 days | Using 5% O2 and 50% CO2 modified atmosphere packaging (MAP) and up to 4 kGy irradiation, Escherichia coli O157:H7, Salmonella enteritidis, and Listeria monocytogenes growth was reduced. | [74] |
| Smoked guinea fowl meat | Gamma irradiation | 2.5, 5 and 7.5 kGy | Stored for 7 weeks in the refrigerator (2–4 °C) | Staphylococcus aureus, Serratia marcescens, and Enterobacter cloacae growth decreased with increasing irradiation doses. | [51] |
| Sample | Treatment | Dose | Sample Storage Conditions | Chemical Characteristics | Ref. |
|---|---|---|---|---|---|
| Smoked guinea fowl meat | Gamma irradiation | 2.5, 5 and 7.5 kGy | Stored in the refrigerator (2–4 °C); storage period of 7 days | The titratable acidity and acid values in meat samples were reduced by irradiation. | [51] |
| Raw ground beef | Electron-beam | 0, 1.5, 3.0 and 4.5 kGy | Vacuum packed and kept at 4 °C for 24 h | After one week at 5–10 °C, the meat’s pH, texture, TVBN (total volatile basic nitrogen), and growth of microorganisms did not change. Therefore, the irradiated products had a longer shelf life. | [56] |
| Minced camel meat | Gamma irradiation | 0, 2, 4 and 6 kGy | Polystyrene trays covered in polyethylene film, stored in a refrigerator at 1–4 °C for up to 6 weeks | Camel meat irradiation slightly affected TVBN and lipid oxidation. | [58] |
| Beef trimmings (20% fat) | Gamma irradiation | 2 to 5 kGy | Sterile bags; polyethylene bags; stored at −18 °C or 2 °C; stored for up to 30 days | 5 kGy irradiation increased the off-flavor intensity of 30-day-aged trimming patties. | [60] |
| Beef loins | Gamma irradiation; chitosan film containing cumin essential oil nanoemulsion | 2.5 kGy | Stored at 3 °C for 21 days in polyethylene pouches | Irradiation and chitosan-containing essential oil nanoemulsion showed slowed changes in water, protein, total lipid, and ash content. | [72] |
| Smoked guinea fowl meat | Gamma irradiation | 2.5, 5 and 7.5 kGy | Stored in the refrigerator (2–4 °C) with a storage period of 7 weeks | Irradiation decreased the titratable acidity and the acid value of meat samples. | [51] |
| Minced camel meat | Gamma irradiation | 0, 2, 4 and 6 kGy | Placed in polystyrene trays and covered with polyethylene film; stored in refrigerator 1–4 °C; storage for up to 6 weeks | Irradiation did not affect camel meat moisture, protein, fat, thiobarbituric acid (TBA) values, total acidity, or fatty acid content. | [58] |
| Rabbit meat | Gamma irradiation | 0, 1.5, and 3 kGy | Packed in polyethylene pouches; stored in the refrigerator 3–5 °C; stored for 3 weeks | Irradiating rabbit meat samples increased the content of thiobarbituric acid reactive substances (TBARS) but did not affect total volatile nitrogen (TVN). Both irradiated and non-irradiated samples had significantly increased TBARS content and TVN during storage. | [69] |
| Turkey breast meat | Gamma irradiation | 0.0, 0.5, 2.0 and 4.0 kGy | Stored at −18 °C; 2 months | Irradiation increased peroxide values but no significant effect was found on TVN content. | [68] |
| Chicken breast fillets | UV-C irradiation; caffeine application (20 mM/g) | 0–15 J/cm2 | Styrofoam boxes; stored at −10 °C | UV-C irradiation and caffeine treatment did not affect protein, crude fat, moisture, ash, total acidity, pH, and absorption coefficient. | [63] |
| Raw ground beef | Electron beam | 0, 1.5, 3.0 and 4.5 kGy | Vacuum packed, stored at 4 °C for 24 h | From 0 to 4.5 kGy, irradiation doses increased lipid and protein oxidation by 156% and 64%, respectively. It suggested that the sample was more prone to lipid oxidation than protein oxidation. | [56] |
| Chicken meat | UV-C light | 0.62, 1.13, and 1.95 mW/cm² | Polyvinyl chloride-wrapped; stored at 4 °C for 9 days | UV-C light increased tyramine, cadaverine, and putrescine levels. | [65] |
| Bovine meat | Gamma irradiation | 0, 0.5, 1, 2 and 3 kGy | Packed in commercial freezing bags; refrigerator storage at 4–7 °C for 3 weeks and freezer storage at −18 °C for 8 months | The chemical characteristics of bovine meat samples subjected to irradiation, specifically the total nitrogen and peroxide values, exhibited no significant differences during frozen storage. | [45] |
| Chicken Meat | Gamma irradiation, kale leaf powder (KLP) and their combination | 3 kGy | Aerobically packaged; refrigerator storage at 4 °C; up to 14 days | 1% and 2% KLP decreased chicken meat pH during storage, but 3 kGy gamma radiation increased pH. The pH of the meat decreased when KLP was combined with 3 kGy irradiation. | [94] |
| Korean native cattle (Hanwoo) beef | UV irradiation | 4.5 mW s/cm2 | Wrapped in polyvinyl chloride; stored at 3–5 °C; stored for 9 days | During storage, irradiated meat had higher TBA values. Volatile basic nitrogen (VBN) values did not change significantly. The physico-chemical properties of the samples were unaffected by UV radiation. | [95] |
| Spicy beef jerky | Gamma irradiation | 0, 0.5, 1.5, 3, 4, 6, and 8 kGy | Vacuum-packed; refrigerator storage (4–8 °C); 1 week | The content of capsanthin in the spicy beef jerky sample decreased, while the level of TBARS increased with an increase in irradiation dose | [62] |
| Meatball | Gamma irradiation | 2 and 4 kGy | Packaged in aerobic packaging and MAP; refrigerator storage 2–4 °C; 0–14 days | The TBARS value of the meatballs showed a significant increase as the irradiation dose increased. | [74] |
| Chicken meat | Gamma irradiation, 0.1 % chitosan and their combination | 0.5 kGy | Packed in polypropylene bags, chilled conditions 4–6 °C; storage up to 14 days. | After 11 days, treated samples showed significant changes in TBARS, trichloroacetic acid-soluble protein (TSP), and trichloroacetic acid (TCA) content. | [96] |
| Goat meat | Gamma irradiation | 4 kGy | Packed in polyethylene bags without vacuum; refrigerator storage at 2–4 °C; 8 days | Compared to non-irradiated goat meat samples, irradiation decreased pH, water-holding capacity, and TBA. However, irradiated samples had higher free fatty acids and a* values. | [73] |
| Beef, Chicken and Salmon Fillets | UV-C irradiation | 360 J/m2 | Vacuum stored; 5 days | The pH of all samples remained unchanged after exposure to irradiation. | [54] |
| Beef | UV-C irradiation | 165, 330 and 398 mJ/cm2 | Vacuum-packed; stored at 4 °C; 8 weeks | Compared to non-irradiated meat, irradiated meat had a lower pH. | [71] |
| Chicken breast meat | Gamma irradiation | 4 kGy | Packaged under MAP; refrigeration storage at 2–6 °C; storage period of 25 days | The TBA value of meats remained below 1 mg of malondialdehyde per kg after 25 days of storage. | [97] |
| Ground beef patties | E-beam irradiation | 1.5, dan 2.0 kGy | Insulated containers; refrigerator storage (4 °C); 0–28 days | In irradiated samples, dried plum puree, rosemary extract, and BHA/BHT antioxidants reduced TBARS levels. | [98] |
| Meatballs | Gamma irradiation | 20, 25 dan 35 kGy | Vacuum-packed + frozen 24 h; room temperature (irradiated samples) and freezer (non-irradiated); 2 months | After irradiation, the pH of meatballs increased before storage but decreased after two months. Irradiated samples had significantly lower Aw values. | [99] |
| Korean native cattle (Hanwoo) beef | UV irradiation | 4.5 mW s/cm2 | Wrapped in polyvinyl chloride; stored at 3–5 °C; 9 days | During storage, pH was not significantly different in all irradiated beef samples. | [95] |
| Chicken, turkey and mixed ground meat | X-ray irradiation | 0.5, 1, 3 and 5 kGy | Plastic bag; stored at −80 °C | Exposure of the meat sample to irradiation led to alterations in the lipidome. | [100] |
| Tegillarca granosa meat | Electron-beam irradiation | 0, 1, 3, 5, 7 or 9 kGy | Vacuum-packed | Irradiation at 7 kGy enhanced the biochemical characteristics of proteins in the meat sample. | [101] |
| Sample | Treatment | Dose | Sample Storage Conditions | Physical Characteristics | Ref. |
|---|---|---|---|---|---|
| Spicy beef jerky | Gamma irradiation | 0, 0.5, 1.5, 3, 4, 6, and 8 kGy | Vacuum-packed; refrigerator storage (4–8 °C); 1 week | The hardness, elasticity, and gumminess of spicy beef jerky samples decreased with increased irradiation. | [62] |
| Beef | UV-C irradiation | 165, 330 and 398 mJ/cm2 | Vacuum-packed; stored at 4 °C; 8 Weeks | Irradiation reduced a* values, but L* and b* values were unaffected. After 8 weeks of storage, L* and a* values were lower than those of non-irradiated samples, but b* values were not significantly different. | [71] |
| Chicken breast meat | Gamma irradiation | 4 kGy | Packaged under MAP; refrigeration storage at 2–6 °C; storage period of 25 days | The irradiation treatment led to a slight increase in the a* values, whereas no significant change was observed in the L* and b* values. | [97] |
| Korean native cattle (Hanwoo) beef | UV irradiation | 4.5 mW s/cm2 | Wrapped in polyvinyl chloride; stored at 3–5 °C; 9 days | During storage, there was an increase in L* and b* values, while the a* value in all samples initially increased until day six and then decreased. | [95] |
| Blue swimming crab meat (Portunas pelagicus) | Gamma irradiation | 2, 4, and 6 kGy | Packed in polypropylene bags; stored at 0–4 for up to 28 days | A low dose of gamma irradiation enhanced the safety of crab meat without causing any undesired changes in texture and L⁎ color value. | [66] |
| Raw ground beef | Electron-beam irradiation | 0, 1.5, 3.0 and 4.5 kGy | Vacuum-packed, stored at 4 °C for 24 h | The color of the samples faded upon irradiation at 4 kGy. | [56] |
| Broiler meat | UV irradiation; activated oxygen (AO) | 9.4, 18.8 and 32.9 mW/s per cm2 | Vacuum bags; stored at 3–5 °C for up to 12 days | The color of the samples treated with UV alone or combined with AO did not exhibit any significant differences. | [61] |
| Beef trimmings (20% fat) | Gamma irradiation | 2 to 5 kGy | Sterile bags; polyethylene bags; stored at −18 °C or 2 °C for up to 30 days | Up to 5 kGy of irradiation did not affect beef patties’ L*, a*, and b* values. | [60] |
| Meatball | Gamma irradiation | 2 and 4 kGy | Packaged in aerobic packaging and MAP; refrigerator storage at 2–4 °C; 0–14 days | During the first 7 days of storage, no significant changes were observed in the a* value, but after 14 days, there was a considerable decrease. In contrast, irradiation did not affect the L* and b* values. | [74] |
| Spicy beef jerky | Gamma irradiation | 0, 0.5, 1.5, 3, 4, 6, and 8 kGy | Vacuum packed; refrigerator storage (4–8 °C); 1 week | The degree of lightness, amount of drip loss, and presence of an off-odor increased as the irradiation dose was increased. | [62] |
| Meatballs | Gamma irradiation | 20, 25 dan 35 kGy | Vacuum-packed + frozen 24 h; room temperature storage (irradiated samples) and freezer storage (non-irradiated samples); 2 months | Meatball firmness decreased as the irradiation dose increased, but L* color values initially did not. After two months of storage, irradiated samples had significantly higher L* values. | [99] |
| Korean native cattle (Hanwoo) beef | UV | 4.5 mWs/cm2 | Wrapped in polyvinyl chloride; stored at 3–5 °C; 9 days | During storage, no significant differences in drip loss and shear force parameters existed among all irradiated beef samples. | [95] |
| Fresh beef | UV irradiation | 12.7, 25.5 and 38.2 W.s/cm² | Packed in polyethylene pouches, and stored in the refrigerator at 3–5 °C for up to 20 days | The color and tenderness of beef samples as well as the water-holding capacity (WHC) percentage decreased with an increase in UV irradiation dose, while the 2-thiobarbituric acid (TBA) level increased. | [92] |
| Chicken meat | UV-C irradiation | 0.62, 1.13, and 1.95 mW/cm² | Packaged in polyvinyl chloride film and kept at 4 °C for 9 days | The most stable b* values were 1.13 and 1.95 mW/cm2. L*, a*, pH, and TBARS values were also similar across groups. | [65] |
| Tegillarca granosa meat | Electron-beam irradiation | 0, 1, 3, 5, 7 or 9 kGy | Vacuum-packed | The texture of Tegillarca granosa meat was increased by irradiation at 3–5 kGy. | [101] |
| Nellore beef | Gamma irradiation and aging combination | 0, 3, 6, and 9 kGy | Vacuum-packed polyethylene | The combination of 9 kGy irradiation and aging improved tenderness. | [113] |
| Sample | Treatment | Dose | Sample Storage Conditions | Shelf-Life | Ref. |
|---|---|---|---|---|---|
| Beef, Chicken and Salmon Fillets | UV-C irradiation | 360 J/m2 | Vacuum storage; 5 days | A combination of vacuum packaging and UV irradiation extended the sample’s shelf life by 66.6%. | [54] |
| Broiler meat | Gamma irradiation | 0, 2 and 3.5 kGy | Stored at −20 °C for 60 days | Broiler meat lasted longer after 2 kGy gamma irradiation. | [126] |
| Ground Beef Patties | Gamma irradiation | 0.0, 1.0, 3.0, 5.0, and 7.0 kGy | Packed in container boxes; stored at 4 °C or −18 °C; maximum 42 days | Irradiation at 5 and 7 kGy increased the storage life of meat samples at 4 °C. | [132] |
| Chicken meat | E-beam irradiation | 3, 4, 5, and 7 kGy | Vacuum-packed in LDPE bags | Irradiation at 4 kGy extended chicken meat shelf life without affecting sensory characteristics. | [131] |
| Marinated pork loin | E-beam irradiation | 0.2–3 kGy | Stored in insulated boxes at 4–8 °C; 0->20 days | Samples stored at 4 °C lasted 7–16 days with a 1 kGy dose. A 2 kGy dose improved shelf life to 20 days. | [133] |
| Chicken meat | Gamma irradiation, 0.1 % chitosan and their combination | 0.5 kGy | Packed in polypropylene bags, and stored in chilled conditions at 4–6 °C; storage for up to 14 days. | With 0.5 kGy gamma irradiation and 0.1% chitosan, chicken meat lasted 14 days in chilled storage. | [96] |
| Chicken breast meat | Gamma irradiation | 4 kGy | Packaged under MAP; refrigeration storage at 2–6 °C; storage period of 25 days | Compared to air-packed samples, MAP (70% CO2/30% N2) and 4 kGy irradiation extended shelf life by 12 days. | [97] |
| Bovine meat | Gamma irradiation | 0, 0.5, 1, 2 and 3 kGy | Commercial freezing bags; storage in the refrigerator at 4–7 °C 3 for weeks and in the freezer at −18 °C for 8 months | Irradiated samples had a shelf life of 14 days when stored at 4–7 °C and exposed to 3 kGy, while non-irradiated samples lasted only three days. | [45] |
| Meatballs | Gamma irradiation | 20, 25 dan 35 kGy | Vacuum packed + frozen for 24 h; room temperature storage (irradiated samples) and freezer storage (non-irradiated); storage for 2 months | The quality of meatballs was maintained for two months at room temperature with 35 kGy irradiation. | [99] |
| Quail meat | Electron-beam irradiation | 1.5, 3, and 5 kGy | Stored at 3–5 °C for 15 days | To maintain quail meat quality and shelf life, 1.5 and 3 kGy irradiation doses are recommended. | [75] |
| Sample | Treatment | Dose | Sample Storage Conditions | Nutritional Characteristics | Ref. |
|---|---|---|---|---|---|
| Goat meat | Gamma irradiation | 0, 1, 2, 4 and 6 kGy | Packed in polystyrene foam boxes, at 2–4 °C | Irradiation increased the core nutritional content of DHA-phosphatidylcholine. The triacylglycerol levels significantly increased after irradiation, while the diacylglycerol levels decreased. | [154] |
| Chicken meat | E-beam irradiation | 3, 4, 5, and 7 kGy | Vacuum-packed in LDPE bags | The vitamin C content decreased following e-beam irradiation at 4 kGy. | [131] |
| Chicken, turkey and mixed ground meat | X-ray irradiation | 0.5, 1, 3, and 5 kGy | Plastic bag storage at −80 °C | Irradiation did not have any impact on the levels of free amino acids. | [125] |
| Chicken, turkey and mixed ground meat | X-ray irradiation | 0.5, 1, 3, and 5 kGy | Plastic bag storage at −80 °C | Following irradiation, taurine became detectable, while the levels of glutathione decreased. Additionally, there was a formation of free fatty acids, but it was relatively insignificant. | [125] |
| Sliced ham | UV-C irradiation | 408, 2040, 4080, and 6120 mJ/cm2 | Refrigerated storage for 0, 7, and 14 days | The antioxidant capacity was higher on day 0 when irradiated with a 4080 mJ/cm2. | [52] |
| Spicy beef jerky | Gamma irradiation | 0, 0.5, 1.5, 3, 4, 6, and 8 kGy | Vacuum-packed; refrigerator storage (4–8 °C) for 1 week | As the irradiation dose increases, free radicals were formed. | [62] |
| Spicy yak jerky | Electron-beam irradiation | 0, 2, 5, 7 and 9 kGy | Vacuum-packed, and stored in the refrigerator at 4–8 °C for 1 week | The protein nutrition was significantly reduced at a high irradiation dose of 9 kGy, while there was no significant impact at doses ranging from 0–7 kGy. | [141] |
| Chicken Meat | Gamma irradiation | 3 kGy | Aerobically packaged; refrigerator storage at 4 °C for up to 14 days | Irradiating chicken meat samples showed reduced amino and fatty acid loss during storage. | [94] |
| Smoked duck meat | Electron-beam irradiation | 0, 1.5, 3.5, and 4.5 kGy | Vacuum-packed; stored at 4 °C for up to 40 days | The quality of smoked duck flesh, including its amino acids, fatty acids, and volatiles, did not change significantly during storage when exposed to <3 kGy irradiation. | [81] |
| Sample | Treatment | Dose | Sample Storage Conditions | Sensory Characteristics | Ref. |
|---|---|---|---|---|---|
| Smoked guinea fowl meat | Gamma irradiation | 2.5, 5 and 7.5 kGy | Stored in the refrigerator (2–4 °C) with a storage period of 7 weeks | Irradiation did not affect aroma, color, tenderness, or taste. | [51] |
| Sliced ham | UV-C irradiation | 408, 2040, 4080, and 6120 mJ/cm2 | Refrigerated storage; 0, 7, and 14 days | The 7th and 14th days of storage showed a slight color change, but consumers did not mind. | [52] |
| Fish and red meat-based ready-to-eat foods | Gamma irradiation | 8 dan 45 kGy | Styrofoam boxes + ice block: non-irradiated foods. Plastic container: room temperature-irradiated foods. Menu cycle: 5 days for 30 days | The sensory characteristics of irradiated food, including overall appearance, texture, color, taste, and aroma, were deemed acceptable by consumers. | [161] |
| Marinated pork loin | E-beam irradiation | 0.2–3 kGy | Stored in insulated boxes at 4–8 °C for 0->20 days | Prolonging the shelf life of irradiated products did not lead to any sensory quality alterations. | [133] |
| Korean native cattle (Hanwoo) beef | UV irradiation | 4.5 mW s/cm2 | Wrapped in polyvinyl chloride; stored at 3–5 °C for 9 days | The results of the sensory test showed no discernible difference between the irradiated and non-irradiated samples. | [95] |
| Rabbit meat | Gamma irradiation | 0, 1.5, and 3 kGy | Packed in polyethylene pouches; stored in the refrigerator 3–5 °C for 3 weeks | Gamma irradiation did not significantly affect rabbit meat’s sensory properties. | [69] |
| Turkey breast meat | Gamma irradiation | 0.0, 0.5, 2.0 and 4.0 kGy | Stored at −18 °C for 2 months | The preference scores given by the panelists for irradiated and non-irradiated samples were the same, indicating that both were equally acceptable in terms of appearance and smell. | [68] |
| Blue swimming crab meat (Portunas pelagicus) | Gamma irradiation | 2, 4, and 6 kGy | Packed in polypropylene bags; stored at 0–4 for up to 28 days | Irradiation at 2–6 kGy in crab meat impacted the overall difference in odor quality. | [66] |
| Broiler meat | UV irradiation and activated oxygen (AO) | 9.4, 18.8 and 32.9 mW/s per cm2 | Vacuum bags; stored at 3–5 °C for up to 12 days | The sensory quality of samples not irradiated, UV-irradiated, or UV+AO-irradiated was not significantly different. | [61] |
| Raw ground beef | Electron-beam irradiation | 0, 1.5, 3.0 and 4.5 kGy | Vacuum-packed and stored at 4 °C for 24 h | Exposing the sample to less than 3 kGy irradiation led to minor alterations in the sensory characteristics. | [56] |
| Ground beef | Gamma irradiation | First assay: 0.26, 0.44, 0.67 and 0.86. Second assay: 1 kGy. Third assay: 2.5 kGy. | Polyethylene bags; freezer storage at −18 °C | The irradiation of samples with 2.5 kGy gamma rays did not affect consumer acceptance results. | [57] |
| Minced camel meat | Gamma irradiation | 0, 2, 4, and 6 kGy | Refrigerator storage at 1–4 °C; 2, 4, and 6 weeks of storage | Irradiated meat samples had similar sensory qualities to non-irradiated ones. | [58] |
| Raw ground beef patties | Electron-beam irradiation | 2, 4, and 6 kGy | Packed with nylon polyethylene bag; stored at −18 °C; storage period of up to 7 months | The sensory characteristics of the beef samples were not affected by electron-beam irradiation. | [59] |
| Minced camel meat | Gamma irradiation | 0, 2, 4 and 6 kGy | Placed in polystyrene trays and covered with polyethylene film; stored in refrigerator 1–4 °C; storage for up to 6 weeks | Irradiated camel meat had similar sensory properties to non-irradiated camel meat. | [58] |
| Beef trimmings (20% fat) | Gamma irradiation | 2 to 5 kGy | Sterile bags (Whirl Pak®); polyethylene bags; stored at (− 18 ± 2) °C or (2 ± 2) °C for up to 30 days | No sensory differences were observed between the patties made from irradiated trimmings aged for 1 or 30 days. | [60] |
| Spicy beef jerky | Gamma irradiation | 0, 0.5, 1.5, 3, 4, 6, and 8 kGy | Vacuum-packed; refrigerator storage (4–8 °C) for 1 week | As the irradiation dose increased, the preference for the color and taste of the spicy beef samples decreased. | [62] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Indiarto, R.; Irawan, A.N.; Subroto, E. Meat Irradiation: A Comprehensive Review of Its Impact on Food Quality and Safety. Foods 2023, 12, 1845. https://doi.org/10.3390/foods12091845
Indiarto R, Irawan AN, Subroto E. Meat Irradiation: A Comprehensive Review of Its Impact on Food Quality and Safety. Foods. 2023; 12(9):1845. https://doi.org/10.3390/foods12091845
Chicago/Turabian StyleIndiarto, Rossi, Arif Nanda Irawan, and Edy Subroto. 2023. "Meat Irradiation: A Comprehensive Review of Its Impact on Food Quality and Safety" Foods 12, no. 9: 1845. https://doi.org/10.3390/foods12091845
APA StyleIndiarto, R., Irawan, A. N., & Subroto, E. (2023). Meat Irradiation: A Comprehensive Review of Its Impact on Food Quality and Safety. Foods, 12(9), 1845. https://doi.org/10.3390/foods12091845








