Evaluation of Steady-State and Time-Resolved Fluorescence Spectroscopy as a Method for Assessing the Impact of Photo-Oxidation on Refined Soybean Oils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Oils
2.2. Accelerated Photo-Oxidation in a Light Chamber
2.3. Oils Analysis
2.4. Fluorescence Measurements
2.4.1. Steady-State
2.4.2. Time-Resolved
2.5. Correlation Analysis between Fluorescence Parameters and PV and CD
3. Results and Discussion
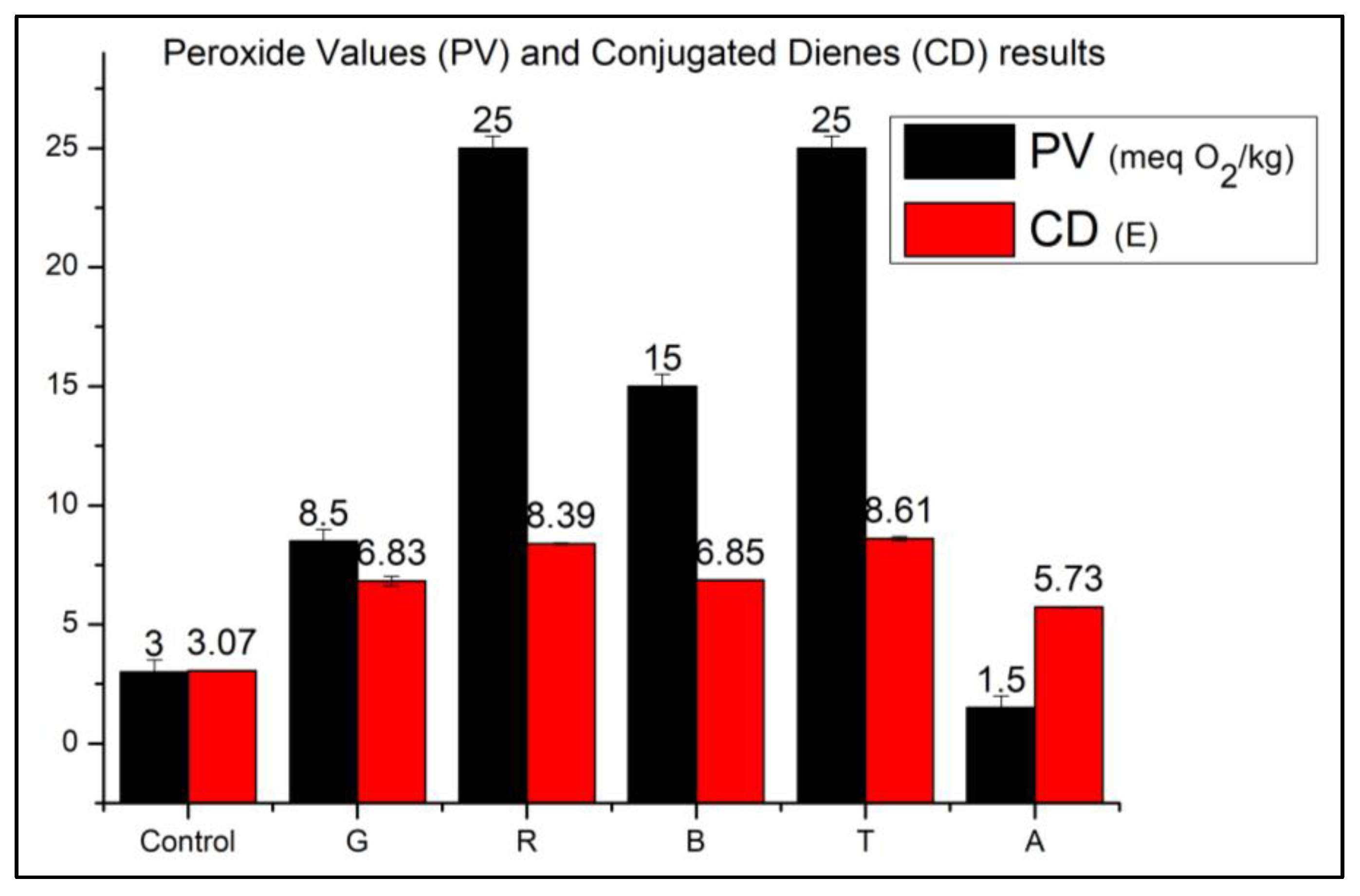
3.1. Analysis of the Oxidation State of the Samples-PV and CD
3.2. Fluorescence Analysis
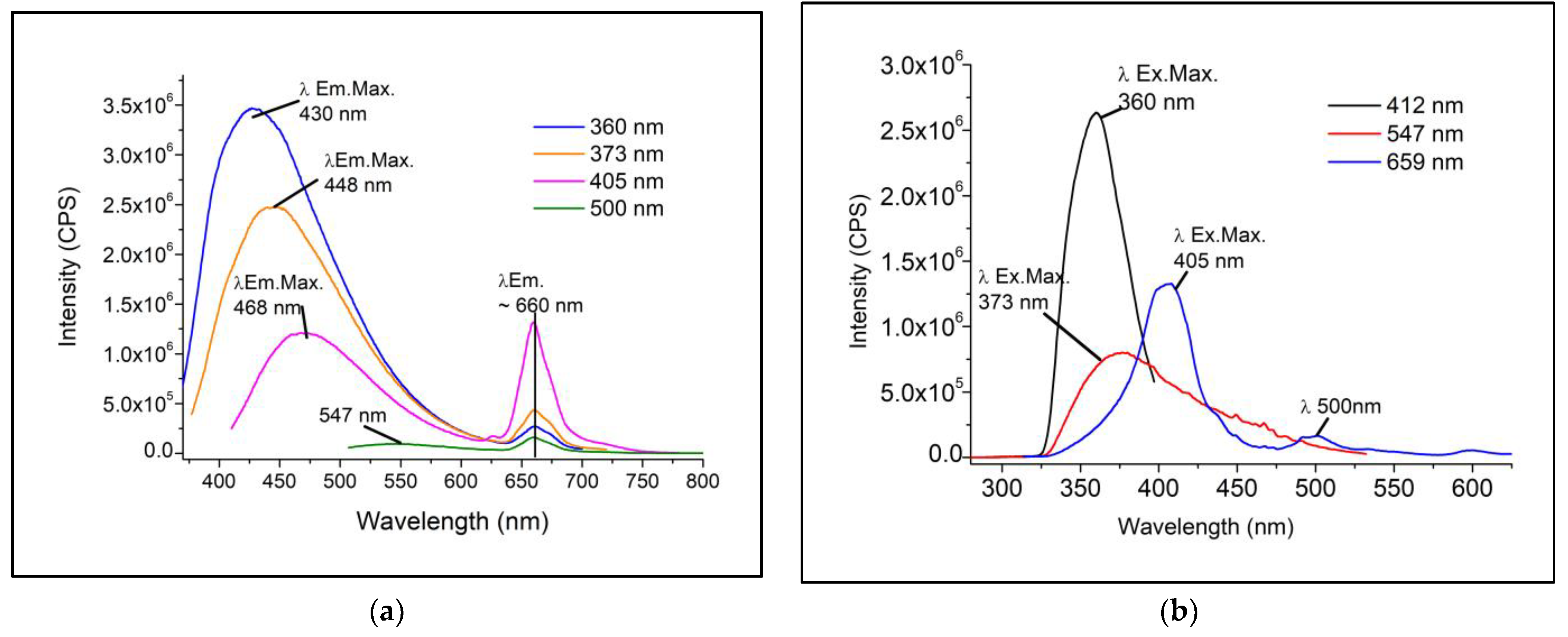
3.2.1. Steady-State
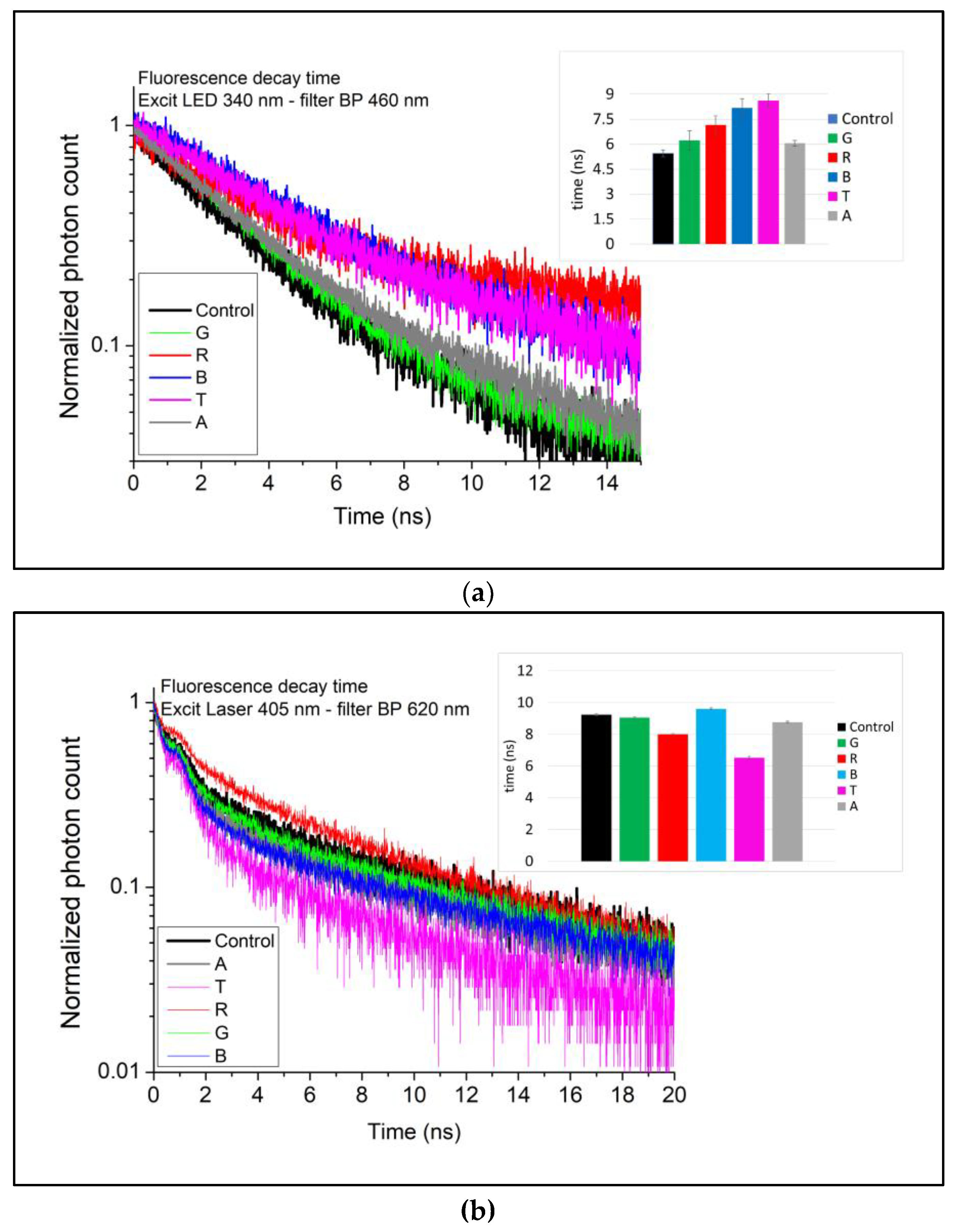
3.2.2. Time-Resolved
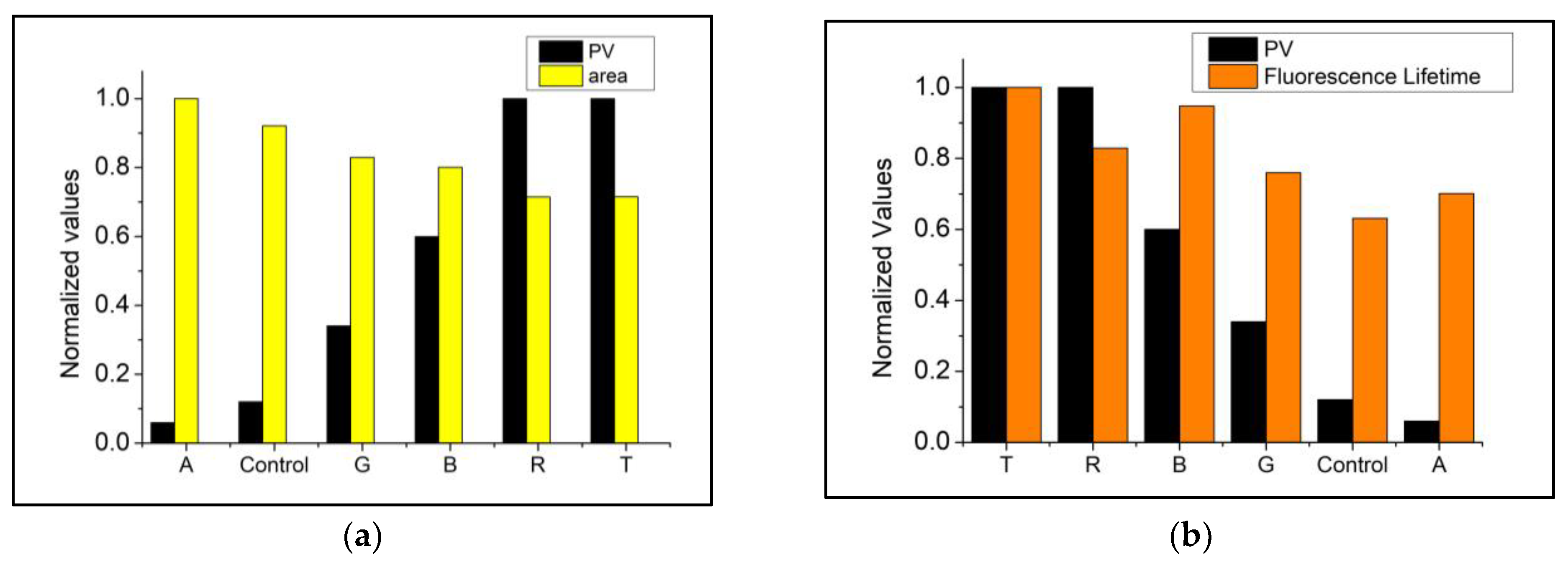
3.3. Correlation between Fluorescence Parameters and PV/CD
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- USDA Foreign Agricultural Service. Available online: https://apps.fas.usda.gov/psdonline/app/index.html#/app/home (accessed on 4 April 2023).
- Grootveld, M.; Percival, B.C.; Leenders, J.; Wilson, P.B. Potential Adverse Public Health Effects Afforded by the Ingestion of Dietary Lipid Oxidation Product Toxins: Significance of Fried Food Sources. Nutrients 2020, 12, 974. [Google Scholar] [CrossRef]
- Monnier, V.M. Dietary Advanced Lipoxidation Products as Risk Factors for Human Health—A Call for Data. Mol. Nutr. Food Res. 2007, 51, 1091–1093. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Yusta, A.; Goicoechea, E.; Guillén, M.D. A Review of Thermo-Oxidative Degradation of Food Lipids Studied by 1H NMR Spectroscopy: Influence of Degradative Conditions and Food Lipid Nature. Compr. Rev. Food Sci. Food Saf. 2014, 13, 838–859. [Google Scholar] [CrossRef]
- CXS 210-1999; Standard for Named Vegetable Oils. Codex Alimentarius: Rome, Italy, 2023.
- Choe, E.; Min, D.B. Mechanisms and Factors for Edible Oil Oxidation. Compr. Rev. Food Sci. Food Saf. 2006, 5, 169–186. [Google Scholar] [CrossRef]
- Ayu, D.F.; Andarwulan, N.; Hariyadi, P.; Purnomo, e.H. Effect of tocopherols, tocotrienols, β-carotene, and chlorophyll on the photo-oxidative stability of red palm oil. Food Sci. Biotechnol. 2016, 25, 401–407. [Google Scholar] [CrossRef]
- Tawfik, M.S.; Huyghebaert, A. Interaction of Packaging Materials and Vegetable Oils: Oil Stability. Food Chem. 1999, 64, 451–459. [Google Scholar] [CrossRef]
- Pristouri, G.; Badeka, A.; Kontominas, M.G. Effect of Packaging Material Headspace, Oxygen and Light Transmission, Temperature and Storage Time on Quality Characteristics of Extra Virgin Olive Oil. Food Control 2010, 21, 412–418. [Google Scholar] [CrossRef]
- BS EN 12464-1:2002; Light and Lighting—Lighting of Work Places—Part 1: Indoor Work. British Standards Institution. BSI: London, UK, 2002.
- ISO/CIE 8995-1; Lighting of Work Places—Part 1: Indoor. International Organization for Standardization (ISO): Geneva, Switzerland, 2002.
- Galave, S.G.; Dadas, S.D.; Bandgar, S.N.; Mangate, S.D. Power Quality Analysis & Characterization of Different Types of Lamp Used for Domestic and Industrial Purpose. In Proceedings of the 2nd International Conference on Trends in Electronics and Informatics (ICOEI), Tirunelveli, India, 11–12 May 2018; pp. 1501–1506. [Google Scholar]
- Liu, L. LED Solar Spectrum Computer Simulation Based on Non-Dominated Sorting Genetic Algorithm. J. Phys. Conf. Ser. 2020, 1578, 12103. [Google Scholar] [CrossRef]
- Bianchi, L.M.; Duncan, S.E.; Webster, J.B.; Neilson, A.P.; O’Keefe, S.F. Contribution of Chlorophyll to Photo-oxidation of Soybean Oil at Specific Visible Wavelengths of Light. J. Food Sci. 2015, 80, C252–C261. [Google Scholar] [CrossRef]
- Barriuso, B.; Astiasarán, I.; Ansorena, D. A review of analytical methods measuring lipid oxidation status in foods: A challenging task. Eur. Food Res. Technol. 2013, 236, 1–15. [Google Scholar] [CrossRef]
- Abeyrathne, E.D.N.S.; Nam, K.; Ahn, D.U. Analytical Methods for Lipid Oxidation and Antioxidant Capacity in Food Systems. Antioxidants 2021, 10, 1587. [Google Scholar] [CrossRef] [PubMed]
- Sadecka, J.; Tothova, J. Fluorescence Spectroscopy and Chemometrics in the Food Classification. Czech J. Food Sci. 2007, 25, 159–173. [Google Scholar] [CrossRef]
- Ahmad, M.H.; Sahar, A.; Hitzmann, B. Fluorescence Spectroscopy for the Monitoring of Food Processes. Adv. Biochem. Eng. Biotechnol. 2017, 161, 121–151. [Google Scholar] [PubMed]
- Ali, H.; Saleen, M.; Anser, M.; Khan, S.; Ullah, R.; Bilal, B. Validation of Fluorescence Spectroscopy to Detect Adulteration of Edible Oil in Extra Virgin Olive Oil (EVOO) by Applying Chemometrics. Appl. Spectrosc. 2018, 72, 1371–1379. [Google Scholar] [CrossRef] [PubMed]
- Al Riza, D.F.; Kondo, N.; Rotich, V.K.; Perone, C.; Giametta, F. Cultivar and Geographical Origin Authentication of Italian Extra Virgin Olive Oil Using Front-Face Fluorescence Spectroscopy and Chemometrics. Food Control 2021, 121, 107604. [Google Scholar] [CrossRef]
- Poulli, K.I.; Mousdis, G.A.; Georgiou, C.A. Synchronous Fluorescence Spectroscopy for Quantitative Determination of Virgin Olive Oil Adulteration with Sunflower Oil. Anal. Bioanal. Chem. 2006, 386, 1571–1575. [Google Scholar] [CrossRef] [PubMed]
- Meenu, M.; Cai, Q.; Xu, B. A Critical Review on Analytical Techniques to Detect Adulteration of Extra Virgin Olive Oil. Trends Food Sci. Technol. 2019, 91, 391–408. [Google Scholar] [CrossRef]
- Milanez, K.D.T.M.; Nóbrega, T.C.A.; Nascimento, D.S.; Insausti, M.; Band, B.S.F.; Pontes, M.J.C. Multivariate Modeling for Detecting Adulteration of Extra Virgin Olive Oil with Soybean Oil Using Fluorescence and UV–Vis Spectroscopies: A Preliminary Approach. LWT—Food Sci. Technol. 2017, 85, 9–15. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, H.; Lu, W. Recent Advances in Analytical Detection of Olive Oil Adulteration. ACS Food Sci. Technol. 2021, 2, 415–424. [Google Scholar] [CrossRef]
- Poulli, K.I.; Chantzos, N.V.; Mousdis, G.A.; Georgiou, C.A. Synchronous Fluorescence Spectroscopy: Tool for Monitoring Thermally Stressed Edible Oils. J. Agric. Food Chem. 2009, 18, 8194–8201. [Google Scholar] [CrossRef]
- Hao, S.; Zhu, L.; Sui, R.; Zuo, M.; Luo, N.; Shi, J.; Zhang, W.; He, X.; Chen, Z. Identification and Quantification of Vegetable Oil Adulteration with Waste Frying Oil by Laser-Induced Fluorescence Spectroscopy. OSA Contin. 2019, 2, 1148. [Google Scholar] [CrossRef]
- Lopes, C.R.B.; da Silva, H.D.T.; Courrol, L.C. Fluorescence Spectroscopy Study of Conventional and Organic Soybean Oil Heated to 270 °C. In Proceedings of the 2021 SBFoton International Optics and Photonics Conference (SBFoton IOPC), São Carlos, Brazil, 31 May–2 June 2021; pp. 1–4. [Google Scholar]
- Michael, P.R.; Johnston, D.E.; Moreno, W. A Conversion Guide: Solar Irradiance and Lux Illuminance. J. Meas. Eng. 2020, 8, 153–166. [Google Scholar] [CrossRef]
- (EEC) No 2568/91; Commission Regulation (EEC) No. 2568/91 on the Characteristics of Olive Oil and Olive-Residue Oil and on the Relevant Methods of Analysis. European Union Commission: Luxembourg, 2016.
- Angerosa, F.; Campestre, C.; Giansante, L. Analysis and Authentication. In Olive Oil: Chemistry and Technology, 2nd ed.; Boskou, D., Ed.; AOCS: New York, NY, USA, 2006; pp. 113–172. [Google Scholar]
- Marmesat, S.; Morales, A.; Velasco, J.; Méndez, M.V.R.; Dobarganes, M.C. Relationship between Changes in Peroxide Value and Conjugated Dienes during Oxidation of Sunflower Oils with Different Degree of Unsaturation. Grasas Y Aceites 2009, 60, 155–160. [Google Scholar]
- Longobardi, F.; Contillo, F.; Catucci, L.; Tommasi, L.; Caponio, F.; Paradiso, V.M. Analysis of peroxide value in olive oils with an easy and green method. Food Control 2021, 130, 108295. [Google Scholar] [CrossRef]
- CODEX STAN 33-1981; Codex Standard for Olive Oil, Virgin and Refined and for Refined Olive-Pomace Oil—Standards for Fats and Oils from Vegetable Sources. Section 2. Codex Alimentarius: Rome, Italy, 2015.
- Smith, S.A.; King, R.E.; Min, D.B. Oxidative and Thermal Stabilities of Genetically Modified High Oleic Sunflower Oil. Food Chem. 2007, 102, 1208–1213. [Google Scholar] [CrossRef]
- Dalaeen, S.; Al-Fraihat, A.; Kassasbeh, E. Effect of Aromatic Plant Essential Oils on Oxidative Stability of Sunflower Oil during Heating and Storage. Pak. J. Nutr. 2011, 10, 864–870. [Google Scholar]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: New York, NY, USA, 2006. [Google Scholar]
- Sayago, A.; Morales, M.T.; Aparicio, R. Detection of Hazelnut Oil in Virgin Olive Oil by a Spectrofluorimetric Method. Eur. Food Res. Technol. 2004, 218, 480–483. [Google Scholar] [CrossRef]
- Zandomeneghi, M.; Carbonaro, L.; Caffarata, C. Fluorescence of Vegetable Oils: Olive Oils. J. Agric. Food Chem. 2005, 53, 759–766. [Google Scholar] [CrossRef]
- Escuderos, M.E.; Sayago, A.; Morales, M.T.; Aparicio, R. Evaluation of α-Tocopherol in Virgin Olive Oil by a Luminiscent Method. Grasas y Aceites 2009, 60, 336–342. [Google Scholar]
- Magalhães, K.F.; Caires, A.R.L.; Silva, M.S.; Alcantara, G.B.; Oliveira, S.L. Endogenous Fluorescence of Biodiesel and Products Thereof: Investigation of the Molecules Responsible for This Effect. Fuel 2014, 119, 120–128. [Google Scholar] [CrossRef]
- Kongbonga, Y.G.M.; Ghalila, H.; Onana, M.B.; Majdi, Y.; Lakhdar, Z.B.; Mezlini, H.; Sevestre-Ghalila, S. Characterization of Vegetable Oils by Fluorescence Spectroscopy. Food Nutr. Sci. 2011, 2, 692–699. [Google Scholar] [CrossRef]
- Guzmán, E.; Baeten, V.; Pierna, J.A.F.; García-Mesa, J.A. Evaluation of the Overall Quality of Olive Oil Using Fluorescence Spectroscopy. Food Chem. 2015, 173, 927–934. [Google Scholar] [CrossRef]
- Sikorska, E.; Gliszczyńska-Świgło, A.; Khmelinskii, I.; Sikorski, M. Synchronous Fluorescence Spectroscopy of Edible Vegetable Oils. Quantification of Tocopherols. J. Agric. Food Chem. 2005, 53, 6988–6994. [Google Scholar] [CrossRef]
- Poulli, K.I.; Mousdis, G.A.; Georgiou, C.A. Monitoring Olive Oil Oxidation under Thermal and UV Stress through Synchronous Fluorescence Spectroscopy and Classical Assays. Food Chem. 2009, 117, 499–503. [Google Scholar] [CrossRef]
- Sikorska, E.; Górecki, T.; Khmelinskii, I.V.; Sikorski, M.; Kozioł, J. Classification of Edible Oils Using Synchronous Scanning Fluorescence Spectroscopy. Food Chem. 2005, 89, 217–225. [Google Scholar] [CrossRef]
- Gaigalas, A.K.; Li, L.; Henderson, O.; Vogt, R.; Barr, J.; Marti, G.; Weaver, J.; Schwartz, A. The Development of Fluorescence Intensity Standards. J. Res. Natl. Inst. Stand. Technol. 2001, 106, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.S.O.; Sinnecker, P.; Tanaka, R.T.; Lanfer-Marquez, U.M. Effect of Harvesting and Drying Conditions on Chlorophyll Levels of Soybean (Glycine max L. Merr). J. Agric. Food Chem. 2003, 51, 1634–1639. [Google Scholar] [CrossRef] [PubMed]
- Gharby, S. Refining Vegetable Oils: Chemical and Physical Refining. Sci. World J. 2022, 2022, 6627013. [Google Scholar] [CrossRef]
- Rezende Souza, E.; Sigoli, F. Fundamental Principia and Models of Inter and Intramolecular Energy Transfer. Quim. Nova 2011, 35, 1841–1847. [Google Scholar] [CrossRef]
- Šubr, M.; Praus, P.; Kočišová, E.; Procházka, M.; Štěpánek, J.; Kuzminova, A.; Kylián, O.; Sureau, F. Magnetron-Sputtered Polytetrafluoroethylene-Stabilized Silver Nanoisland Surface for Surface-Enhanced Fluorescence. Nanomaterials 2020, 10, 773. [Google Scholar] [CrossRef]
- Viljanen, K.; Sundberg, S.; Ohshima, T.; Heinonen, M. Carotenoids as Antioxidants to Prevent Photo-oxidation. Eur. J. Lipid Sci. Technol. 2002, 104, 353–359. [Google Scholar] [CrossRef]
- Veberg, A.; Vogt, G.; Wold, J.P. Fluorescence in Aldehyde Model Systems Related to Lipid Oxidation. LWT—Food Sci. Technol. 2006, 39, 562–570. [Google Scholar] [CrossRef]
- Wybranowski, T.; Ziomkowska, B.; Cyrankiewicz, M.; Bosek, M.; Pyskir, J.; Napiórkowska, M.; Kruszewski, S. A Study of the Oxidative Processes in Human Plasma by Time-Resolved Fluorescence Spectroscopy. Sci. Rep. 2022, 12, 9012. [Google Scholar] [CrossRef] [PubMed]
- Lingvay, M.; Akhtar, P.; Sebők-Nagy, K.; Páli, T.; Lambrev, P.H. Photobleaching of Chlorophyll in Light-Harvesting Complex II Increases in Lipid. Environ. Front. Plant Sci. 2020, 11, 849. [Google Scholar] [CrossRef] [PubMed]
- Coltro, L.; Padula, M.; Saron, E.S.; Borghetti, J.; Buratin, A.E.P. Evaluation of a UV Absorber Added to PET Bottles for Edible Oil Packaging. Packag. Technol. Sci. 2003, 16, 15–20. [Google Scholar] [CrossRef]


| Sample | Label |
|---|---|
| Soybean oil before the photo-oxidation experiment. | Control |
| Green bottle | G |
| Red bottle | R |
| Blue bottle | B |
| Colorless bottle | T |
| Bottle covered with aluminum foil | A |
| λEx./λEm.Max. (nm) | 373/~448 | 373/~660 | ||||
|---|---|---|---|---|---|---|
| Marker | Area | λEm.Max. | Intensity | Area | λEm.Max. | Intensity |
| PV | −0.96 | −0.95 | −0.88 | −0.85 | 0.75 | −0.88 |
| CD | −0.79 | −0.91 | −0.71 | −0.68 | 0.66 | −0.72 |
| λEx./λEm.Max. (nm) | 405/~468 | 405/~659 | ||||
| Marker | Area | λEm.Max. | Intensity | Area | λEm.Max. | Intensity |
| PV | −0.89 | −0.87 | −0.93 | −0.87 | 0.89 | −0.88 |
| CD | −0.79 | −0.76 | −0.82 | −0.70 | 0.73 | −0.72 |
| λEx./λEm.Max. (nm) | 500/~547 | 500/~659 | ||||
| Marker | Area | λEm.Max. | Intensity | Area | λEm.Max. | Intensity |
| PV | −0.52 | 0.28 | −0.59 | −0.83 | 0.87 | −0.86 |
| CD | −0.62 | 0.04 | −0.68 | −0.65 | 0.69 | −0.69 |
| λEx./λEm.Max (nm) | 340/460 | 405/620 | ||||
| Marker | Fluorescence lifetime | Fluorescence lifetime | ||||
| PV | 0.81 | −0.69 | ||||
| CD | 0.80 | −0.64 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, C.R.B.; Courrol, L.C. Evaluation of Steady-State and Time-Resolved Fluorescence Spectroscopy as a Method for Assessing the Impact of Photo-Oxidation on Refined Soybean Oils. Foods 2023, 12, 1862. https://doi.org/10.3390/foods12091862
Lopes CRB, Courrol LC. Evaluation of Steady-State and Time-Resolved Fluorescence Spectroscopy as a Method for Assessing the Impact of Photo-Oxidation on Refined Soybean Oils. Foods. 2023; 12(9):1862. https://doi.org/10.3390/foods12091862
Chicago/Turabian StyleLopes, Carla Regina Borges, and Lilia Coronato Courrol. 2023. "Evaluation of Steady-State and Time-Resolved Fluorescence Spectroscopy as a Method for Assessing the Impact of Photo-Oxidation on Refined Soybean Oils" Foods 12, no. 9: 1862. https://doi.org/10.3390/foods12091862





