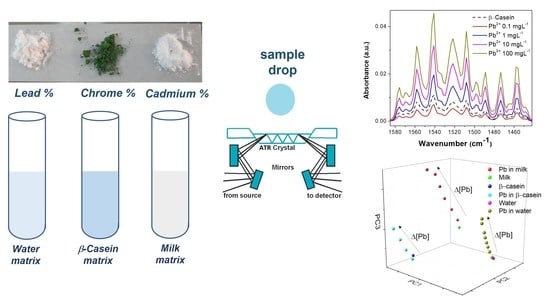
A Study of the Interactions of Heavy Metals in Dairy Matrices Using Fourier Transform Infrared Spectroscopy, Chemometric, and In Silico Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
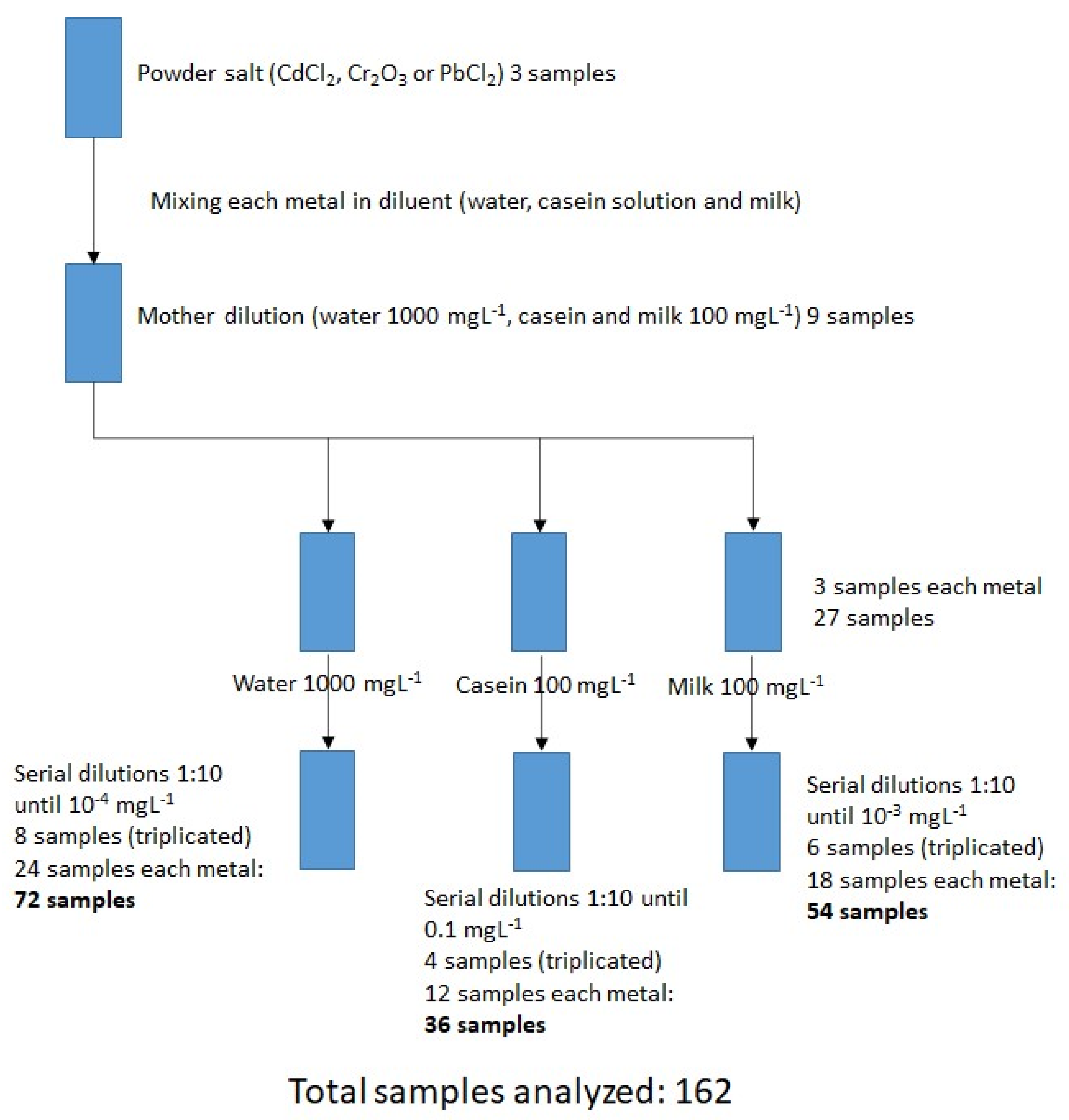
2.2. Heavy Metal Concentrations
2.3. Sample Preparation
2.4. Infrared Spectroscopy
2.5. Data Analysis
2.6. In-Silico Analysis
3. Results
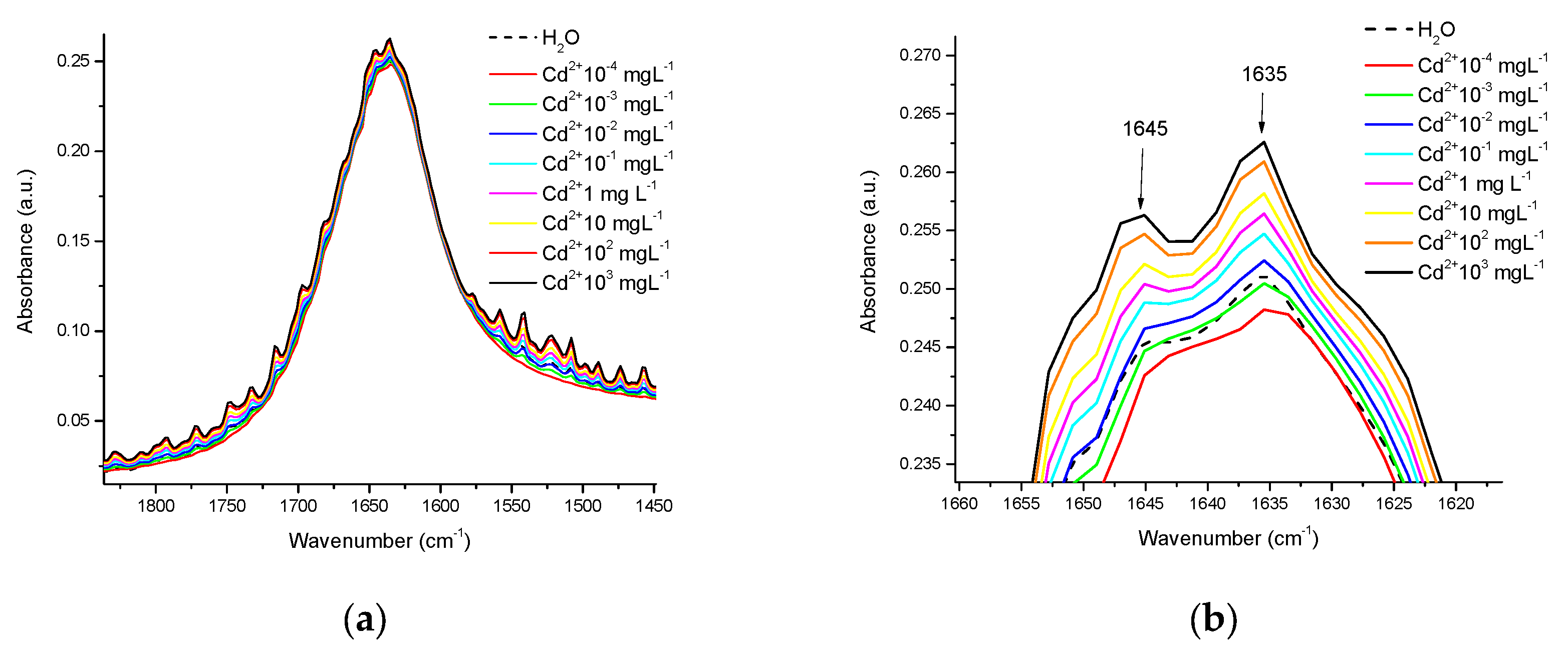
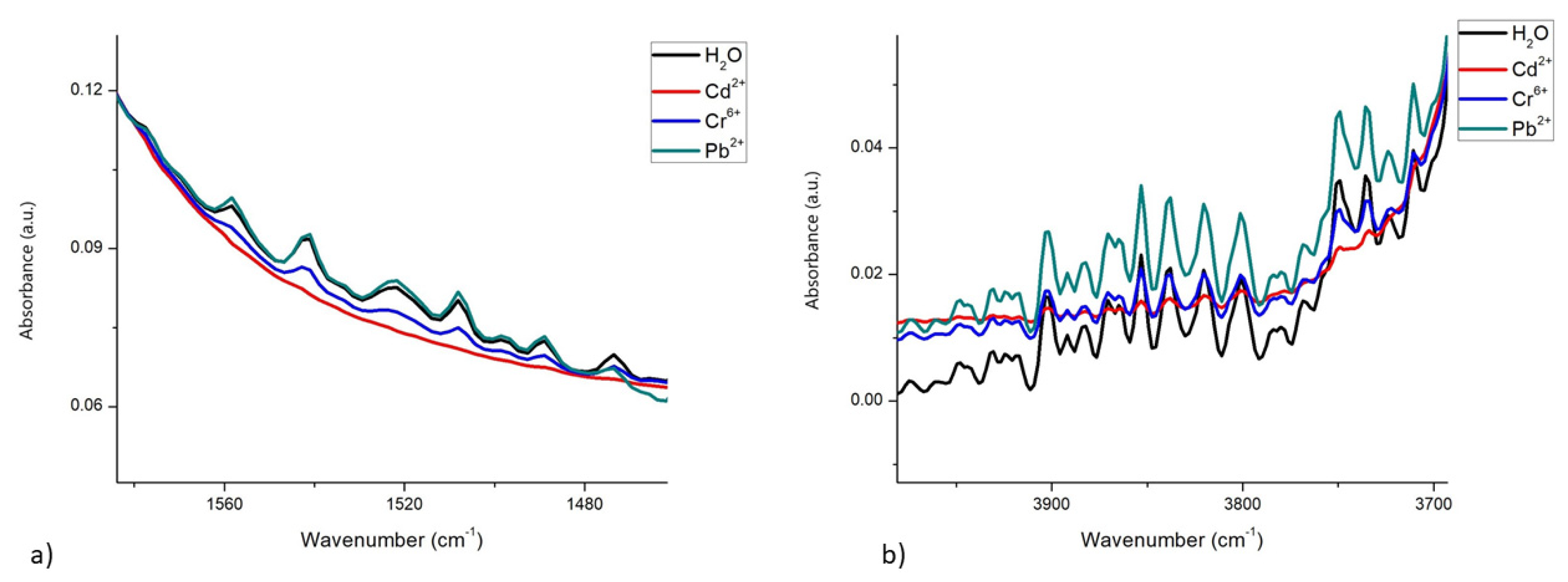
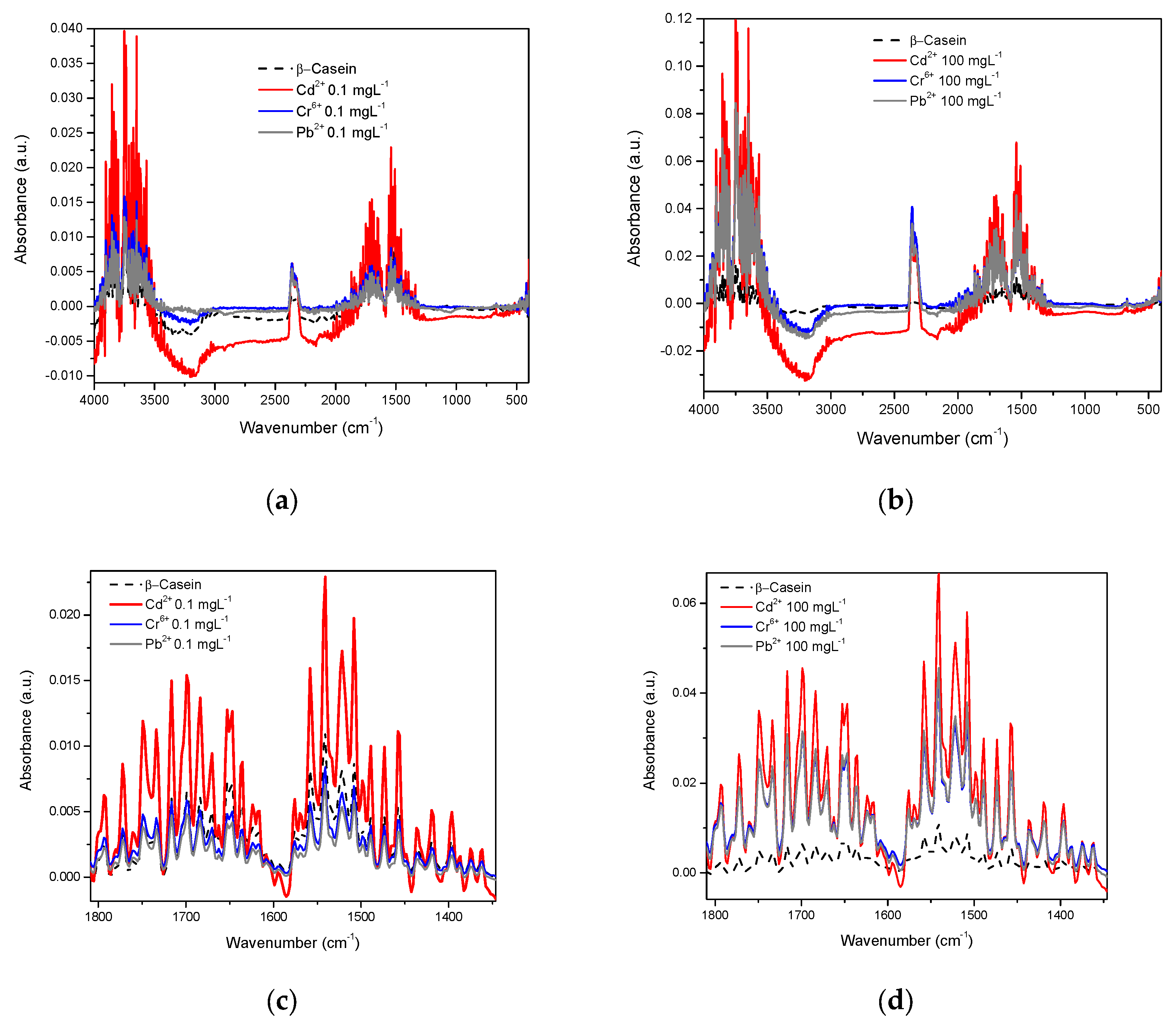
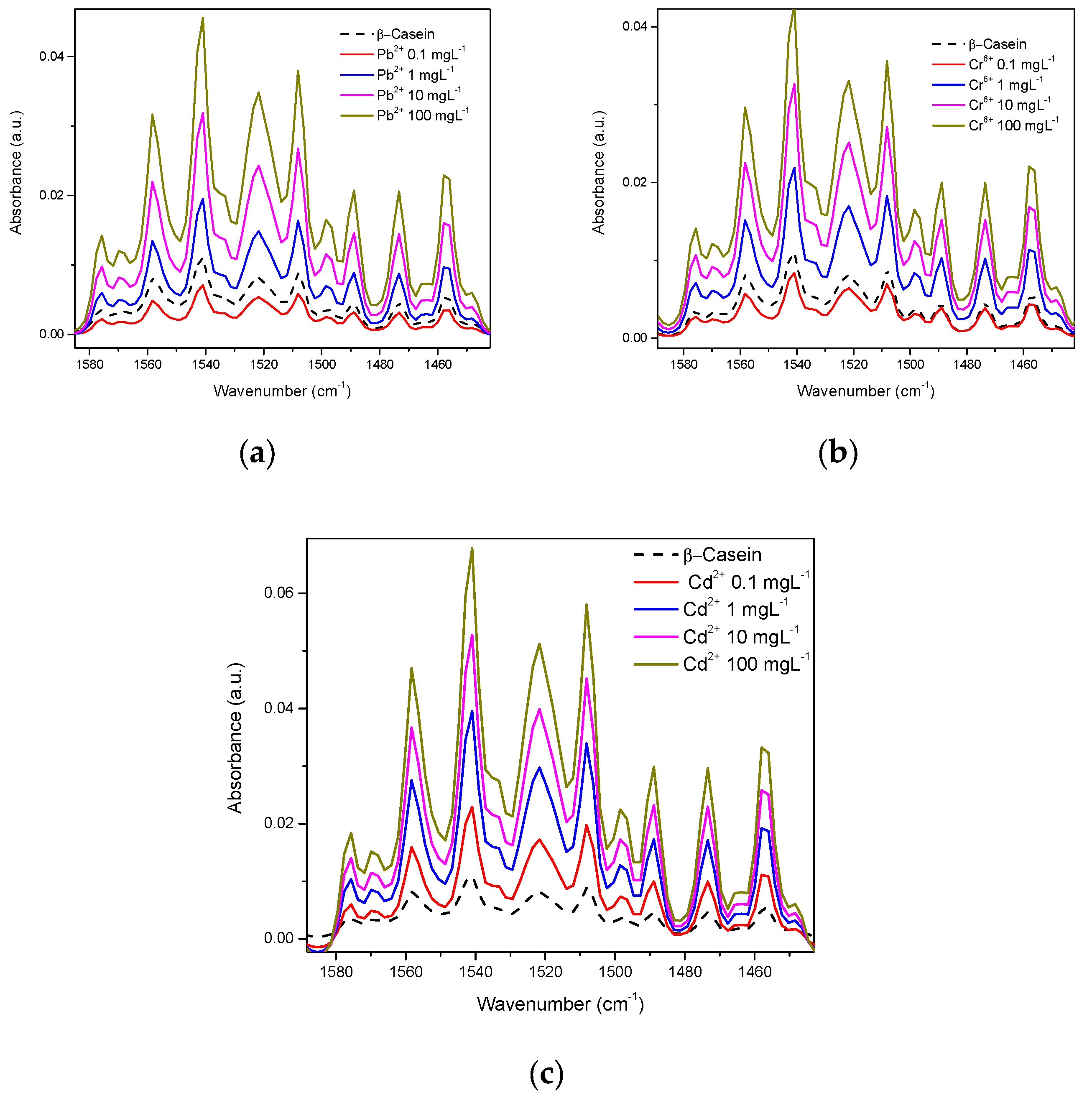
3.1. Spectra in Water
3.2. Spectra in β-Casein
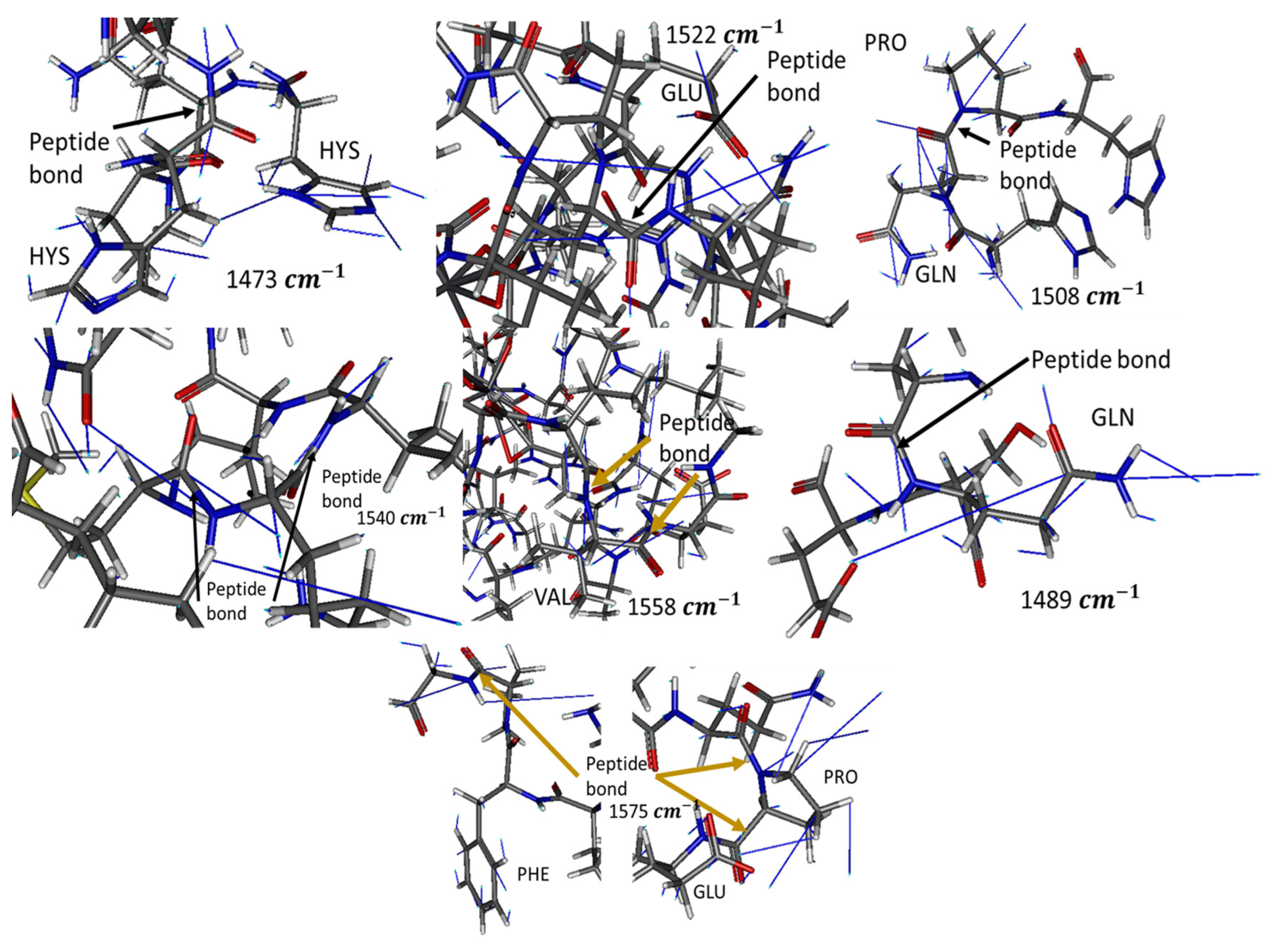
In-Silico Analysis
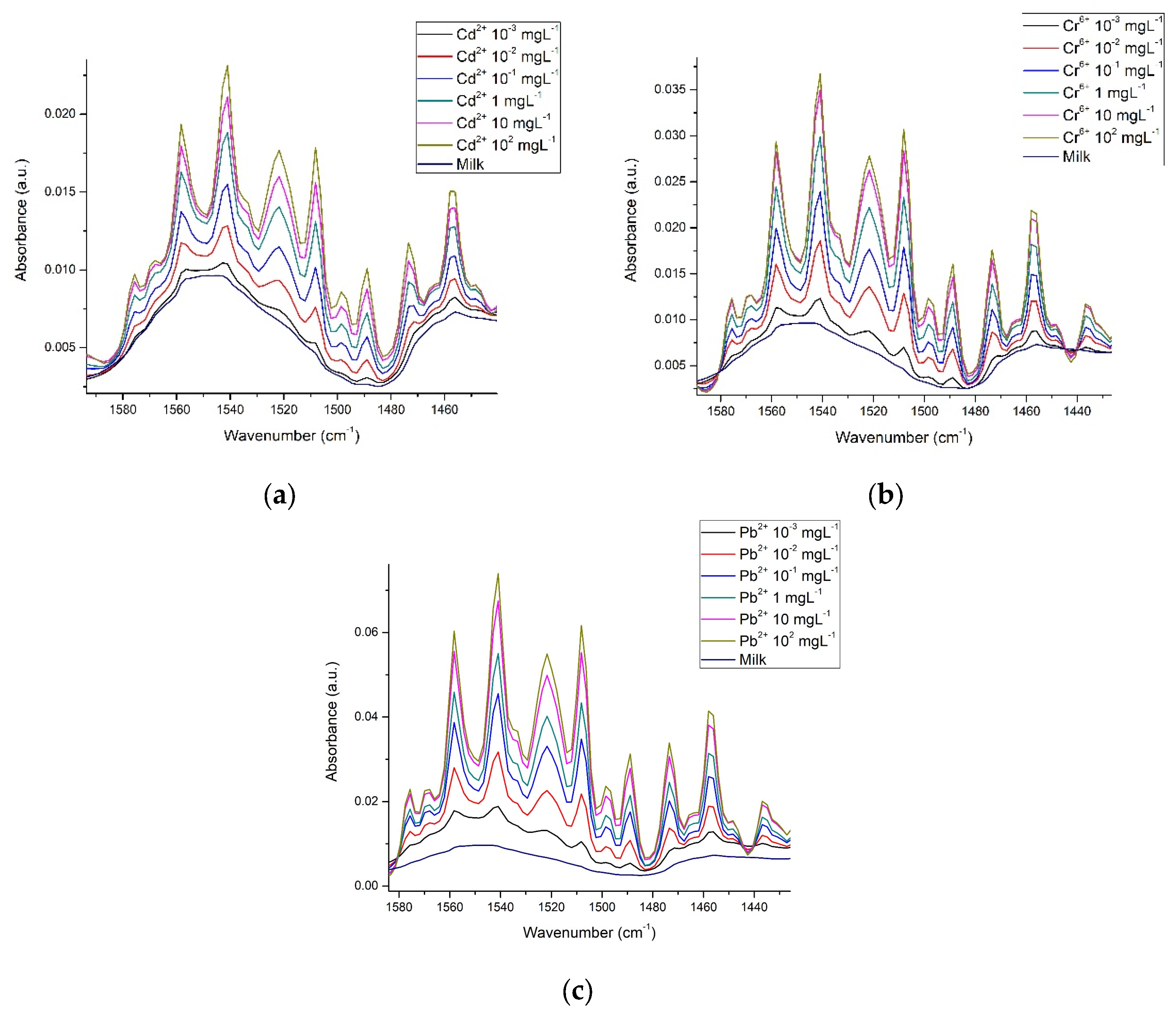
3.3. Spectra in Milk
3.4. Chemometric Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Năstăsescu, V.; Mititelu, M.; Goumenou, M.; Docea, A.O.; Renieri, E.; Udeanu, D.I.; Oprea, E.; Arsene, A.L.; Dinu-Pîrvu, C.E.; Ghica, M. Heavy Metal and Pesticide Levels in Dairy Products: Evaluation of Human Health Risk. Food Chem. Toxicol. 2020, 146, 111844. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Pandita, S.; Setia, R. A Meta-Analysis of Potential Ecological Risk Evaluation of Heavy Metals in Sediments and Soils. Gondwana Res. 2022, 103, 487–501. [Google Scholar] [CrossRef]
- Khan, S.; Cao, Q.; Zheng, Y.M.; Huang, Y.Z.; Zhu, Y.G. Health Risks of Heavy Metals in Contaminated Soils and Food Crops Irrigated with Wastewater in Beijing, China. Environ. Pollut. 2008, 152, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Benítez-Rojas, A.C.; Delgado-Macuil, R.J.; Amador-Espejo, G.G.; Eustaquio-Rosales, E.; Martinez-Martinez, Y.L. Evaluation of Microbiological and Toxicological Quality (Heavy Metals) in Fresh Artisan Cheese Commercialized in Puebla City, Mexico. Int. J. Food Eng. 2019, 5, 276–281. [Google Scholar] [CrossRef]
- Hashami, Z.; Chabook, N.; Javanmardi, F.; Mohammadi, R.; Bashiry, M.; Mousavi Khaneghah, A. The Concentration and Prevalence of Potentially Toxic Elements (PTEs) in Cheese: A Global Systematic Review and Meta-Analysis. Int. J. Environ. Health Res. 2022, 1–20. [Google Scholar] [CrossRef]
- Meshref, A.M.S.; Moselhy, W.A.; Hassan, N.E.-H.Y. Heavy Metals and Trace Elements Levels in Milk and Milk Products. J. Food Meas. Charact. 2014, 8, 381–388. [Google Scholar] [CrossRef]
- Aggarwal, A.; Verma, T.; Ghosh, S. Heavy Metal Residues in Milk and Milk Products and Their Detection Method. In Trends and Innovations in Food Science; IntechOpen: London, UK, 2022. [Google Scholar]
- Iqbal, H.; Ishfaq, M.; Abbas, M.N.; Wahab, A.; Qayum, M.; Mehsud, S. Pathogenic Bacteria and Heavy Metals Toxicity Assessments in Evaluating Unpasteurized Raw Milk Quality through Biochemical Tests Collected from Dairy Cows. Asian Pac. J. Trop. Dis. 2016, 6, 868–872. [Google Scholar] [CrossRef]
- Baianu, I.C.; You, T. High-Resolution near-Infrared and Nuclear Magnetic Resonance Analysis of Food and Grain Composition. Handb. Food Anal. Instrum. 2009, 12, 247–279. [Google Scholar]
- Custers, D.; Cauwenbergh, T.; Bothy, J.L.; Courselle, P.; De Beer, J.O.; Apers, S.; Deconinck, E. ATR-FTIR Spectroscopy and Chemometrics: An Interesting Tool to Discriminate and Characterize Counterfeit Medicines. J. Pharm. Biomed. Anal. 2015, 112, 181–189. [Google Scholar] [CrossRef]
- Santos, M.C.; Nascimento, P.A.M.; Guedes, W.N.; Pereira-Filho, E.R.; Filletti, É.R.; Pereira, F.M.V. Chemometrics in Analytical Chemistry–an Overview of Applications from 2014 to 2018. Eclét. Quím. J. 2019, 44, 11–25. [Google Scholar] [CrossRef]
- Adams, M.J. Chemometrics in Analytical Spectroscopy; Royal Society of Chemistry: London, UK, 2007. [Google Scholar]
- Brereton, R.G. Applied Chemometrics for Scientists; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Wold, S.; Esbensen, K.; Geladi, P. Principal Component Analysis. Chemom. Intell. Lab. Syst. 1987, 2, 37–52. [Google Scholar] [CrossRef]
- Bauer, R.; Nieuwoudt, H.; Bauer, F.F.; Kossmann, J.; Koch, K.R.; Esbensen, K.H. FTIR Spectroscopy for Grape and Wine Analysis; ACS Publications: Washington, DC, USA, 2008. [Google Scholar]
- Rohman, A.; Che Man, Y.B.; Hashim, P.; Ismail, A. FTIR Spectroscopy Combined with Chemometrics for Analysis of Lard Adulteration in Some Vegetable Oils. Cyta-J. Food 2011, 9, 96–101. [Google Scholar] [CrossRef]
- Rohman, A.; Che Man, Y.B. FTIR Spectroscopy Combined with Chemometrics for Analysis of Lard in the Mixtures with Body Fats of Lamb, Cow, and Chicken. Int. Food Res. J. 2010, 17, 519–526. [Google Scholar]
- Pereira, R.C.C.; Skrobot, V.L.; Castro, E.V.R.; Fortes, I.C.P.; Pasa, V.M.D. Determination of Gasoline Adulteration by Principal Components Analysis-Linear Discriminant Analysis Applied to FTIR Spectra. Energy Fuels 2006, 20, 1097–1102. [Google Scholar] [CrossRef]
- Yu, S.; Sheng, L.; Zhang, C.; Deng, H. Physiological Response of Arundo Donax to Cadmium Stress by Fourier Transform Infrared Spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 198, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Kepenek, E.S.; Severcan, M.; Gozen, A.G.; Severcan, F. Discrimination of Heavy Metal Acclimated Environmental Strains by Chemometric Analysis of FTIR Spectra. Ecotoxicol. Environ. Saf. 2020, 202, 110953. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.S.; Sawant, S.G. Identification of CaCO3 Polymorphs of Shellfish by FTIR Spectroscopy and Evaluation of Metals Adsorption by Powdered Exoskeleton Shell. Indian J. Geo-Marine Sci. 2022, 51, 304–309. [Google Scholar]
- Wang, Q.; Li, L.; Tian, Y.; Kong, L.; Cai, G.; Zhang, H.; Zhang, J.; Zuo, W.; Wen, B. Shapeable Amino-Functionalized Sodium Alginate Aerogel for High-Performance Adsorption of Cr (VI) and Cd (II): Experimental and Theoretical Investigations. Chem. Eng. J. 2022, 446, 137430. [Google Scholar] [CrossRef]
- Rohman, A.; Man, Y.B.C. The Use of Fourier Transform Mid Infrared (FT-MIR) Spectroscopy for Detection and Quantification of Adulteration in Virgin Coconut Oil. Food Chem. 2011, 129, 583–588. [Google Scholar] [CrossRef]
- Iñon, F.A.; Garrigues, S.; de la Guardia, M. Nutritional Parameters of Commercially Available Milk Samples by FTIR and Chemometric Techniques. Anal. Chim. Acta 2004, 513, 401–412. [Google Scholar] [CrossRef]
- Duarte, I.F.; Barros, A.; Delgadillo, I.; Almeida, C.; Gil, A.M. Application of FTIR Spectroscopy for the Quantification of Sugars in Mango Juice as a Function of Ripening. J. Agric. Food Chem. 2002, 50, 3104–3111. [Google Scholar] [CrossRef] [PubMed]
- Anjos, O.; Campos, M.G.; Ruiz, P.C.; Antunes, P. Application of FTIR-ATR Spectroscopy to the Quantification of Sugar in Honey. Food Chem. 2015, 169, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Gurbanov, R.; Gozen, A.G.; Severcan, F. Rapid Classification of Heavy Metal-Exposed Freshwater Bacteria by Infrared Spectroscopy Coupled with Chemometrics Using Supervised Method. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 189, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Williams, S. Official Methods of Analysis; AOAC Inc.: Rockville, MD, USA, 1984. [Google Scholar]
- Paré, J.R.J.; Bélanger, J.M.R. Instrumental Methods in Food Analysis; Elsevier: Amsterdam, The Netherlands, 1997. [Google Scholar]
- Pishva, P.; Bayazt, M.K.; Kurt, H.; Yüce, M. The Efficacy of Staphylococcus Aureus Dry Biomass in the Detection of Cd (II) Heavy Metal Ions. Emergent Mater. 2022, 5, 1745–1755. [Google Scholar] [CrossRef]
- Mozgawa, W. The Influence of Some Heavy Metals Cations on the FTIR Spectra of Zeolites. J. Mol. Struct. 2000, 555, 299–304. [Google Scholar] [CrossRef]
- Fischer, W.B.; Fedorowicz, A.; Koll, A. Structured Water around Ions—FTIR Difference Spectroscopy and Quantum-Mechanical Calculations. Phys. Chem. Chem. Phys. 2001, 3, 4228–4234. [Google Scholar] [CrossRef]
- Barthel, J.; Deser, R. FTIR Study of Ion Solvation and Ion-Pair Formation in Alkaline and Alkaline Earth Metal Salt Solutions in Acetonitrile. J. Solut. Chem. 1994, 23, 1133–1146. [Google Scholar] [CrossRef]
- Iqbal, M.; Saeed, A.; Zafar, S.I. FTIR Spectrophotometry, Kinetics and Adsorption Isotherms Modeling, Ion Exchange, and EDX Analysis for Understanding the Mechanism of Cd2+ and Pb2+ Removal by Mango Peel Waste. J. Hazard Mater. 2009, 164, 161–171. [Google Scholar] [CrossRef]
- Bandopadhyay, A.K. Determination of Quartz Content for Indian Coals Using an FTIR Technique. Int. J. Coal Geol. 2010, 81, 73–78. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.-H.; Zhao, L.-J. ATR-FTIR Spectroscopic Studies on Aqueous LiClO4, NaClO4, and Mg (ClO4)2 Solutions. Phys. Chem. Chem. Phys. 2004, 6, 537–542. [Google Scholar] [CrossRef]
- Mozgawa, W.; Król, M.; Bajda, T. Application of IR Spectra in the Studies of Heavy Metal Cations Immobilization on Natural Sorbents. J. Mol. Struct. 2009, 924, 427–433. [Google Scholar] [CrossRef]
- Nateras-Ramírez, O.; López-Cervantes, J.; Sánchez-Machado, D.I.; Aguilar-Ruiz, R.J.; Martínez-Macias, M.R. Kinetic Modeling of Cd (II) and Pb (II) Biosorption from Aqueous Solution by Inactive Biomass of Nannochloropsis Oculata Microalgae. Water Air Soil Pollut. 2022, 233, 184. [Google Scholar] [CrossRef]
- Raj, A.F.P.; Krajnc, S.; Bauman, M.; Lakić, M.; Gutmaher, A.; Lobnik, A.; Košak, A. Removal of Pb2+, Cr3+ and Hg2+ Ions from Aqueous Solutions Using SiO2 and Amino-Functionalized SiO2 Particles. J. Solgel Sci. Technol. 2022, 103, 290–308. [Google Scholar] [CrossRef]
- Merlino, V.M.; Renna, M.; Nery, J.; Muresu, A.; Ricci, A.; Maggiolino, A.; Celano, G.; de Ruggieri, B.; Tarantola, M. Are Local Dairy Products Better? Using Principal Component Analysis to Investigate Consumers’ Perception towards Quality, Sustainability, and Market Availability. Animals 2022, 12, 1421. [Google Scholar] [CrossRef]
- Vojnovic, D.; Procida, G.; Favretto, L.G. Chemometric Differentiation of Raw and Commercial Milk by Trace Elements Using Principal Component Analysis. Food Addit. Contam. 1991, 8, 343–349. [Google Scholar] [CrossRef]
- Luiz, L.d.C.; Bell, M.J.V.; Rocha, R.A.d.; Leal, N.L.; Anjos, V.d.C. Detection of Veterinary Antimicrobial Residues in Milk through Near-Infrared Absorption Spectroscopy. J. Spectrosc. 2018, 2018, 5152832. [Google Scholar] [CrossRef]
- Ali, Z.; O’Hare, W.T.; Theaker, B.J. Detection of Bacterial Contaminated Milk by Means of a Quartz Crystal Microbalance Based Electronic Nose. J. Therm. Anal. Calorim. 2003, 71, 155–161. [Google Scholar] [CrossRef]
- Farrugia, J.; Griffin, S.; Valdramidis, V.P.; Camilleri, K.; Falzon, O. Principal Component Analysis of Hyperspectral Data for Early Detection of Mould in Cheeselets. Curr. Res. Food Sci. 2021, 4, 18–27. [Google Scholar] [CrossRef]
- Allouche, A.-R. Gabedit-A Graphical User Interface for Computational Chemistry Softwares. J. Comput. Chem. 2011, 32, 174–182. [Google Scholar] [CrossRef]
- Farrell, H.M., Jr.; Wickham, E.D.; Unruh, J.J.; Qi, P.X.; Hoagland, P.D. Secondary Structural Studies of Bovine Caseins: Temperature Dependence of β-Casein Structure as Analyzed by Circular Dichroism and FTIR Spectroscopy and Correlation with Micellization. Food Hydrocoll. 2001, 15, 341–354. [Google Scholar] [CrossRef]
- Verma, P.K.; Kundu, A.; Puretz, M.S.; Dhoonmoon, C.; Chegwidden, O.S.; Londergan, C.H.; Cho, M. The Bend+Libration Combination Band Is an Intrinsic, Collective, and Strongly Solute-Dependent Reporter on the Hydrogen Bonding Network of Liquid Water. J. Phys. Chem. B 2018, 122, 2587–2599. [Google Scholar] [CrossRef] [PubMed]
- Swaisgood, H.E. Review and Update of Casein Chemistry. J. Dairy Sci. 1993, 76, 3054–3061. [Google Scholar] [CrossRef] [PubMed]
- De Kruif, C.G.; Grinberg, V.Y. Micellisation of β-Casein. Colloids Surf. A Physicochem. Eng. Asp. 2002, 210, 183–190. [Google Scholar] [CrossRef]
- O’Connell, J.E.; Grinberg, V.Y.; de Kruif, C.G. Association Behavior of β-Casein. J. Colloid Interface Sci. 2003, 258, 33–39. [Google Scholar] [CrossRef]
- Indrayanto, G.; Rohman, A. The Use of FTIR Spectroscopy Combined with Multivariate Analysis in Food Composition Analysis. In Spectroscopic Techniques & Artificial Intelligence for Food and Beverage Analysis; Springer: Singapore, 2020; pp. 25–51. [Google Scholar]
- Barbano, D.M.; Dellavalle, M.E. Rapid Method for Determination of Milk Casein Content by Infrared Analysis. J. Dairy Sci. 1987, 70, 1524–1528. [Google Scholar] [CrossRef]
- Max, J.-J.; Gessinger, V.; van Driessche, C.; Larouche, P.; Chapados, C. Infrared Spectroscopy of Aqueous Ionic Salt Solutions at Low Concentrations. J. Chem. Phys. 2007, 126, 184507. [Google Scholar] [CrossRef]
- Innocenzi, P.; Malfatti, L.; Carboni, D.; Takahashi, M. Sol-to-Gel Transition in Fast Evaporating Systems Observed by in Situ Time-Resolved Infrared Spectroscopy. ChemPhysChem 2015, 16, 1933–1939. [Google Scholar] [CrossRef]
- Mojet, B.L.; Ebbesen, S.D.; Lefferts, L. Light at the Interface: The Potential of Attenuated Total Reflection Infrared Spectroscopy for Understanding Heterogeneous Catalysis in Water. Chem. Soc. Rev. 2010, 39, 4643–4655. [Google Scholar] [CrossRef] [PubMed]
- Barth, A. Infrared Spectroscopy of Proteins. Biochim. Et Biophys. Acta (BBA)-Bioenerg. 2007, 1767, 1073–1101. [Google Scholar] [CrossRef]
- Gerbino, E.; Mobili, P.; Tymczyszyn, E.; Fausto, R.; Gómez-Zavaglia, A. FTIR Spectroscopy Structural Analysis of the Interaction between Lactobacillus Kefir S-Layers and Metal Ions. J. Mol. Struct. 2011, 987, 186–192. [Google Scholar] [CrossRef]
- Nahar, S.; Tajmir-Riahi, H.A. Complexation of Heavy Metal Cations Hg, Cd, and Pb with Proteins of PSII: Evidence for Metal–Sulfur Binding and Protein Conformational Transition by FTIR Spectroscopy. J. Colloid Interface Sci. 1996, 178, 648–656. [Google Scholar] [CrossRef]
- Nara, M.; Morii, H.; Tanokura, M. Coordination to Divalent Cations by Calcium-Binding Proteins Studied by FTIR Spectroscopy. Biochim. Et Biophys. Acta (BBA)-Biomembr. 2013, 1828, 2319–2327. [Google Scholar] [CrossRef] [PubMed]
- Sawalha, M.F.; Peralta-Videa, J.R.; Saupe, G.B.; Dokken, K.M.; Gardea-Torresdey, J.L. Using FTIR to Corroborate the Identity of Functional Groups Involved in the Binding of Cd and Cr to Saltbush (Atriplex Canescens) Biomass. Chemosphere 2007, 66, 1424–1430. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.; Li, J.; Cai, W.; Shao, X. Simultaneous Determination of Heavy Metal Ions in Water Using Near-Infrared Spectroscopy with Preconcentration by Nano-Hydroxyapatite. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012, 96, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Rauh, F.; Mizaikoff, B. Simultaneous Determination of Monoatomic Ions via Infrared Attenuated Total Reflection Spectroscopy in Aqueous Solution at Different Temperatures. Appl. Spectrosc. 2016, 70, 1214–1227. [Google Scholar] [CrossRef]
- Saqib, S.; Nazeer, A.; Ali, M.; Zaman, W.; Younas, M.; Shahzad, A.; Nisar, M. Catalytic Potential of Endophytes Facilitates Synthesis of Biometallic Zinc Oxide Nanoparticles for Agricultural Application. BioMetals 2022, 35, 967–985. [Google Scholar] [CrossRef]
- Smith, D.K.; Radivojac, P.; Obradovic, Z.; Dunker, A.K.; Zhu, G. Improved Amino Acid Flexibility Parameters. Protein Sci. 2003, 12, 1060–1072. [Google Scholar] [CrossRef]
- Bhaskaran, R.; Ponnuswamy, P.K. Positional Flexibilities of Amino Acid Residues in Globular Proteins. Int. J. Pept. Protein Res. 2009, 32, 241–255. [Google Scholar] [CrossRef]
- Le Meste, M.; Colas, B.; Simatos, D.; Closs, B.; Courthaudon, J.-L.; Lorient, D. Contribution of Protein Flexibility to the Foaming Properties of Casein. J. Food Sci. 1990, 55, 1445–1447. [Google Scholar] [CrossRef]
- Brooks, D.J.; Fresco, J.R.; Lesk, A.M.; Singh, M. Evolution of Amino Acid Frequencies in Proteins Over Deep Time: Inferred Order of Introduction of Amino Acids into the Genetic Code. Mol. Biol. Evol. 2002, 19, 1645–1655. [Google Scholar] [CrossRef]
- Gaur, R.K. Amino Acid Frequency Distribution Among Eukaryotic Proteins. IIOAB 2014, 5, 6–11. [Google Scholar]
- Kumar, V.; Sharma, A.; Kumar, R.; Bhardwaj, R.; Kumar Thukral, A.; Rodrigo-Comino, J. Assessment of Heavy-Metal Pollution in Three Different Indian Water Bodies by Combination of Multivariate Analysis and Water Pollution Indices. Hum. Ecol. Risk Assess. Int. J. 2020, 26, 1–16. [Google Scholar] [CrossRef]

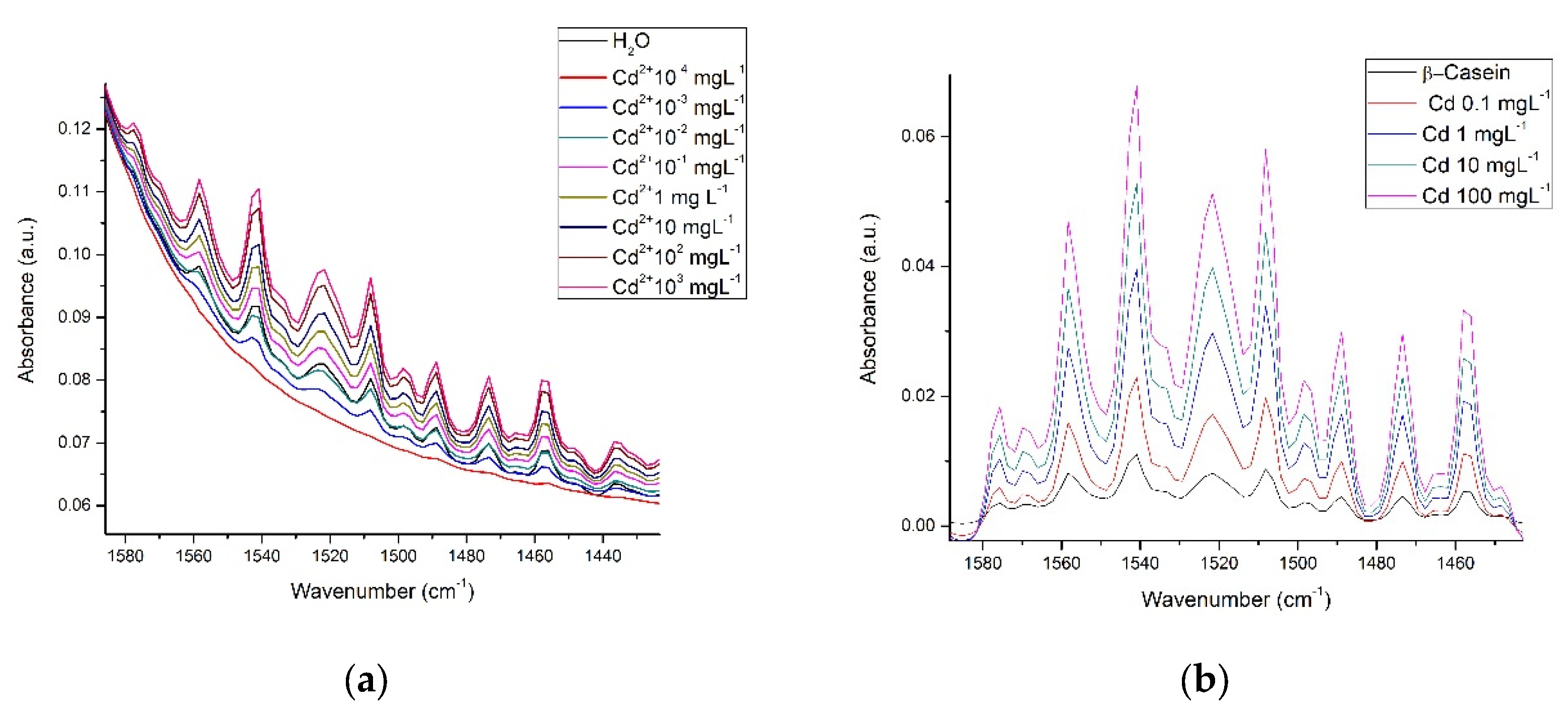

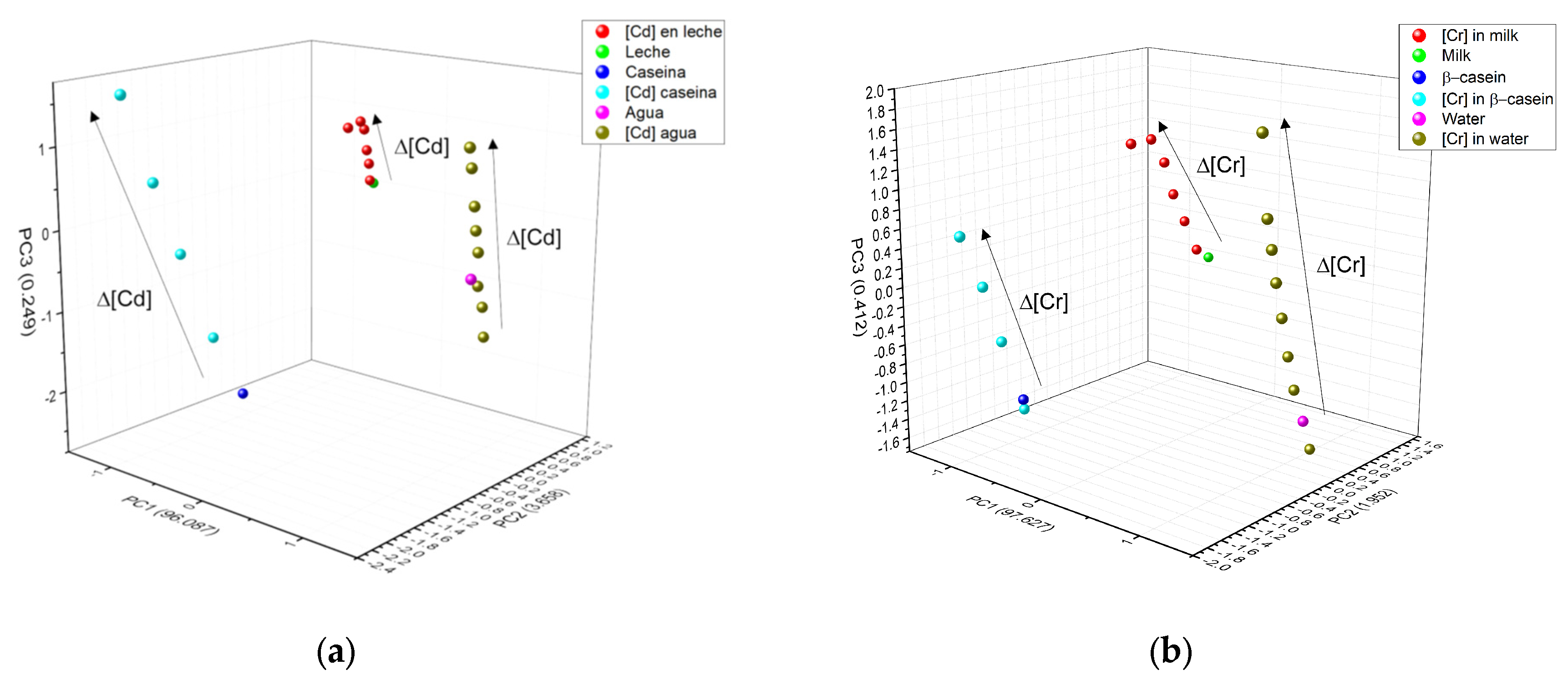

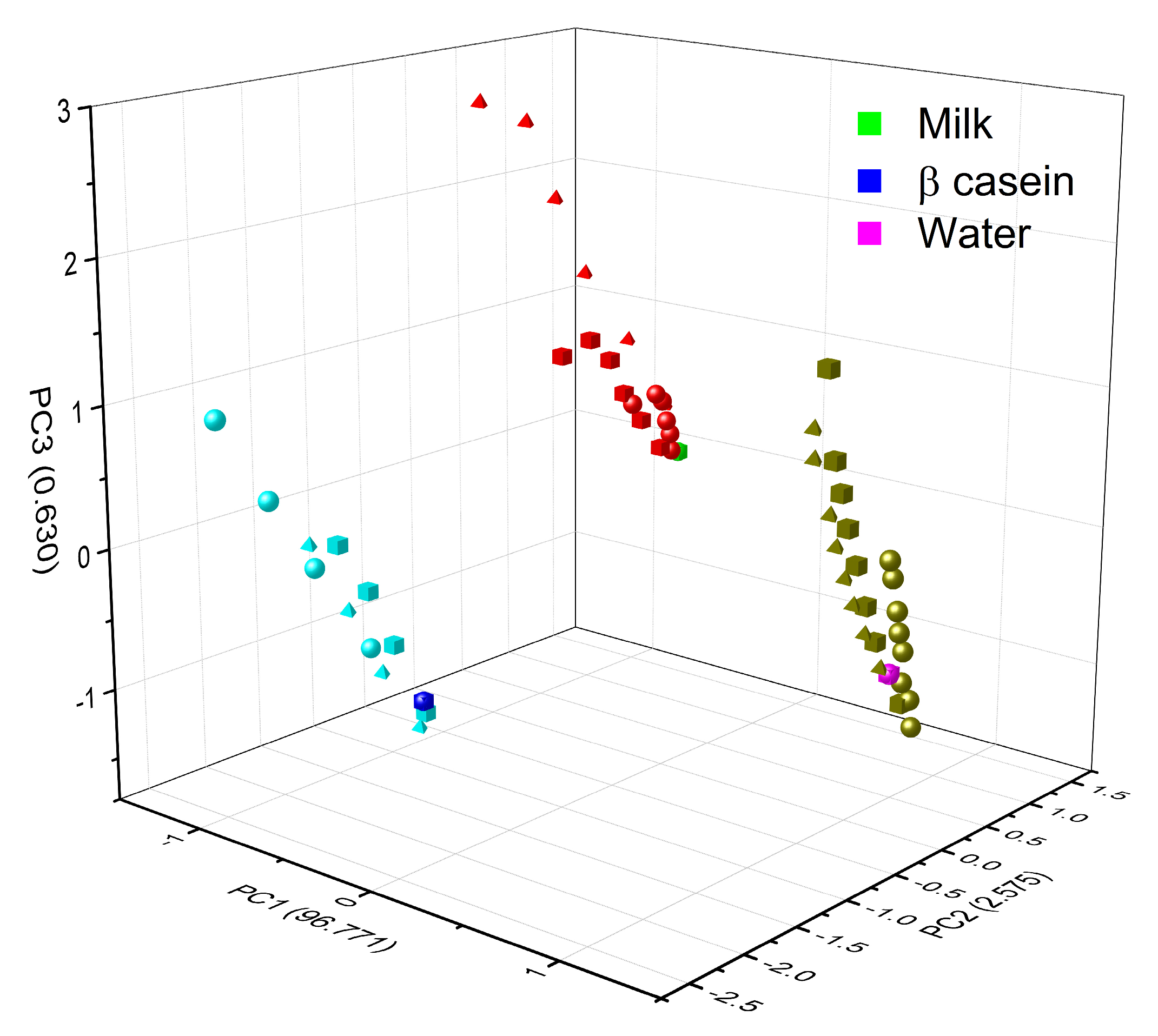
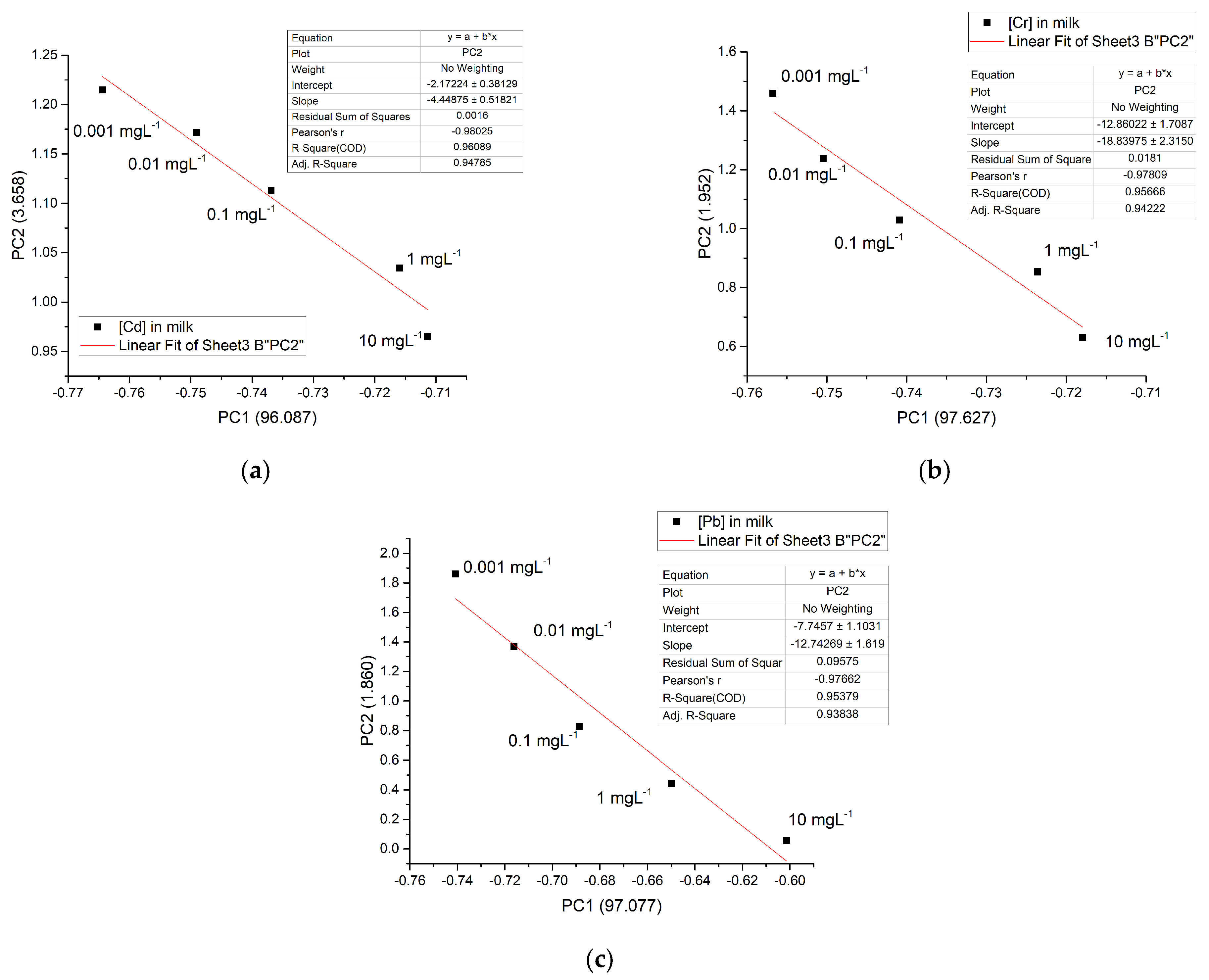
| Wavenumber (cm−1) | Group | Description |
|---|---|---|
| 1473 | Gln, Hys radicals, and peptide bonds | When Gln or Hys radicals lie in the same plane as another amide, including peptide bonds that are antiparallel to each other, at a distance of 4.5 Å |
| 1489 | Gln or Glu radical with a peptide bond | When the radical amide of Gln and the peptide bond are at an angle ≈ 90° and at 4.5 Å or when the plane of the carboxyl radical of Glu and the peptide bond lie in the same plane |
| 1508 | Imide group of proline with any amide | Imide and the peptide bond on the same plane and another amide in parallel at 4.5 Å |
| 1522 | Glu, Gln radicals, and peptide bond | The amide planes are perpendicular to the plane of the carboxylic acid of Glu or to the plane formed by a C carbonyl, its O, and a sp3 C |
| 1540 | Peptide bond and radical amide from Gln | Two amides at an angle of 100° with the vertex being a C sp3 or the plane formed by a carbonyl, its O and a C sp3 |
| 1558 | Peptide bond | When pairs of two antiparallel peptide bonds separated by a Cα from Val or Glu (such as a hairpin motif) are close to each other |
| 1575 | Peptide bond from Pro, radical benzene from Phe, and carboxylic from Glu | The benzene plane of Phe lies parallel to its C-Ter peptide bond and perpendicular to the next peptide bond, which is from Pro |
| Region | Intervals in Wavenumber (cm−1) | Justification 1 |
|---|---|---|
| 1 | 3500–3000 | Stretching of -OH |
| 2500–2000 | Scissoring band | |
| 2000–1500 | Scissoring band | |
| 2 | 3500–3000 | Stretching of -OH |
| 2000–1500 | Scissoring band | |
| 3 | 3400–3200 | Stretching of -OH |
| 2400–2250 | Scissoring band | |
| 1800–1450 | Scissoring band | |
| 4 | 3400–3200 | Stretching of -OH |
| 1800–1450 | Scissoring band |
| Region | Intervals in Wavenumber (cm−1) | Justification 2 |
|---|---|---|
| 1 | 3500–3000 | Stretching of -NH |
| 1600–1750 | Carbonyl stretching | |
| 2000–1500 | Bending vibration -NH | |
| 2 | 3500–3000 | Stretching of -NH |
| 1500–1300 | C-N vibrations | |
| 3 | 3400–3200 | Stretching of -NH |
| 2650–2000 | -N-C-N- stretching | |
| 1600–1250 | Carboxylic acids, aromatics | |
| 4 | 3400–3200 | Stretching of -NH |
| 1800–1450 | Bending vibration -NH |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benítez-Rojas, A.C.; Jaramillo-Flores, M.E.; Zaca-Moran, O.; Quiroga-Montes, I.; Delgado-Macuil, R.J. A Study of the Interactions of Heavy Metals in Dairy Matrices Using Fourier Transform Infrared Spectroscopy, Chemometric, and In Silico Analysis. Foods 2023, 12, 1919. https://doi.org/10.3390/foods12091919
Benítez-Rojas AC, Jaramillo-Flores ME, Zaca-Moran O, Quiroga-Montes I, Delgado-Macuil RJ. A Study of the Interactions of Heavy Metals in Dairy Matrices Using Fourier Transform Infrared Spectroscopy, Chemometric, and In Silico Analysis. Foods. 2023; 12(9):1919. https://doi.org/10.3390/foods12091919
Chicago/Turabian StyleBenítez-Rojas, Alfredo C., María E. Jaramillo-Flores, Orlando Zaca-Moran, Israel Quiroga-Montes, and Raúl J. Delgado-Macuil. 2023. "A Study of the Interactions of Heavy Metals in Dairy Matrices Using Fourier Transform Infrared Spectroscopy, Chemometric, and In Silico Analysis" Foods 12, no. 9: 1919. https://doi.org/10.3390/foods12091919
APA StyleBenítez-Rojas, A. C., Jaramillo-Flores, M. E., Zaca-Moran, O., Quiroga-Montes, I., & Delgado-Macuil, R. J. (2023). A Study of the Interactions of Heavy Metals in Dairy Matrices Using Fourier Transform Infrared Spectroscopy, Chemometric, and In Silico Analysis. Foods, 12(9), 1919. https://doi.org/10.3390/foods12091919