Evaluating the Potential Safety Risk of Plant-Based Meat Analogues by Analyzing Microbial Community Composition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Sample Preparation
2.3. Bacterial and Fungal Genomic DNA Extraction
2.4. High-Throughput Sequencing
2.5. Date Processing and Bioinformatics Analysis
3. Results
3.1. High-Throughput Sequencing Results and Diversity Analysis
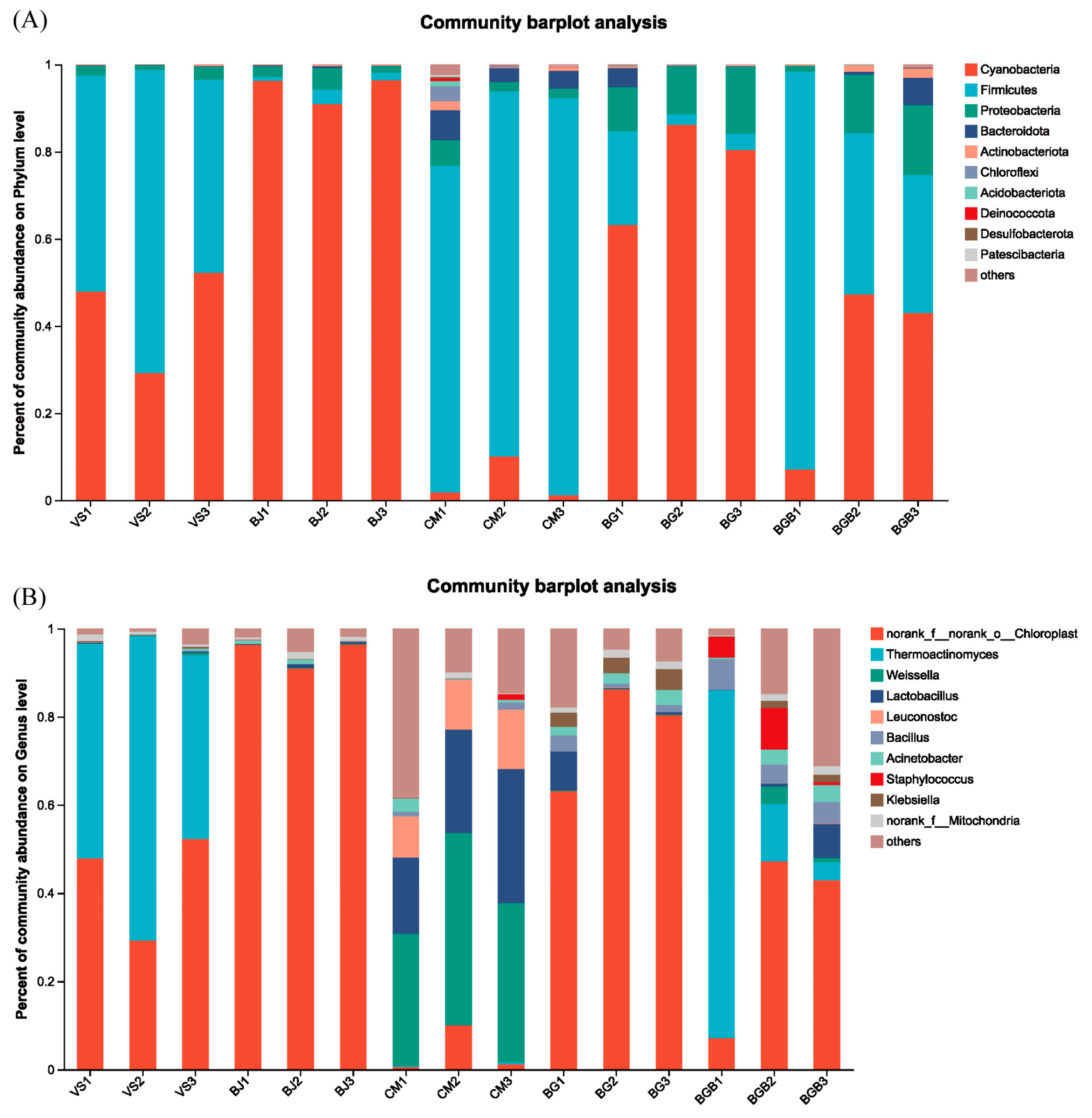
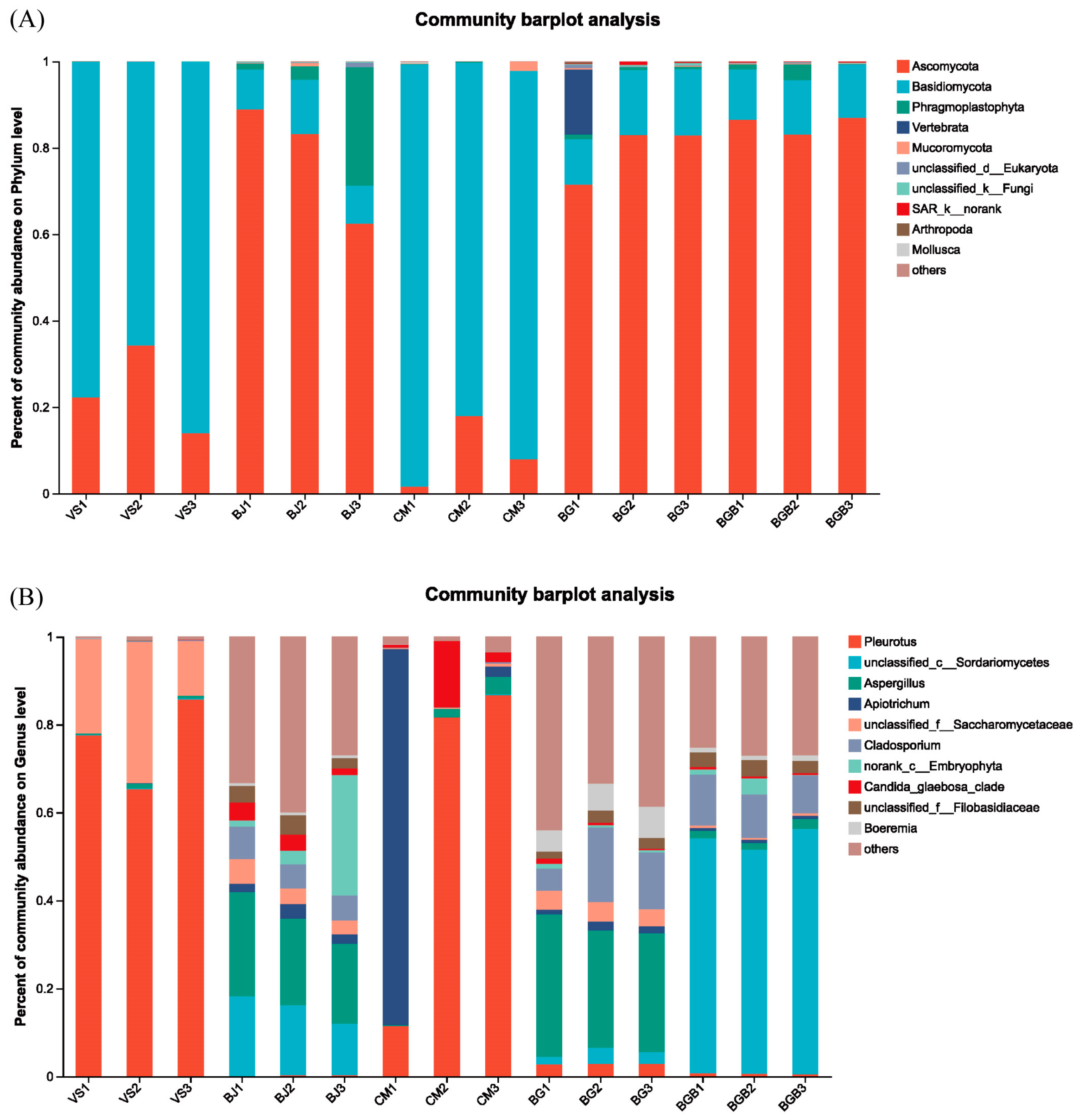
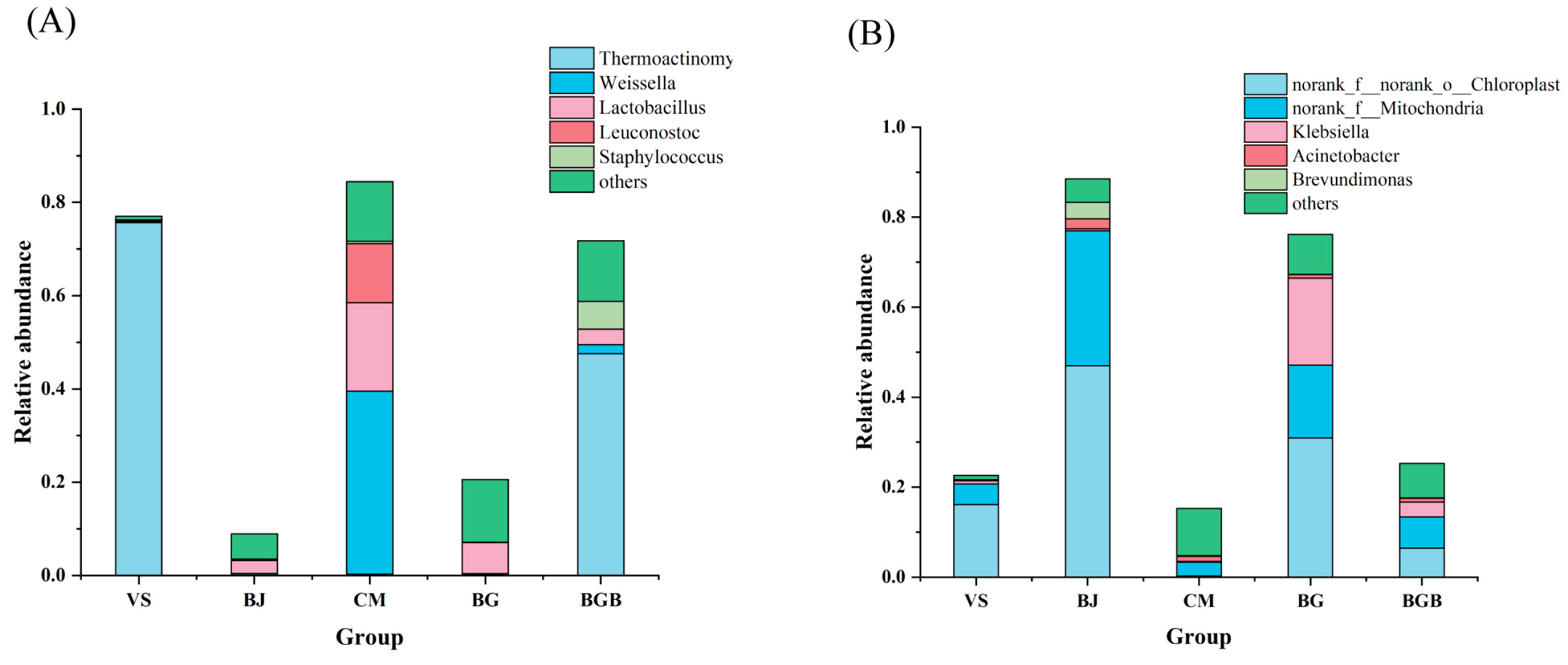
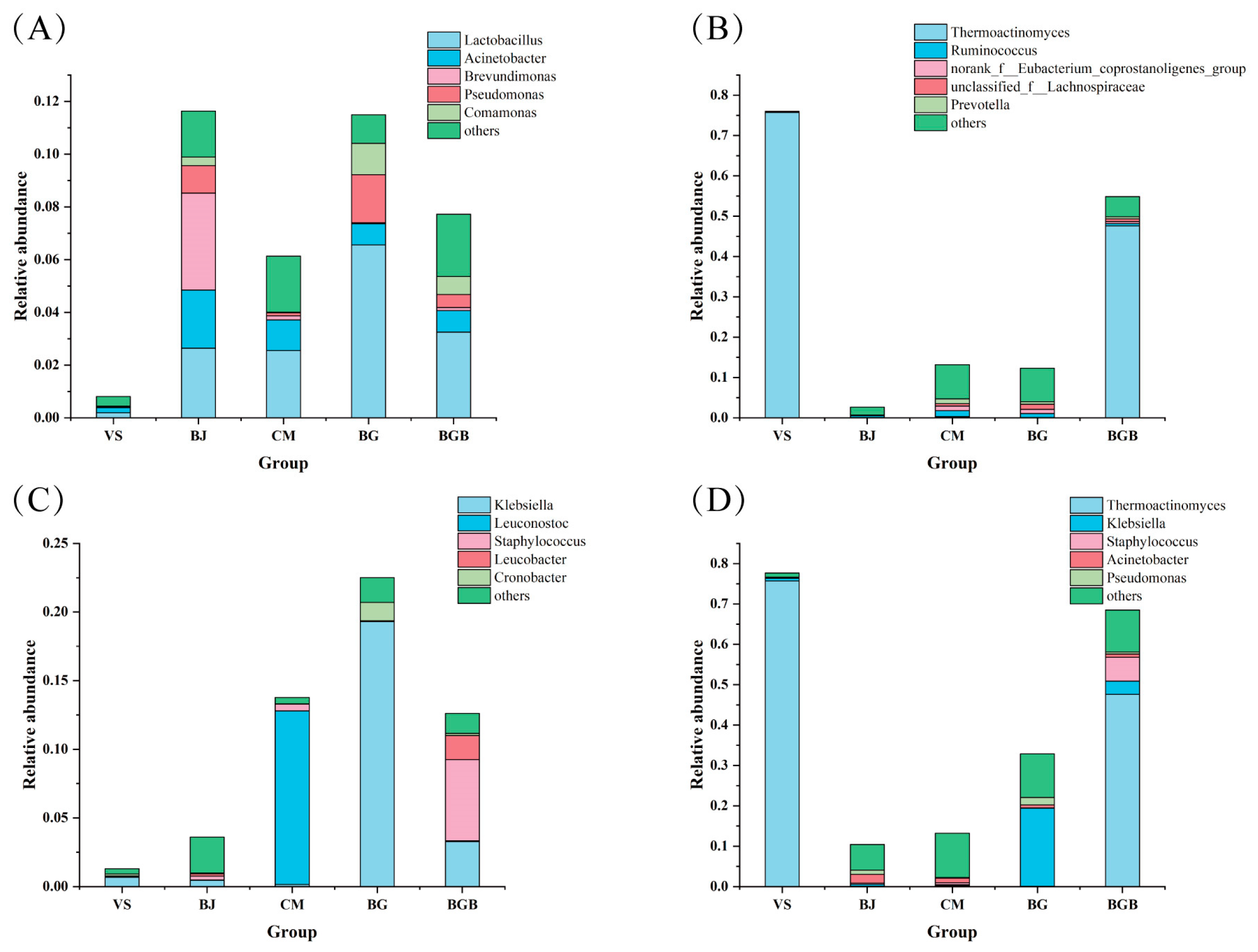
3.2. Taxonomic Composition of Microbial Community
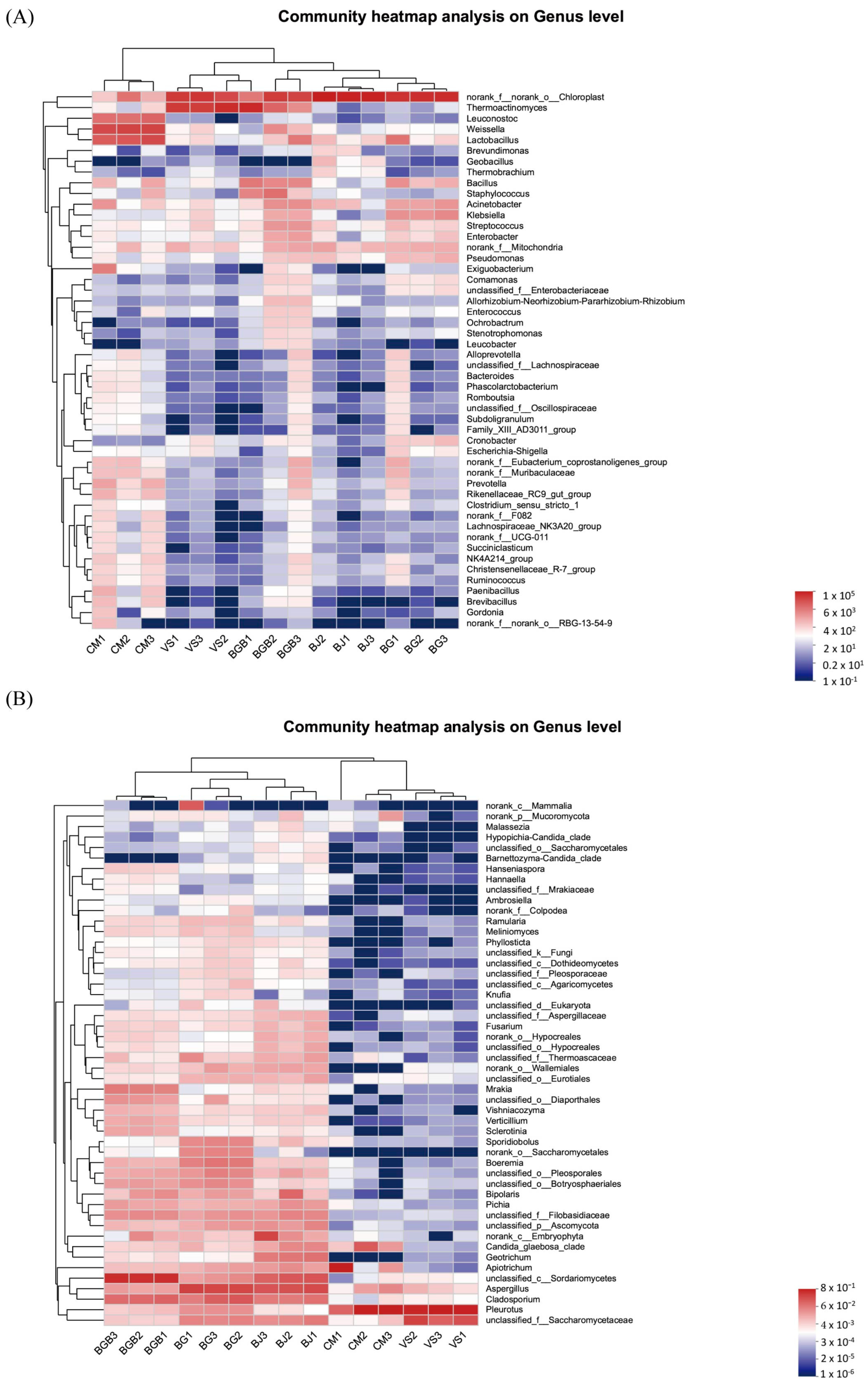
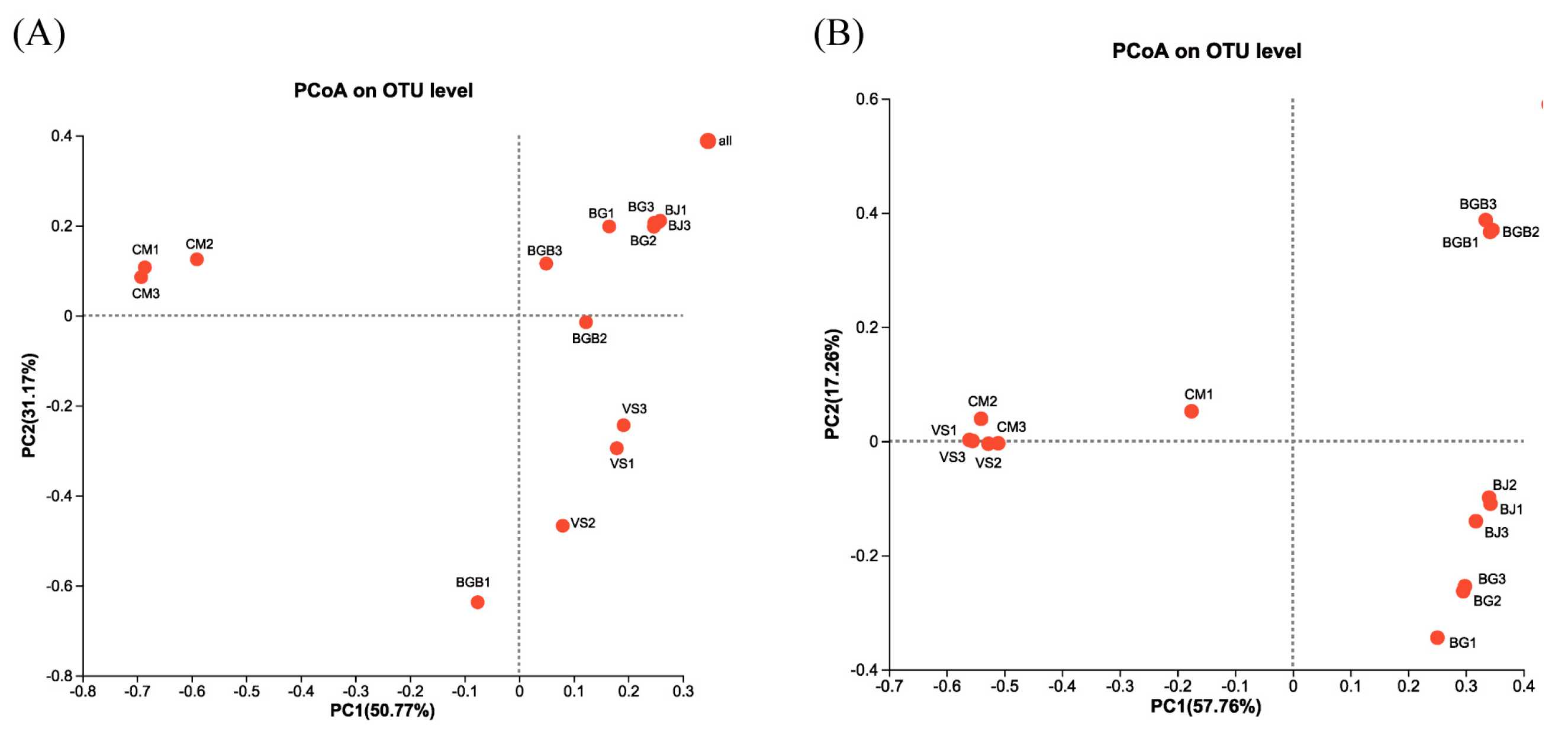
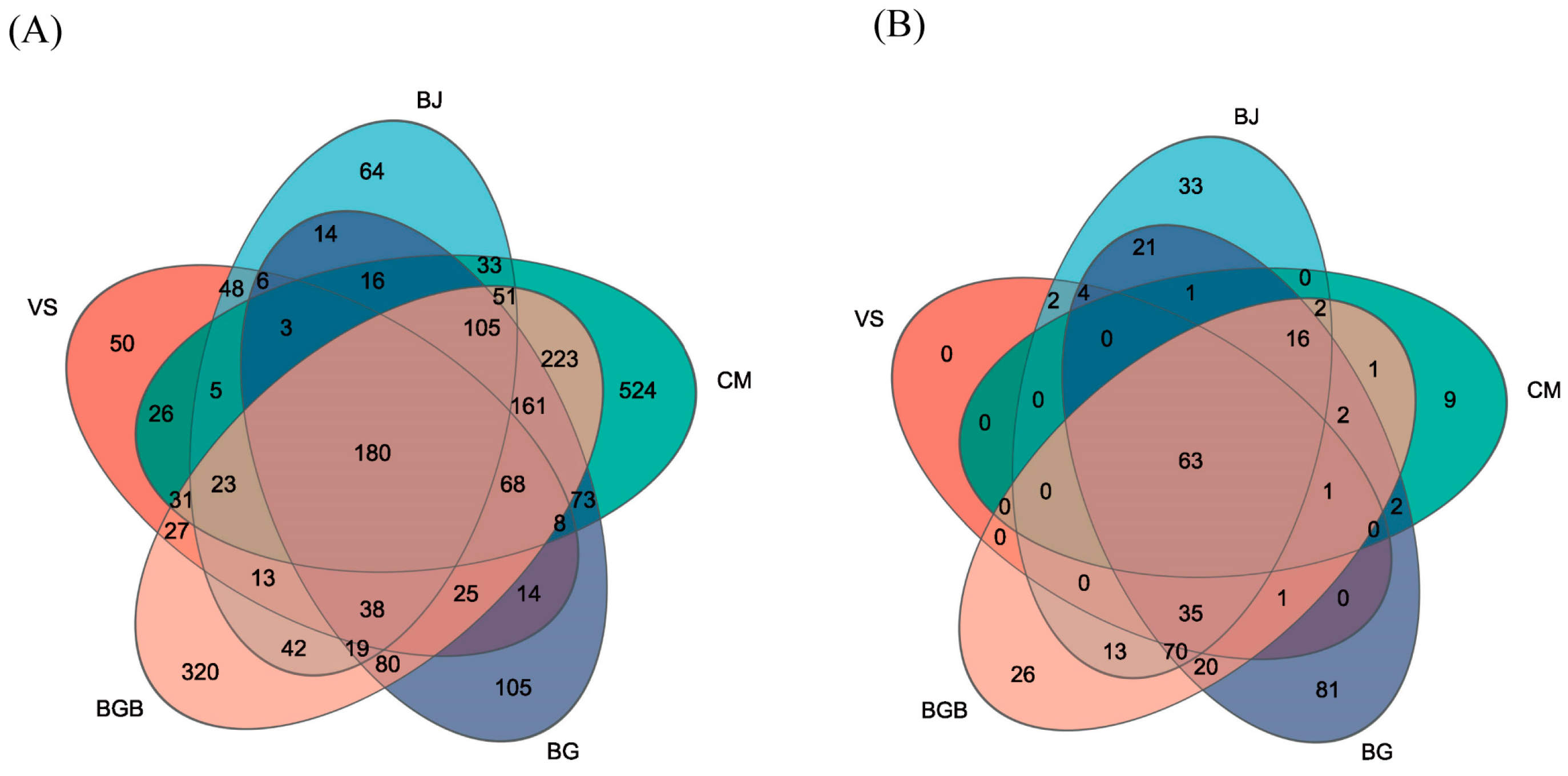
3.3. The Similarity of Microbial Community
3.4. The Relative Abundance of Gram-Positive and Gram-Negative Bacteria
3.5. The Relative Abundance of the Aerobic, Anaerobic, and Facultative Anaerobic Bacteria
3.6. The Relative Abundance of the Potential Pathogenic Bacteria
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boland, M.J.; Rae, A.N.; Vereijken, J.M.; Meuwissen, M.P.M.; Fischer, A.R.H.; van Boekel, M.A.J.S.; Rutherfurd, S.M.; Gruppen, H.; Moughan, P.J.; Hendriks, W.H. The future supply of animal-derived protein for human consumption. Trends Food Sci. Technol. 2013, 29, 62–73. [Google Scholar] [CrossRef]
- Mekonnen, M.M.; Hoekstra, A.Y. A Global Assessment of the Water Footprint of Farm Animal Products. Ecosystems 2012, 15, 401–415. [Google Scholar] [CrossRef]
- Ogino, A.; Orito, H.; Shimada, K.; Hirooka, H. Evaluating environmental impacts of the Japanese beef cow?calf system by the life cycle assessment method. Anim. Sci. J. 2007, 78, 424–432. [Google Scholar] [CrossRef]
- Walker, P.; Rhubart-Berg, P.; McKenzie, S.; Kelling, K.; Lawrence, R.S. Public health implications of meat production and consumption. Public Health Nutr. 2005, 8, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Micha, R.; Wallace, S.K.; Mozaffarian, D. Red and Processed Meat Consumption and Risk of Incident Coronary Heart Disease, Stroke, and Diabetes Mellitus a Systematic Review and Meta-Analysis. Circulation 2010, 121, 2271–2283. [Google Scholar] [CrossRef] [PubMed]
- Layton, D.S.; Choudhary, A.; Bean, A.G.D. Breaking the chain of zoonoses through biosecurity in livestock. Vaccine 2017, 35, 5967–5973. [Google Scholar] [CrossRef] [PubMed]
- Tomley, F.M.; Shirley, M.W. Livestock infectious diseases and zoonoses. Philos. Trans. R. Soc. B 2009, 364, 2637–2642. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Chatli, M.K.; Mehta, N.; Singh, P.; Malav, O.P.; Verma, A.K. Meat analogues: Health promising sustainable meat substitutes. Crit. Rev. Food Sci. Nutr. 2017, 57, 923–932. [Google Scholar] [CrossRef]
- Lusk, J.L.; Blaustein-Rejto, D.; Shah, S.; Tonsor, G.T. Impact of plant-based meat alternatives on cattle inventories and greenhouse gas emissions. Environ. Res. Lett. 2022, 17, 024035. [Google Scholar] [CrossRef]
- Wang, Y.; Cai, W.; Li, L.; Gao, Y.; Lai, K.H. Recent Advances in the Processing and Manufacturing of Plant-Based Meat. J. Agric. Food Chem. 2023, 71, 1276–1290. [Google Scholar] [CrossRef]
- Bryant, C.; Barnett, J. Consumer acceptance of cultured meat: A systematic review. Meat Sci. 2018, 143, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Hocquette, J.F. Is in vitro meat the solution for the future? Meat Sci. 2016, 120, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Boukid, F.; Rosell, C.M.; Castellari, M. Pea protein ingredients: A mainstream ingredient to (re)formulate innovative foods and beverages. Trends Food Sci. Technol. 2021, 110, 729–742. [Google Scholar] [CrossRef]
- Ahnen, R.T.; Jonnalagadda, S.S.; Slavin, J.L. Role of plant protein in nutrition, wellness, and health. Nutr. Rev. 2019, 77, 735–747. [Google Scholar] [CrossRef] [PubMed]
- Asgar, M.A.; Fazilah, A.; Huda, N.; Bhat, R.; Karim, A.A. Nonmeat Protein Alternatives as Meat Extenders and Meat Analogs. Compr. Rev. Food Sci. Food Saf. 2010, 9, 513–529. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.H.; Loveday, S.M.; Hardacre, A.K.; Parker, M.E. Effects of soy protein to wheat gluten ratio on the physicochemical properties of extruded meat analogues. Food Struct. 2019, 19, 100102. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Zhang, L.J.; He, S.D.; Li, X.J.; Jin, R.S.; Liu, Q.; Chen, S.G.; Sun, H.J. High-moisture Extrusion Technology Application in the Processing of Textured Plant Protein Meat Analogues: A Review. Food Rev. Int. 2022, 39, 4873–4908. [Google Scholar] [CrossRef]
- Hadi, J.; Brightwell, G. Safety of Alternative Proteins: Technological, Environmental and Regulatory Aspects of Cultured Meat, Plant-Based Meat, Insect Protein and Single-Cell Protein. Foods 2021, 10, 1226. [Google Scholar] [CrossRef]
- Luchansky, J.B.; Shoyer, B.A.; Jung, Y.; Shane, L.E.; Osoria, M.; Porto-Fett, A.C.S. Viability of Shiga Toxin-Producing Escherichia coli, Salmonella, and Listeria monocytogenes within Plant versus Beef Burgers during Cold Storage and following Pan Frying. J. Food Prot. 2020, 83, 434–442. [Google Scholar] [CrossRef]
- Xiang, N.; Lyu, Y.; Zhu, X.; Bhunia, A.K.; Narsimhan, G. Effect of physicochemical properties of peptides from soy protein on their antimicrobial activity. Peptides 2017, 94, 10–18. [Google Scholar] [CrossRef]
- Blagojevic, B.; Nesbakken, T.; Alvseike, O.; Vagsholm, I.; Antic, D.; Johler, S.; Houf, K.; Meemken, D.; Nastasijevic, I.; Pinto, M.V.; et al. Drivers, opportunities, and challenges of the European risk-based meat safety assurance system. Food Control 2021, 124, 107870. [Google Scholar] [CrossRef]
- Tóth, A.J.; Dunay, A.; Battay, M.; Illés, C.B.; Bittsánszky, A.; Süth, M. Microbial Spoilage of Plant-Based Meat Analogues. Appl. Sci. 2021, 11, 8309. [Google Scholar] [CrossRef]
- Liu, Z.; Shaposhnikov, M.; Zhuang, S.; Tu, T.; Wang, H.; Wang, L. Growth and survival of common spoilage and pathogenic bacteria in ground beef and plant-based meat analogues. Food Res. Int. 2023, 164, 112408. [Google Scholar] [CrossRef] [PubMed]
- Cameron, E.S.; Schmidt, P.J.; Tremblay, B.J.M.; Emelko, M.B.; Muller, K.M. Enhancing diversity analysis by repeatedly rarefying next generation sequencing data describing microbial communities. Sci. Rep. 2021, 11, 22302. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhu, L.; Zhang, Y.; Liang, R.; Luo, X. Microbial community dynamics analysis by high-throughput sequencing in chilled beef longissimus steaks packaged under modified atmospheres. Meat Sci. 2018, 141, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Bassey, A.P.; Chen, Y.; Zhu, Z.; Odeyemi, O.A.; Frimpong, E.B.; Ye, K.; Li, C.; Zhou, G. Assessment of quality characteristics and bacterial community of modified atmosphere packaged chilled pork loins using 16S rRNA amplicon sequencing analysis. Food Res. Int. 2021, 145, 110412. [Google Scholar] [CrossRef] [PubMed]
- Fernandes Rde, P.; Freire, M.T.; de Paula, E.S.; Kanashiro, A.L.; Catunda, F.A.; Rosa, A.F.; Balieiro, J.C.; Trindade, M.A. Stability of lamb loin stored under refrigeration and packed in different modified atmosphere packaging systems. Meat Sci. 2014, 96, 554–561. [Google Scholar] [CrossRef]
- Odeyemi, O.A.; Alegbeleye, O.O.; Strateva, M.; Stratev, D. Understanding spoilage microbial community and spoilage mechanisms in foods of animal origin. Compr. Rev. Food Sci. Food Saf. 2020, 19, 311–331. [Google Scholar] [CrossRef]
- Podschun, R.; Ullmann, U. Klebsiella spp. as nosocomial pathogens: Epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin. Microbiol. Rev. 1998, 11, 589–603. [Google Scholar] [CrossRef]
- Carvalheira, A.; Silva, J.; Teixeira, P. Acinetobacter spp. in food and drinking water—A review. Food Microbiol. 2021, 95, 103675. [Google Scholar] [CrossRef]
- Cheung, G.Y.C.; Bae, J.S.; Otto, M. Pathogenicity and virulence of Staphylococcus aureus. Virulence 2021, 12, 547–569. [Google Scholar] [CrossRef] [PubMed]
- Wild, F.; Czerny, M.; Janssen, A.M.; Kole, A.P.W.; Zunabovic, M.; Domig, K.J. The evolution of a plant-based alternative to meat From niche markets to widely accepted meat alternatives. Agro FOOD Ind. Hi Tech 2014, 25, 45–49. [Google Scholar]
| Plant-Based Meat Analogues | Main Content |
|---|---|
| Beef jerky | soy brushed protein, water, golden sugar, soy protein isolate, soybean oil, brewed soy sauce, flavor, spices, lactic acid, salt, malt extract, round psyllium husk, yeast extract, methylcellulose, gum arabic, citrus fiber, soy protein powder |
| Vegetable sausage | vegetable oil, water, oyster mushroom, soy tissue protein, edible salt, monosodium glutamate, food flavor, white vinegar, carotene, gum arabic, ginger powder, methylcellulose, soy protein powder, guar gum, locust bean gum. |
| Beef grains | soy brushed protein (low-temperature desolventizing edible soybean meal, gluten, soy protein isolate, cornstarch), water, trehalose, spicy beef flavored compound sauce, soy protein isolate, soybean oil, round bud psyllium husk, malt extract, lactic acid |
| Crab mayonnaise | vegetable oil, water, oyster mushroom, soy tissue protein, edible salt, monosodium glutamate, food flavor, white vinegar, carotene, gum arabic, ginger powder, methylcellulose, soy protein powder, guar gum, locust bean gum. |
| Beef grains billet | soy brushed protein (low-temperature edible soybean meal, gluten powder, soy protein isolate, cornstarch), water, trehalose, spicy beef flavor compound sauce, soy protein isolate, soybean oil, round bud psyllium husk, malt extract, lactic acid |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hai, D.; Guo, B.; Qiao, M.; Jiang, H.; Song, L.; Meng, Z.; Huang, X. Evaluating the Potential Safety Risk of Plant-Based Meat Analogues by Analyzing Microbial Community Composition. Foods 2024, 13, 117. https://doi.org/10.3390/foods13010117
Hai D, Guo B, Qiao M, Jiang H, Song L, Meng Z, Huang X. Evaluating the Potential Safety Risk of Plant-Based Meat Analogues by Analyzing Microbial Community Composition. Foods. 2024; 13(1):117. https://doi.org/10.3390/foods13010117
Chicago/Turabian StyleHai, Dan, Baodang Guo, Mingwu Qiao, Haisheng Jiang, Lianjun Song, Ziheng Meng, and Xianqing Huang. 2024. "Evaluating the Potential Safety Risk of Plant-Based Meat Analogues by Analyzing Microbial Community Composition" Foods 13, no. 1: 117. https://doi.org/10.3390/foods13010117





