Optimization and In Vitro Digestion of a Guava (Psidium guajava), Mamey (Pouteria sapota) and Stevia (Stevia rebaudiana) Functional Beverage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Standards and Reagents
2.2. Plant Material
2.3. Stevia Solutions
2.4. Beverage Preparation
2.4.1. Experimental Design, Data Analysis and Verification of the Model
2.4.2. Constrained Formulation
2.4.3. D-Optimal Mixture Design and Analysis
2.5. Methanolic Extracts
2.6. Total Phenolic Content (TPC) by Fast Blue BB (FBBB)
2.7. DPPH● Radical Scavenging Activity
2.8. Trolox Equivalent Antioxidant Capacity (TEAC) Assay
2.9. Ferric Reducing Antioxidant Power (FRAP) Assay
2.10. Total Carotenoids (TC)
2.11. Sensory Analysis
2.12. Phenolic Profile
2.13. Carotenoid Profile
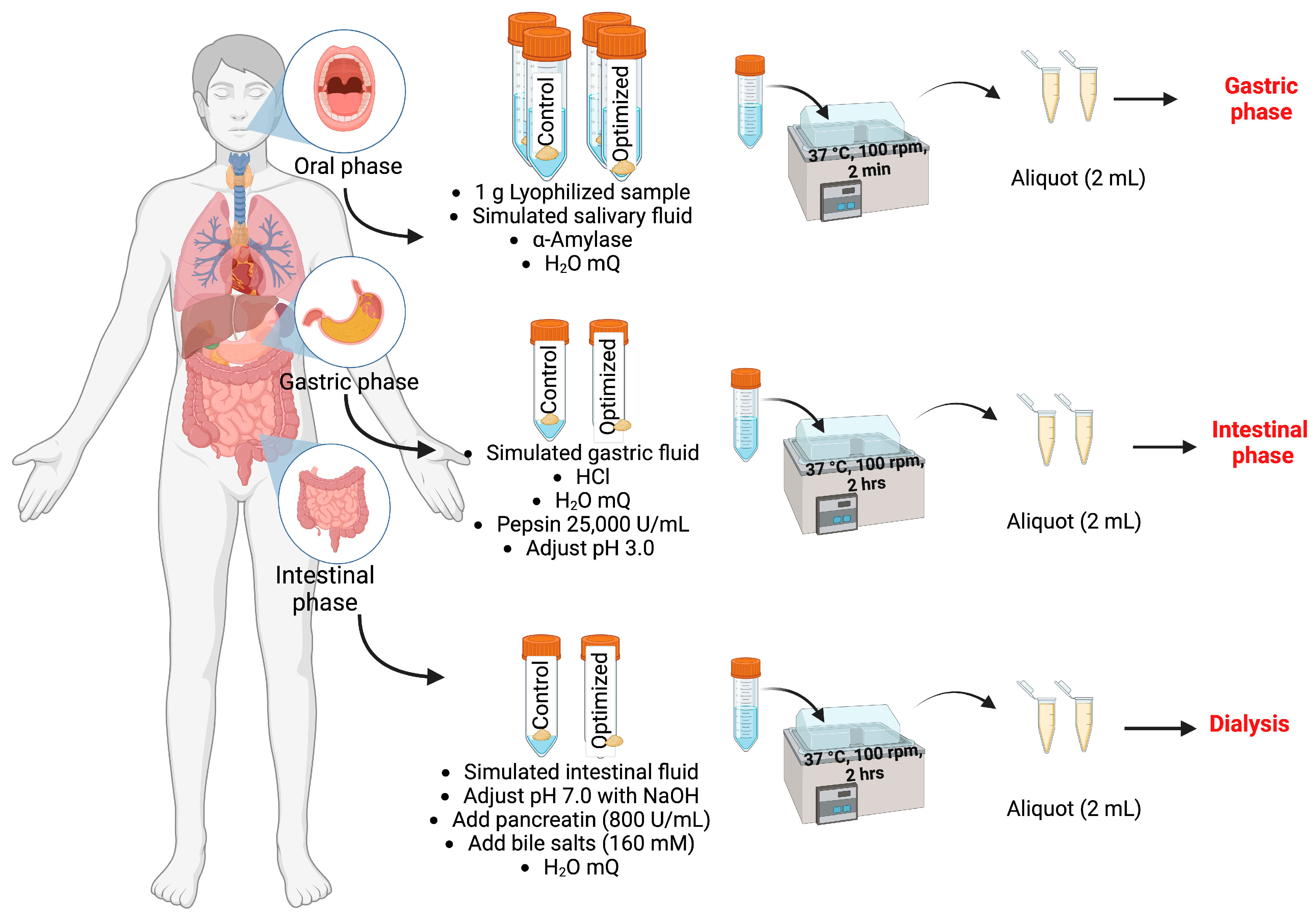
2.14. In Vitro Digestion
2.15. Statistical Analysis
3. Results
3.1. Total Phenolics and Carotenoids Content
3.2. Antioxidant Capacity
3.3. Model Development and Validation
3.3.1. Numerical Optimization Computation
3.3.2. Optimization Overview
3.4. Post Analysis and Model Simulation
3.4.1. Point Prediction
3.4.2. Model Simulation
3.5. Phenolic and Carotenoid Profile, and Their Release during In Vitro Digestion
3.6. Sensory Acceptability
4. Limitations and Impacts
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ardid-Ruiz, A.; Harazin, A.; Barna, L.; Walter, F.R.; Bladé, C.; Suárez, M.; Deli, M.A.; Aragonès, G. The Effects of Vitis Vinifera L. Phenolic Compounds on a Blood-Brain Barrier Culture Model: Expression of Leptin Receptors and Protection against Cytokine-Induced Damage. J. Ethnopharmacol. 2020, 247, 112253. [Google Scholar] [CrossRef] [PubMed]
- López-Molina, R.; Parra-Cabrera, S.; López-Ridaura, R.; González-Villalpando, M.E.; Ferrannini, E.; González-Villalpando, C. Sweetened Beverages Intake, Hyperuricemia and Metabolic Syndrome. The Mexico City Diabetes Study. Salud Publica Mex. 2013, 55, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Vilca, W.C.; Yovera-Juarez, E.A.; Tarazona-Meza, C.; Garc, V.; Carrillo-larco, R.M. Sugar-Sweetened Beverage Consumption in Adults: Evidence from a National Health Survey in Peru. Nutrients 2022, 14, 582. [Google Scholar] [CrossRef] [PubMed]
- Malik, V.S.; Hu, F.B. Fructose and Cardiometabolic Health: What the Evidence from Sugar-Sweetened Beverages Tells Us. J. Am. Coll. Cardiol. 2015, 66, 1615–1624. [Google Scholar] [CrossRef] [PubMed]
- Chaput, J.P.; Tremblay, M.S.; Katzmarzyk, P.T.; Fogelholm, M.; Hu, G.; Maher, C.; Maia, J.; Olds, T.; Onywera, V.; Sarmiento, O.L.; et al. Sleep Patterns and Sugar-Sweetened Beverage Consumption among Children from around the World. Public Health Nutr. 2018, 21, 2385–2393. [Google Scholar] [CrossRef] [PubMed]
- Pan American Health Organization. Sugar-Sweetened Beverage Taxation in the Region of the Americas; Pan American Health Organization: Washington, DC, USA, 2020; ISBN 978-92-75-12300-3. [Google Scholar]
- Buniowska, M.; Carbonell-Capella, J.M.; Frigola, A.; Esteve, M.J. Bioaccessibility of Bioactive Compounds after Non-Thermal Processing of an Exotic Fruit Juice Blend Sweetened with Stevia Rebaudiana. Food Chem. 2017, 221, 1834–1842. [Google Scholar] [CrossRef] [PubMed]
- Carbonell-Capella, J.M.; Buniowska, M.; Esteve, M.J.; Frígola, A. Effect of Stevia Rebaudiana Addition on Bioaccessibility of Bioactive Compounds and Antioxidant Activity of Beverages Based on Exotic Fruits Mixed with Oat Following Simulated Human Digestion. Food Chem. 2015, 184, 122–130. [Google Scholar] [CrossRef]
- Curi, P.N.; De Almeida, A.B.; Tavares, B.D.S.; Nunes, C.A.; Pio, R.; Pasqual, M.; De Souza, V.R. Optimization of Tropical Fruit Juice Based on Sensory and Nutritional Characteristics. Food Sci. Technol. 2017, 37, 308–314. [Google Scholar] [CrossRef]
- Akonor, P.T. Optimization of a Fruit Juice Cocktail Containing Soursop, Pineapple, Orange and Mango Using Mixture Design. Sci. African 2020, 8, e00368. [Google Scholar] [CrossRef]
- Ongphimai, N.; Lilitchan, S.; Aryusuk, K.; Bumrungpert, A.; Krisnangkura, K. Phenolic Acids Content and Antioxidant Capacity of Fruit Extracts from Thailand. Chiang Mai J. Sci. 2013, 40, 636–642. [Google Scholar]
- Angulo-López, J.E.; Flores-Gallegos, A.C.; Torres-León, C.; Ramírez-Guzmán, K.N.; Martínez, G.A.; Aguilar, C.N. Guava (Psidium guajava L.) Fruit and Valorization of Industrialization by-Products. Processes 2021, 9, 1075. [Google Scholar] [CrossRef]
- Murillo, E.; Agócs, A.; Nagy, V.; Király, S.B.; Kurtán, T.; Toribio, E.M.; Lakey-Beitia, J.; Deli, J. Isolation and Identification of Sapotexanthin 5,6-Epoxide and 5,8-Epoxide from Red Mamey (Pouteria Sapota). Chirality 2020, 32, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Gulyás-Fekete, G.; Murillo, E.; Kurtán, T.; Papp, T.; Illyés, T.Z.; Drahos, L.; Visy, J.; Agócs, A.; Turcsi, E.; Deli, J. Cryptocapsinepoxide-Type Carotenoids from Red Mamey, Pouteria sapota. J. Nat. Prod. 2013, 76, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Pacifico, S.; Piccolella, S.; Nocera, P.; Tranquillo, E.; Dal Poggetto, F.; Catauro, M. New Insights into Phenol and Polyphenol Composition of Stevia Rebaudiana Leaves. J. Pharm. Biomed. Anal. 2019, 163, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Žlabur, J.Š.; Dobričević, N.; Brnčić, M.; Barba, F.J.; Lorenzo, J.M.; Franco, D.; Atanasov, A.G.; Voća, S.; Brnčić, S.R. Evaluation of the Behavior of Phenolic Compounds and Steviol Glycosides of Sonicated Strawberry Juice Sweetened with Stevia (Stevia Rebaudiana Bertoni). Molecules 2019, 24, 1202. [Google Scholar] [CrossRef] [PubMed]
- Granato, D.; de Castro, I.A.; Ellendersen, L.S.N.; Masson, M.L. Physical Stability Assessment and Sensory Optimization of a Dairy-Free Emulsion Using Response Surface Methodology. J. Food Sci. 2010, 75, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Chacón-Ordóñez, T.; Esquivel, P.; Quesada, S.; Jiménez, R.R.; Cordero, A.; Carle, R.; Schweiggert, R. Mamey Sapote Fruit and Carotenoid Formulations Derived Thereof Are Dietary Sources of Vitamin A—A Comparative Randomized Cross-over Study. Food Res. Int. 2019, 122, 340–347. [Google Scholar] [CrossRef]
- Preciado-Saldaña, A.M.; Domínguez-Avila, J.A.; Ayala-Zavala, J.F.; Astiazaran-Garcia, H.F.; Montiel-Herrera, M.; Villegas-Ochoa, M.A.; González-Aguilar, G.A.; Wall-Medrano, A. Mango “Ataulfo” Peel Extract Improves Metabolic Dysregulation in Prediabetic Wistar Rats. Life 2022, 12, 532. [Google Scholar] [CrossRef]
- Medina, M.B. Simple and Rapid Method for the Analysis of Phenolic Compounds in Beverages and Grains. J. Agric. Food Chem. 2011, 59, 1565–1571. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Xiang, J.; Yuan, Y.; Du, L.; Zhang, Y.; Li, C.; Beta, T. Modification on Phenolic Profiles and Enhancement of Antioxidant Activity of Proso Millets during Germination. Food Chem. X 2023, 18, 100628. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, Y.; Xiang, J.; Wang, C.; Johnson, J.B.; Beta, T. Diverse Polyphenol Components Contribute to Antioxidant Activity and Hypoglycemic Potential of Mulberry Varieties. LWT 2023, 173, 114308. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. Ferric Reducing/Antioxidant Power Assay: Direct Measure of Total Antioxidant Activity of Biological Fluids and Modified Version for Simultaneous Measurement of Total Antioxidant Power and Ascorbic Acid Concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.R.; Collins, J.; Fish, W.W.; Tadmor, Y.; Webber, C.L.; Perkins-Veazie, P. Rapid Method for Total Carotenoid Detection in Canary Yellow-Fleshed Watermelon. J. Food Sci. 2007, 72, S319–S323. [Google Scholar] [CrossRef]
- Stone, H.; Sidel, J.L. Sensory Evaluation Practices, 3rd ed.; Taylor, S.L., Ed.; Tragon Corporation: Redwood City, CA, USA, 2004; ISBN 9780123741868. [Google Scholar]
- de Oliveira Rocha, I.F.; Bolini, H.M.A. Passion Fruit Juice with Different Sweeteners: Sensory Profile by Descriptive Analysis and Acceptance. Food Sci. Nutr. 2015, 3, 129–139. [Google Scholar] [CrossRef]
- Preciado-Saldaña, A.M.; Abraham Domínguez-Avila, J.; Fernando Ayala-Zavala, J.; Villegas-Ochoa, M.A.; Sáyago-Ayerdi, S.G.; Wall-Medrano, A.; González-Córdova, A.F.; González-Aguilar, G.A. Formulation and Characterization of an Optimized Functional Beverage from Hibiscus (Hibiscus Sabdariffa L.) and Green Tea (Camellia Sinensis L.). Food Sci. Technol. Int. 2019, 25, 547–561. [Google Scholar] [CrossRef]
- Mattila, P.; Kumpulainen, J. Determination of Free and Total Phenolic Acids in Plant-Derived Foods by HPLC with Diode-Array Detection. J. Agric. Food Chem. 2002, 50, 3660–3667. [Google Scholar] [CrossRef]
- Yeum, K.J.; Booth, S.L.; Sadowski, J.A.; Liu, C.; Tang, G.; Krinsky, N.I.; Russell, R.M. Human Plasma Carotenoid Response to the Ingestion of Controlled Diets High in Fruits and Vegetables. Am. J. Clin. Nutr. 1996, 64, 594–602. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-lacanal, C.; Boutrou, R.; Carrière, F. INFOGEST Static in Vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Gaweł-Bȩben, K.; Bujak, T.; Nizioł-Łukaszewska, Z.; Antosiewicz, B.; Jakubczyk, A.; Karaś, M.; Rybczyńska, K. Stevia Rebaudiana Bert. Leaf Extracts as a Multifunctional Source of Natural Antioxidants. Molecules 2015, 20, 5468–5486. [Google Scholar] [CrossRef] [PubMed]
- Pico, J.; Pismag, R.Y.; Laudouze, M.; Martinez, M.M. Systematic Evaluation of the Folin-Ciocalteu and Fast Blue BB Reactions during the Analysis of Total Phenolics in Legumes, Nuts and Plant Seeds. Food Funct. 2020, 11, 9868–9880. [Google Scholar] [CrossRef]
- Flores, G.; Wu, S.B.; Negrin, A.; Kennelly, E.J. Chemical Composition and Antioxidant Activity of Seven Cultivars of Guava (Psidium guajava) Fruits. Food Chem. 2015, 170, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Chacón-Ordóñez, T.; Schweiggert, R.M.; Bosy-Westphal, A.; Jiménez, V.M.; Carle, R.; Esquivel, P. Carotenoids and Carotenoid Esters of Orange- and Yellow-Fleshed Mamey Sapote (Pouteria sapota (Jacq.) H.E. Moore & Stearn) Fruit and Their Post-Prandial Absorption in Humans. Food Chem. 2017, 221, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Medina, M.B. Determination of the Total Phenolics in Juices and Superfruits by a Novel Chemical Method. J. Funct. Foods 2011, 3, 79–87. [Google Scholar] [CrossRef]
- Thummajitsakul, S.; Nuanphong, P.; Photo, J.; Mantong, S.; Kosuwin, R.; Taejarernwiriyakul, O.; Silprasit, K. Evaluation of Total Phenolic Content, Antioxidant Activity and Anti-Amylase Activity of Different Vegetable and Fruit Mixtures. Sci. Technol. Asia 2021, 26, 197–209. [Google Scholar] [CrossRef]
- Busuioc, A.C.; Botezatu, A.D.; Furdui, B.; Vinatoru, C.; Maggi, F.; Caprioli, G.; Dinica, R. Comparative Study of the Chemical Compositions and Antioxidant Activities of Fresh Juices from Romanian Cucurbitaceae Varieties. Molecules 2020, 25, 5468. [Google Scholar] [CrossRef]
- Castro-López, C.; Sánchez-Alejo, E.; Saucedo-Pompa, S.; Rojas, R.; Aranda-Ruiz, J.; Martínez-Avila, G. Fluctuations in Phenolic Content, Ascorbic Acid and Total Carotenoids and Antioxidant Activity of Fruit Beverages during Storage. Heliyon 2016, 2, e00152. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B.; Nutti, M.R.; Viana de Carvalho, J.L. Chapter 28—Carotenoids of Sweet Potato, Cassava, and Maize and Their Use in Bread and Flour Fortification. In Flour Breads and Their Fortifification in Health and Disease Prevention, 1st ed.; Preedy, V.R., Watson, R.R., Patel, V.B., Eds.; Academic Press: Cambridge, MA, USA, 2011; Volume 1, pp. 301–311. [Google Scholar] [CrossRef]
- Gupta, D. Methods for Determination of Antioxidant Capacity: A Review. Int. J. Pharm. Sci. Res. 2015, 6, 546–566. [Google Scholar] [CrossRef]
- Gomes, W.F.; Kumar, B.; Rodriguez, Ó.; Sousa, E.; Brito, D.; André, F.; Fernandes, N.; Rodrigues, S. Effect of Ultrasound Followed by High Pressure Processing on Prebiotic Cranberry Juice. Food Chem. 2017, 218, 261–268. [Google Scholar] [CrossRef]
- Martono, Y.; Soetjipto, H. Bioactive Components and Antioxidant Properties of Stevia Beverage. In Proceedings of the 9th Joint Conference on Chemistry, Semarang, Indonesia, 12–13 November 2014; pp. 363–368. [Google Scholar]
- Lu, Y.; Mu, K.; McClements, D.J.; Liang, X.; Liu, X.; Liu, F. Fermentation of Tomato Juice Improves in Vitro Bioaccessibility of Lycopene. J. Funct. Foods 2020, 71, 104020. [Google Scholar] [CrossRef]
- Hardin, R.H.; Sloane, N.J.A. A New Approach to the Construction of Optimal Designs. J. Stat. Plan. Inference 1993, 37, 339–369. [Google Scholar] [CrossRef]
- Aju, D.E.; Onyelowe, K.C.; Alaneme, G.U. Constrained Vertex Optimization and Simulation of the Unconfined Compressive Strength of Geotextile Reinforced Soil for Flexible Pavement Foundation Construction. Clean. Eng. Technol. 2021, 5, 100287. [Google Scholar] [CrossRef]
- Yahia, E.M.; Gutiérrez-Orozco, F.; Arvizu-de Leon, C. Phytochemical and Antioxidant Characterization of Mamey (Pouteria sapota Jacq. H.E. Moore & Stearn) Fruit. Food Res. Int. 2011, 44, 2175–2181. [Google Scholar] [CrossRef]
- Ma, J.; Yang, H.; Basile, M.J.; Kennelly, E.J. Analysis of Polyphenolic Antioxidants from the Fruits of Three Pouteria Species by Selected Ion Monitoring Liquid Chromatography-Mass Spectrometry. J. Agric. Food Chem. 2004, 52, 5873–5878. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Lu, W.; Zhou, X. Phenolic Compounds and In Vitro Antibacterial and Antioxidant Activities of Three Tropic Fruits: Persimmon, Guava, and Sweetsop. BioMed Res. Int. 2016, 2016, 4287461. [Google Scholar] [CrossRef]
- Velderrain-Rodríguez, G.R.; Palafox-Carlos, H.; Wall-Medrano, A.; Ayala-Zavala, J.F.; Chen, C.Y.O.; Robles-Sánchez, M.; Astiazaran-García, H.; Alvarez-Parrilla, E.; González-Aguilar, G.A. Phenolic Compounds: Their Journey after Intake. Food Funct. 2014, 5, 189–197. [Google Scholar] [CrossRef]
- Harrison, E.H.; Kopec, R.E. Digestion and Intestinal Absorption of Dietary Carotenoids and Vitamin A. In Physiology of the Gastrointestinal Tract, 6th ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; Volume 2, pp. 1133–1151. ISBN 9780128099544. [Google Scholar]
- Pinto, T.; Vilela, A.; Cosme, F. Chemical and Sensory Characteristics of Fruit Juice and Fruit Fermented Beverages and Their Consumer Acceptance. Beverages 2022, 8, 33. [Google Scholar] [CrossRef]
- Chopda, C.A.; Barrett, D.M. Optimization of Guava Juice and Powder Production. J. Food Process. Preserv. 2001, 25, 411–430. [Google Scholar] [CrossRef]
- Schiassi, M.C.E.V.; de Souza, V.R.; Lago, A.M.T.; Campos, L.G.; Queiroz, F. Fruits from the Brazilian Cerrado Region: Physico-Chemical Characterization, Bioactive Compounds, Antioxidant Activities, and Sensory Evaluation. Food Chem. 2018, 245, 305–311. [Google Scholar] [CrossRef]
- Küster-Boluda, I.; Vidal-Capilla, I. Consumer Attitudes in the Election of Functional Foods. Spanish J. Mark.-ESIC 2017, 21, 65–79. [Google Scholar] [CrossRef]
- Silliman, K.; Rodas-Fortier, K.; Neyman, M. Survey of Dietary and Exercise Habits and Perceived Barriers to Following a Healthy Lifestyle in a College Population. Californian J. Health Promot. 2004, 2, 82–91. [Google Scholar] [CrossRef]

| Mixture | Space Type | Guava Pulp (g) | Mamey Pulp (g) | Stevia Solution (%) |
|---|---|---|---|---|
| 1 | Vertex | 17.77 | 18.73 | 1.50 |
| 2 | CentEdge | 12.22 | 24.53 | 1.25 |
| 3 | AxialCB | 16.38 | 20.24 | 1.37 |
| 4 | Vertex | 12.22 | 24.28 | 1.50 |
| 5 | CentEdge | 14.99 | 21.50 | 1.50 |
| 6 | Center | 14.99 | 21.75 | 1.25 |
| 7 | Vertex | 17.77 | 19.23 | 1.00 |
| 8 | Vertex | 17.77 | 18.73 | 1.50 |
| 9 | Interior | 13.60 | 23.14 | 1.25 |
| 10 | CentEdge | 14.99 | 22.00 | 1.00 |
| 11 | Vertex | 17.77 | 19.23 | 1.00 |
| 12 | Vertex | 12.22 | 24.28 | 1.50 |
| 13 | CentEdge | 17.77 | 18.98 | 1.25 |
| 14 | CentEdge | 14.99 | 22.00 | 1.00 |
| 15 | Vertex | 12.22 | 24.78 | 1.00 |
| 16 | Vertex | 12.22 | 24.78 | 1.00 |
| Sample | TPC mg GAE/100 g | TC µg β-Carotene/ g | DPPH mg TE/100 g | TEAC mg TE/100 g | FRAP mg TE/100 g |
|---|---|---|---|---|---|
| Guava pulp | 3632.02 ± 216.55 | 4.92 ± 0.20 | 21.98 ± 2.14 | 68.50 ± 0.66 | 74.84 ± 2.26 |
| Mamey pulp | 316.55 ± 3.59 | 49.29 ± 1.42 | 12.35 ± 1.09 | 46.83 ± 4.45 | 18.89 ± 1.11 |
| Stevia leaves | 540.74 ± 8.98 | 36.97 ± 0.14 | 297.63 ± 2.13 | 102.72 ± 4.40 | 235.81 ± 7.84 |
| Mixture | X1 (g) | X2 (g) | X3 (%) | TPC (mg GAE/100 mL) | TC (mg β-Carotene/100 mL) | DPPH (mg TE/100 mL) | TEAC (mg TE/100 mL) | FRAP (mg TE/100 mL) |
|---|---|---|---|---|---|---|---|---|
| 1 | 17.77 | 18.73 | 1.50 | 261.73 ± 15.83 | 0.11 ± 0.00 | 186.94 ± 9.93 | 54.88 ± 1.35 | 90.47 ± 1.40 |
| 2 | 12.22 | 24.53 | 1.25 | 360.96 ± 11.13 | 0.13 ± 0.01 | 188.82 ± 5.09 | 65.03 ± 1.95 | 77.60 ± 1.94 |
| 3 | 16.38 | 20.24 | 1.37 | 534.34 ± 37.82 | 0.17 ± 0.01 | 248.47 ± 3.20 | 83.24 ± 3.00 | 155.33 ± 12.03 |
| 4 | 12.22 | 24.28 | 1.50 | 261.56 ± 14.92 | 0.14 ± 0.01 | 213.86 ± 5.48 | 59.95 ± 0.73 | 91.10 ± 4.71 |
| 5 | 14.99 | 21.50 | 1.50 | 261.22 ± 10.23 | 0.12 ± 0.01 | 156.17 ± 3.24 | 66.02 ± 2.85 | 94.75 ± 3.97 |
| 6 | 14.99 | 21.75 | 1.25 | 368.59 ± 13.91 | 0.15 ± 0.00 | 241.00 ± 3.69 | 69.51 ± 3.52 | 61.16 ± 1.90 |
| 7 | 17.77 | 19.23 | 1.00 | 364.58 ± 17.51 | 0.25 ± 0.01 | 253.56 ± 1.17 | 85.01 ± 6.02 | 159.31 ± 3.64 |
| 8 | 17.77 | 18.73 | 1.50 | 248.01 ± 8.21 | 0.14 ± 0.00 | 165.71 ± 3.36 | 81.84 ± 5.34 | 90.80 ± 5.51 |
| 9 | 13.60 | 23.14 | 1.25 | 377.24 ± 16.81 | 0.16 ± 0.00 | 242.23 ± 3.83 | 66.39 ± 1.70 | 76.61 ± 2.60 |
| 10 | 14.99 | 22.00 | 1.00 | 352.74 ± 13.65 | 0.17 ± 0.00 | 247.90 ± 3.79 | 87.97 ± 3.32 | 145.79 ± 2.86 |
| 11 | 17.77 | 19.23 | 1.00 | 463.73 ± 9.62 | 0.14 ± 0.00 | 257.66 ± 2.10 | 92.83 ± 5.24 | 133.915 ± 1.04 |
| 12 | 12.22 | 24.28 | 1.50 | 260.21 ± 5.31 | 0.15 ± 0.00 | 157.73 ± 5.91 | 63.52 ± 5.87 | 91.51 ± 5.07 |
| 13 | 17.77 | 18.98 | 1.25 | 479.81 ± 15.12 | 0.14 ± 0.01 | 253.81 ± 2.17 | 67.49 ± 8.82 | 165.53 ± 15.20 |
| 14 | 14.99 | 22.00 | 1.00 | 404.79 ± 32.66 | 0.25 ± 0.01 | 252.01 ± 2.99 | 90.43 ± 4.91 | 125.52 ± 3.45 |
| 15 | 12.22 | 24.78 | 1.00 | 393.21 ± 20.40 | 0.37 ± 0.01 | 241.29 ± 1.79 | 89.93 ± 4.78 | 103.13 ± 3.07 |
| 16 | 12.22 | 24.78 | 1.00 | 386.83 ± 20.82 | 0.36 ± 0.03 | 243.04 ± 1.80 | 85.35 ± 6.67 | 125.90 ± 6.17 |
| Responses | Third-Order Polynomial Equations | p-Value | R2 | Lack of Fit |
|---|---|---|---|---|
| DPPH (Y1) | Y = 5.56X1 + 5.45X2 − 66.60X3 + 0.1626X1X2 + 73.88X1X3 + 74.75X2X3 − 2.75X1X2X3 | 0.03 | 0.72 | 0.2348 |
| TEAC (Y2) | Y = 4.48X1 + 4.46X2 + 55.47X3 + 0.0672X1X2 − 59.04X1X3 − 60.31X2X3 + 4.60X1X2X3 | 0.04 | 0.70 | 0.6803 |
| FRAP (Y3) | Y = 33.35 X1 + 16.39 X2 + 3028.02 X3 − 62.26 X1X2 − 3867.78X1X3 − 3467.90X2X3 + 1612.22X1X2X3 | 0.32 | 0.47 | 0.0037 |
| TPC (Y4) | Y = 8.41X1 + 8.24X2 − 135.15X3 − 0.42X1X2 + 149.89X1X3 + 150.76X2X3 + 11.37X1X2X3 | 0.02 | 0.73 | 0.0396 |
| TC (Y5) | Y = −1.62X1 − 1.08X2 + 94.39X3 − 0.90X1X2 − 110.21X1X3 − 116.49X2X3 + 16.44X1X2X3 | 0.01 | 0.75 | 0.4886 |
| Variable | Goal | Lower Limit | Upper Limit | Lower Weight | Upper Weight | Importance |
|---|---|---|---|---|---|---|
| A: Guava pulp (g) | is in range | 12.22 | 17.77 | 1 | 1 | 3 |
| B: Mamey pulp (g) | is in range | 18.73 | 24.78 | 1 | 1 | 3 |
| C: Stevia solution (%) | is in range | 1.0 | 1.5 | 1 | 1 | 3 |
| DPPH (mg TE/100 mL) | maximize | 156.17 | 257.66 | 1 | 1 | 3 |
| TEAC (mg TE/100 mL) | maximize | 54.88 | 92.83 | 1 | 1 | 3 |
| FRAP (mg TE/100 mL) | maximize | 61.16 | 165.53 | 1 | 1 | 3 |
| TPC (mg GAE/100 mL) | maximize | 248.01 | 534.34 | 1 | 1 | 3 |
| TC (mg β-carotene/100 mL) | maximize | 0.11 | 0.37 | 1 | 1 | 3 |
| Number | Guava (g) | Mamey (g) | Stevia (%) | DPPH (mg TE/100 mL) | TEAC (mg TE/100 mL) | FRAP (mg TE/100 mL) | TPC (mg GAE/100 mL) | TC (mg β-Carotene/100 mL) | Desirability | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 17.77 | 19.230 | 1.000 | 262.157 | 89.389 | 171.286 | 433.043 | 0.198 | 0.785 | Selected |
| 2 | 12.220 | 24.780 | 1.000 | 233.653 | 87.492 | 114.199 | 382.257 | 0.350 | 0.729 |
| Response | Predicted Mean | Predicted Median | Std Dev | SE Mean | 95% CI Low for Mean | 95% CI High for Mean |
|---|---|---|---|---|---|---|
| DPPH (mg TE/100 mL) | 262.15 | 260.13 | 32.77 | N/A | 216.73 | 317.09 |
| TEAC (mg TE/100 mL) | 89.38 | 88.75 | 10.73 | N/A | 74.75 | 107.31 |
| FRAP (mg TE/100 mL) | 171.28 | 164.73 | 48.80 | N/A | 111.79 | 262.47 |
| TPC (mg GAE/100 mL) | 433.04 | 420.76 | 69.31 | N/A | 339.63 | 552.14 |
| TC (mg β-carotene/100 mL) | 0.19 | 0.19 | 0.04 | N/A | 0.13 | 0.28 |
| Variable | DPPH (mg TE/100 mL) | TEAC (mg TE/100 mL) | FRAP (mg TE/100 mL) | TPC (mg GAE/100 mL) | TC (mg β-Carotene/100 mL) |
|---|---|---|---|---|---|
| Predicted | 262.15 ± 32.77 | 89.38 ± 10.73 | 171.28 ± 48.80 | 433.04 ± 69.31 | 0.19 ± 0.04 |
| Experimental | 213.58 ± 5.06 | 78.90 ± 4.76 | 234.03 ± 13.76 | 418.21 ± 2.74 | 0.20 ± 0.00 |
| Phenolic Compound | Retention Time (min) | Free Compound | Alkaline Released | Acid Released | Intestinal Phase In Vitro Digestion | Concentration (μg/mL) |
|---|---|---|---|---|---|---|
| Gallic acid | 2.93 | ✓ | ✓ | - | - | 932.50 ± 0.73 |
| Gallocatechin gallate | 3.05 | ✓ | - | - | - | 4.57 ± 0.05 |
| p-coumaric acid | 10.27 | ✓ | ✓ | - | NQ | 11.75 ± 0.24 |
| Quercetin 3-β-d-glucoside | 13.87 | ✓ | - | - | - | 1.74 ± 0.08 |
| Ferulic acid | 11.53 | - | ✓ | - | NQ | 1.53 ± 0.12 |
| Cinnamic acid | 18.96 | - | ✓ | - | - | 2.48 ± 0.06 |
| Chlorogenic acid | 10.77 | - | - | - | NQ | NQ |
| Vanillic acid | 4.20 | NQ | - | - | - | NQ |
| Catechin | 4.45 | NQ | - | - | - | NQ |
| Carotenoid | Optimized Beverage | Oral Phase | Gastric Phase | Intestinal Phase |
|---|---|---|---|---|
| Cryptoxanthin | 0.01 ± 0.00 | 0.99 ± 0.00 | 0.25 ± 0.00 | - |
| α-carotene | 0.36 ± 0.02 | 1.38 ± 0.00 | 0.38 ± 0.00 | - |
| β-carotene | 0.43 ± 0.00 | 0.21 ± 0.00 | 0.03 ± 0.00 | - |
| Retinol | - | 8.84 ± 0.00 | 04.45 ± 0.00 | 5.76 ± 0.00 |
| Lutein | NQ | - | - | - |
| Zeaxanthin | NQ | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belmonte-Herrera, B.H.; Domínguez-Avila, J.A.; Ayala-Zavala, J.F.; Valenzuela-Melendres, M.; Tortoledo-Ortiz, O.; González-Aguilar, G.A. Optimization and In Vitro Digestion of a Guava (Psidium guajava), Mamey (Pouteria sapota) and Stevia (Stevia rebaudiana) Functional Beverage. Foods 2024, 13, 142. https://doi.org/10.3390/foods13010142
Belmonte-Herrera BH, Domínguez-Avila JA, Ayala-Zavala JF, Valenzuela-Melendres M, Tortoledo-Ortiz O, González-Aguilar GA. Optimization and In Vitro Digestion of a Guava (Psidium guajava), Mamey (Pouteria sapota) and Stevia (Stevia rebaudiana) Functional Beverage. Foods. 2024; 13(1):142. https://doi.org/10.3390/foods13010142
Chicago/Turabian StyleBelmonte-Herrera, Beatriz Haydee, J. Abraham Domínguez-Avila, J. Fernando Ayala-Zavala, Martín Valenzuela-Melendres, Orlando Tortoledo-Ortiz, and Gustavo A. González-Aguilar. 2024. "Optimization and In Vitro Digestion of a Guava (Psidium guajava), Mamey (Pouteria sapota) and Stevia (Stevia rebaudiana) Functional Beverage" Foods 13, no. 1: 142. https://doi.org/10.3390/foods13010142
APA StyleBelmonte-Herrera, B. H., Domínguez-Avila, J. A., Ayala-Zavala, J. F., Valenzuela-Melendres, M., Tortoledo-Ortiz, O., & González-Aguilar, G. A. (2024). Optimization and In Vitro Digestion of a Guava (Psidium guajava), Mamey (Pouteria sapota) and Stevia (Stevia rebaudiana) Functional Beverage. Foods, 13(1), 142. https://doi.org/10.3390/foods13010142










