The Optimization of Assay Conditions and Characterization of the Succinic Semialdehyde Dehydrogenase Enzyme of Germinated Tartary Buckwheat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Tartary Buckwheat Germination Test
2.3. SSADH Extraction
2.4. Fractional Precipitation of SSADH
2.5. Optimization of SSADH Enzyme Activity Determination Conditions
2.6. Optimization of SSADH Enzyme Activity Determination Conditions
2.6.1. Stability Analysis of SSADH
2.6.2. Effects of Different Metal Ions on SSADH
2.6.3. Determination of Enzymatic Reaction Kinetics Parameters of SSADH
2.7. Determination Indicators and Methods
2.7.1. Determination of Protein Content
2.7.2. Determination of SSADH Activity
2.8. Statistical Analysis
3. Results and Discussion
3.1. (NH4)2SO4 Fractional Precipitation
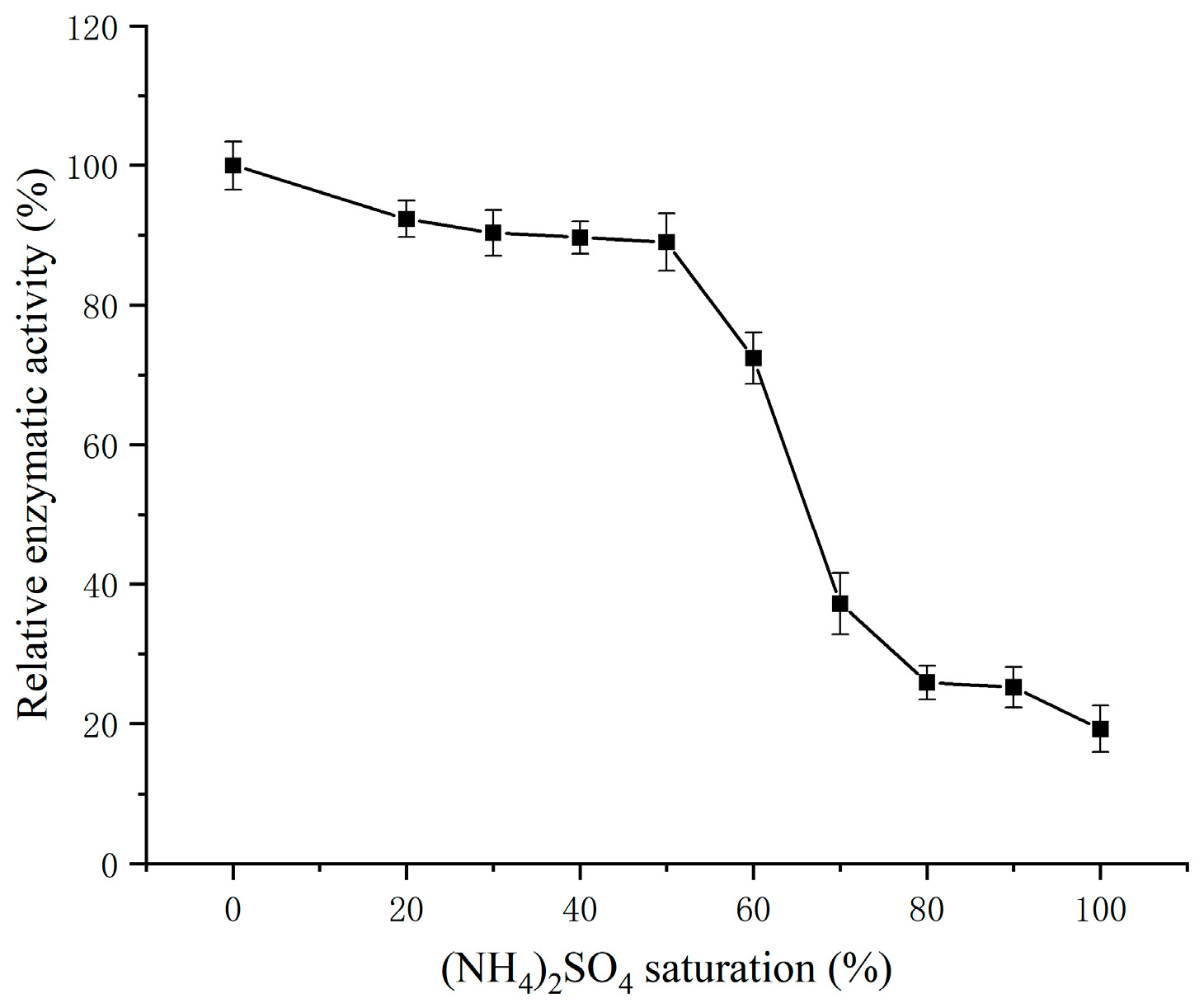
3.1.1. Determination of (NH4)2SO4 Saturation
3.1.2. (NH4)2SO4 Fractional Precipitation Results
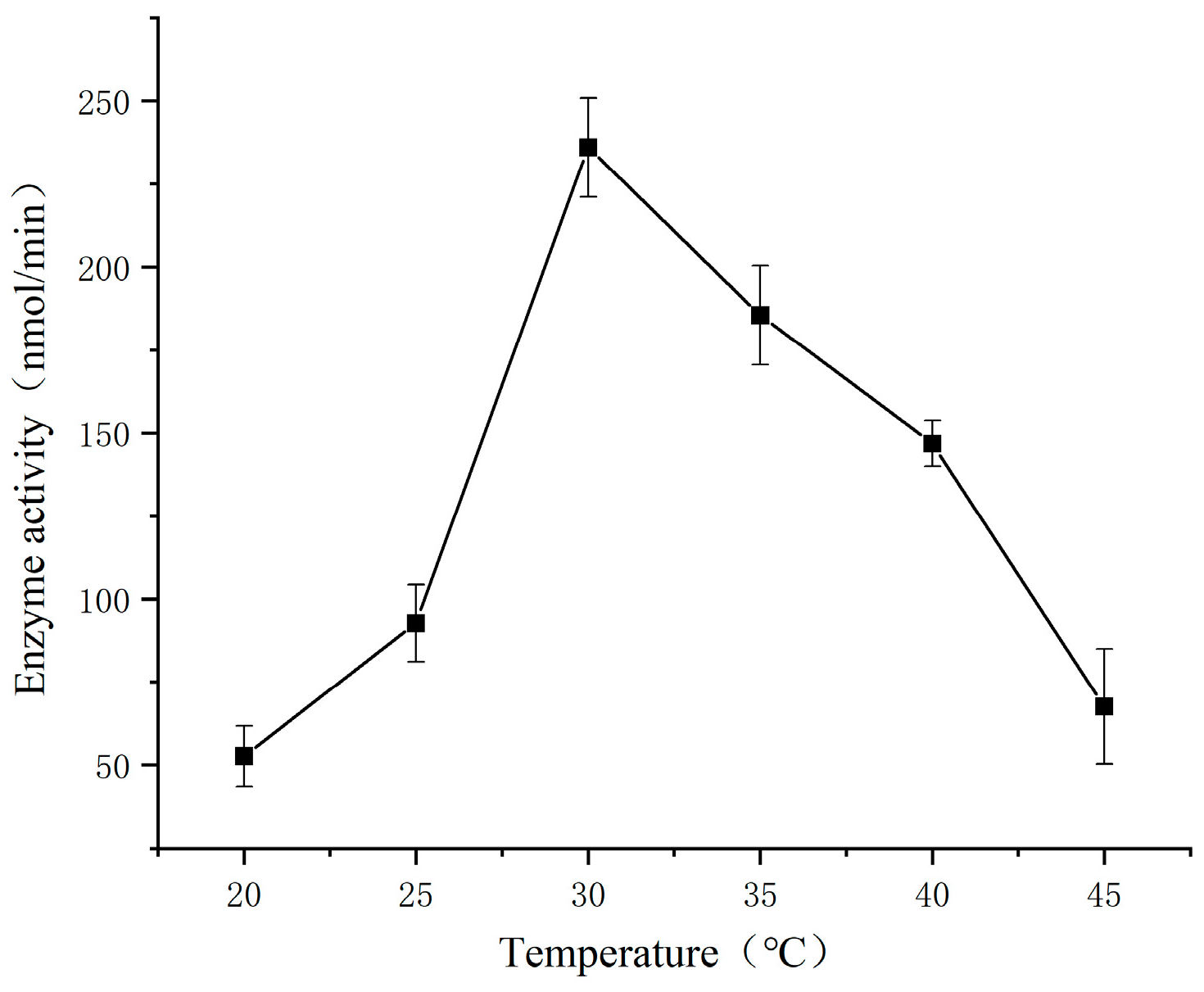
3.2. Effect of Temperature on The Determination of SSADH Activity in Germinated Tartary Buckwheat
3.3. Effect of pH on the Determination of SSADH Activity in Germinated Tartary Buckwheat
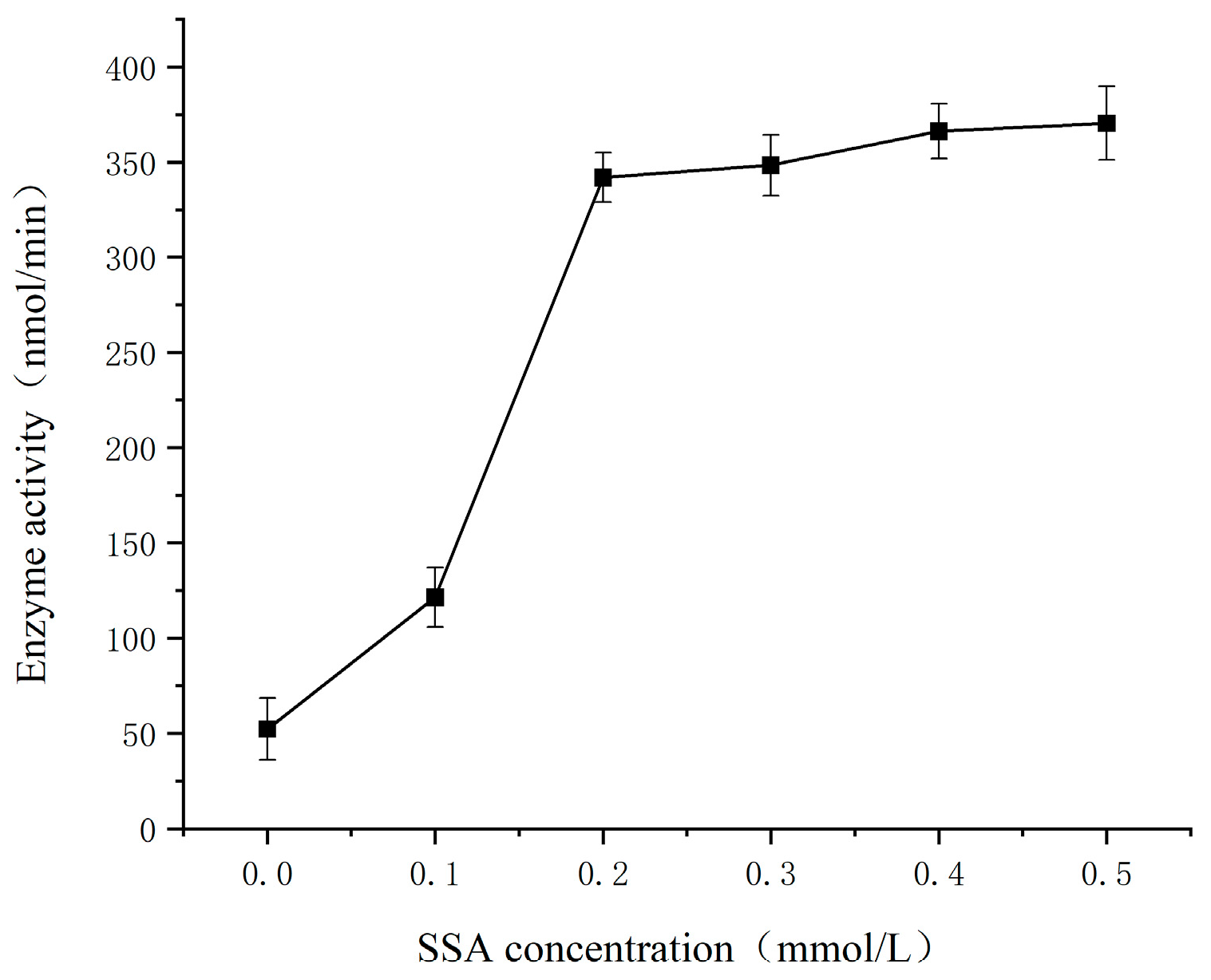
3.4. Effect of Substrate SSA Concentration on the SSADH Activity of Germinated Tartary Buckwheat
3.5. Determination Conditions of SSADH Enzyme Activity Box–Behnken Experimental Model Analysis of Variance
3.5.1. Response Surface Experimental Design and Results
3.5.2. Regression Model and Variance Analysis
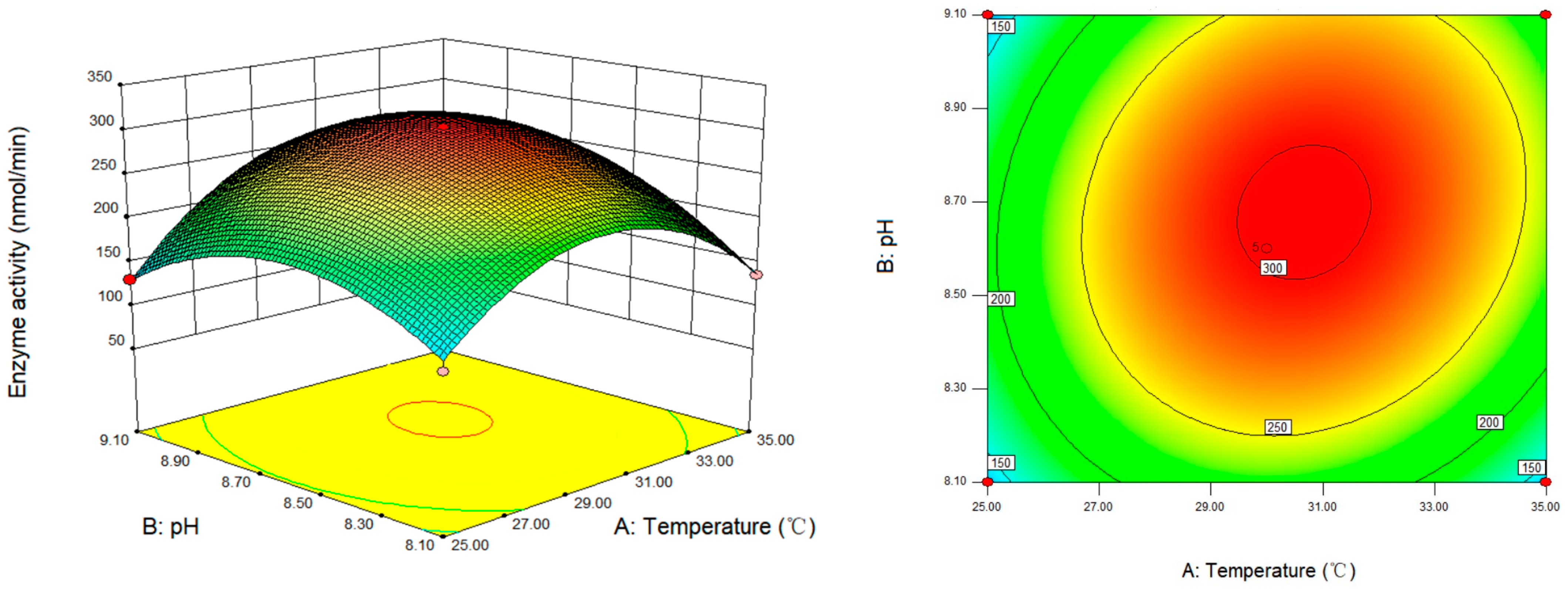
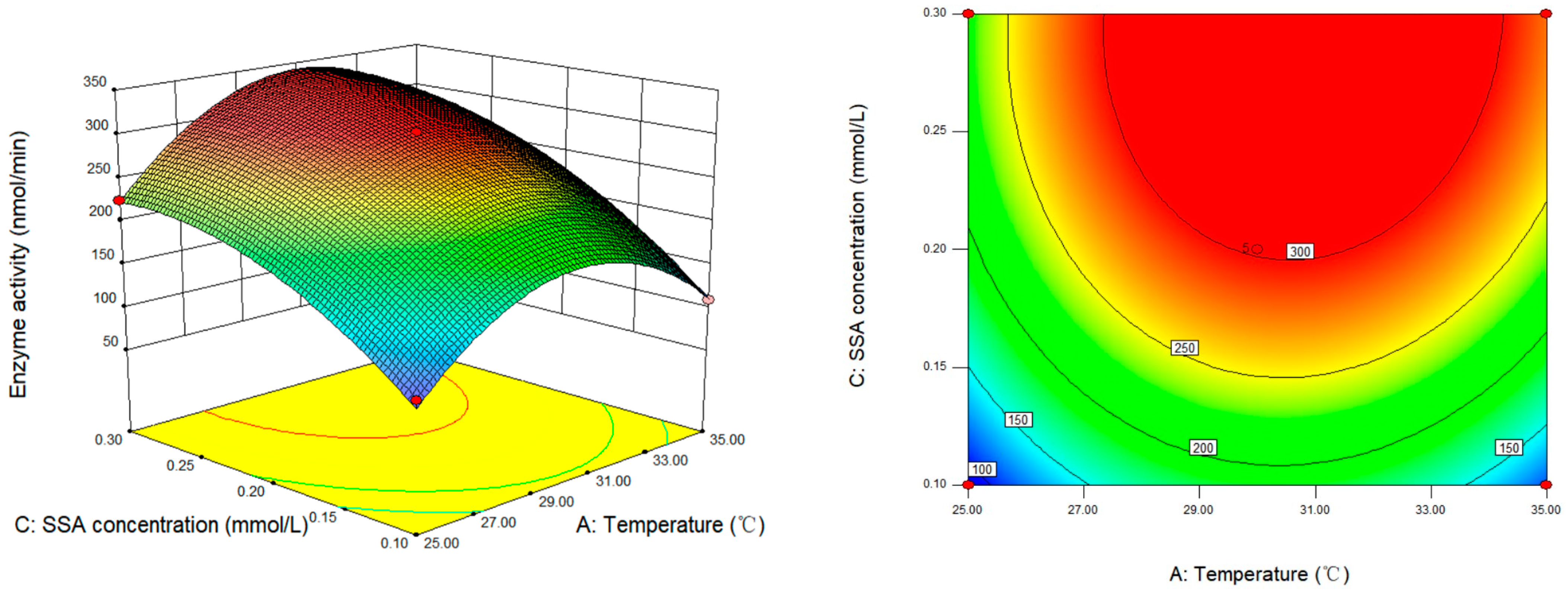
3.6. Response Surface Analysis and Optimization of SSADH Activity Determination Conditions of Germinated Tartary Buckwheat
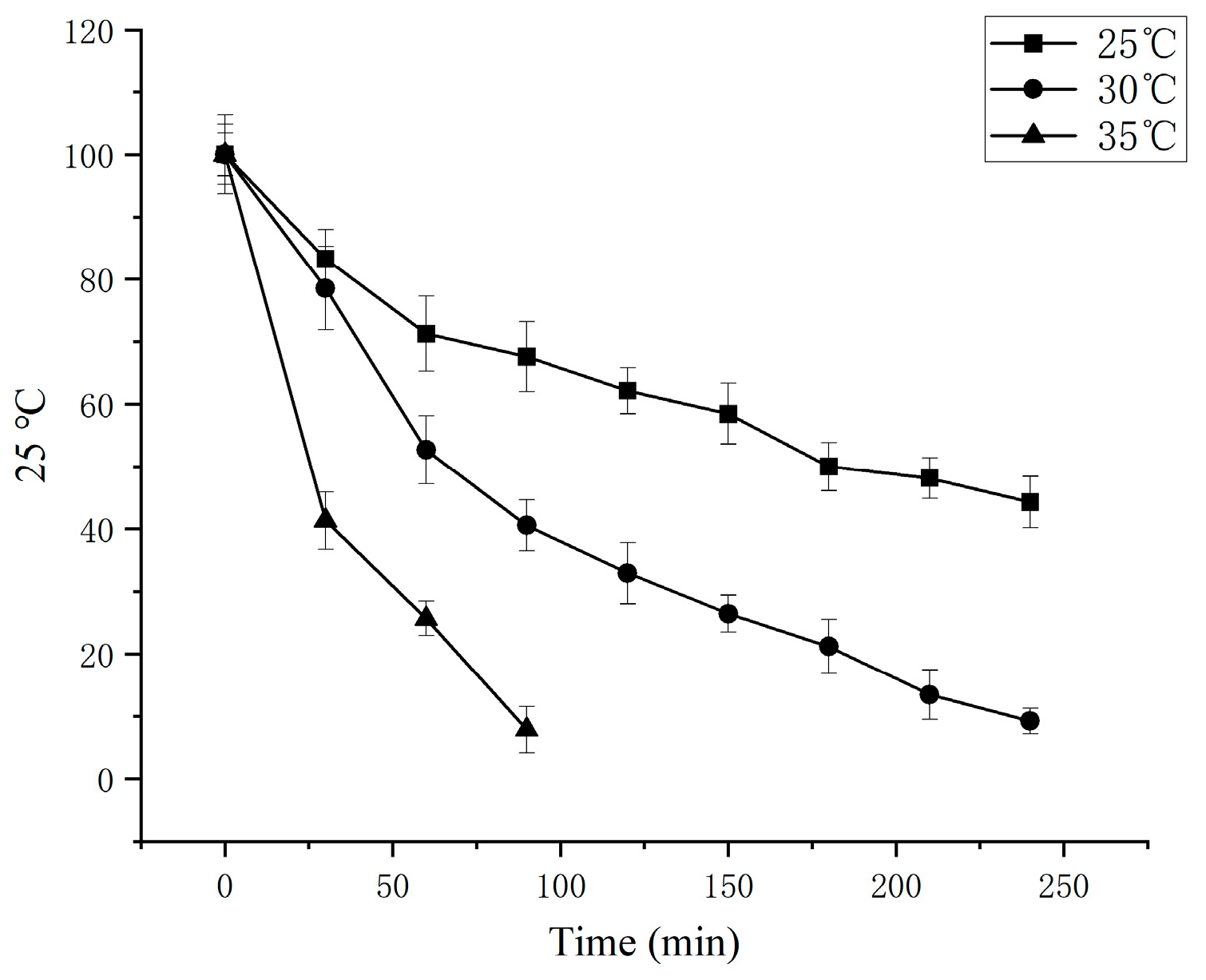
3.7. Thermal Stability and pH Stability of Germinated Tartary Buckwheat SSADH
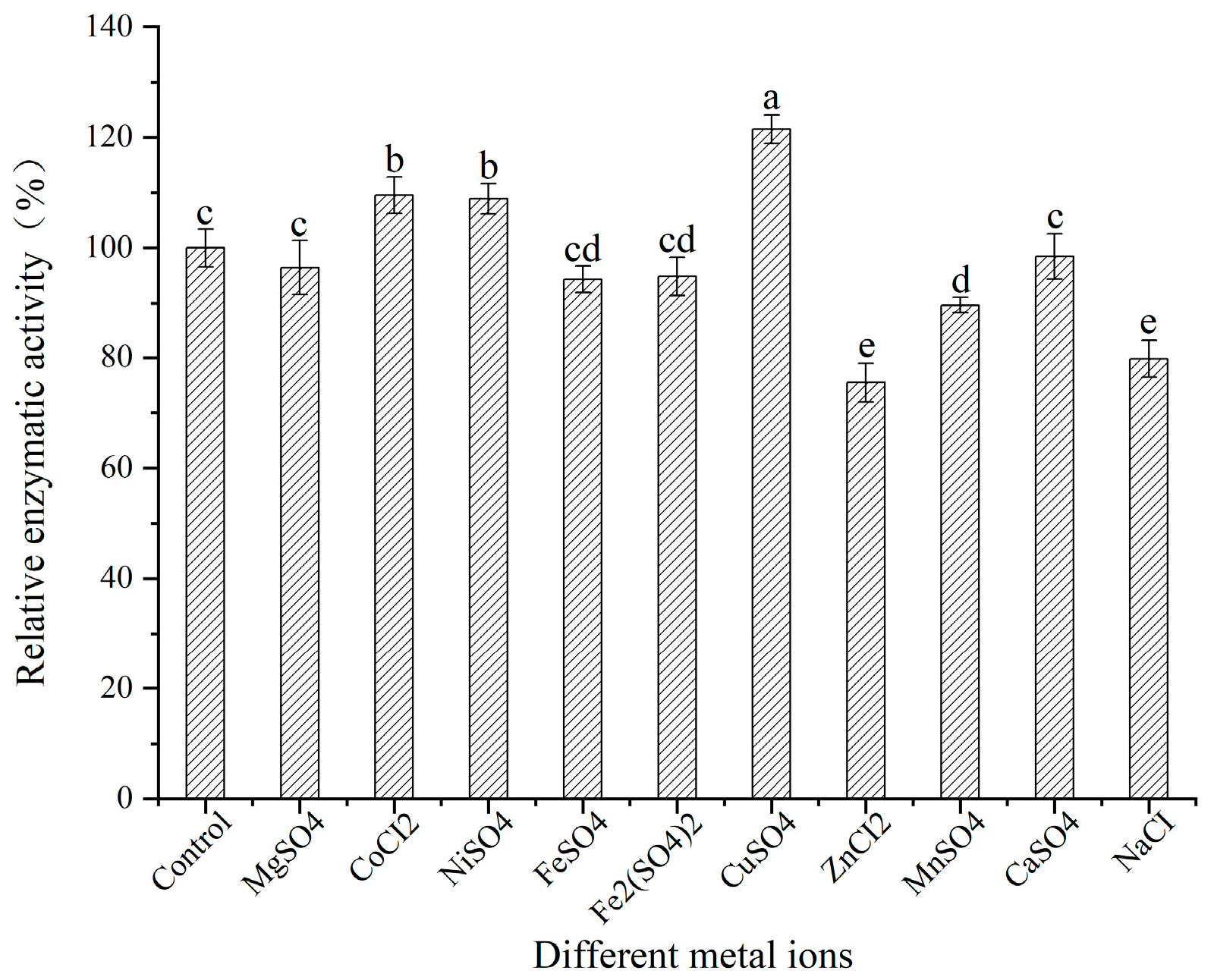
3.8. Effects of Metal Ions on SSADH Activity in Germinated Tartary Buckwheat
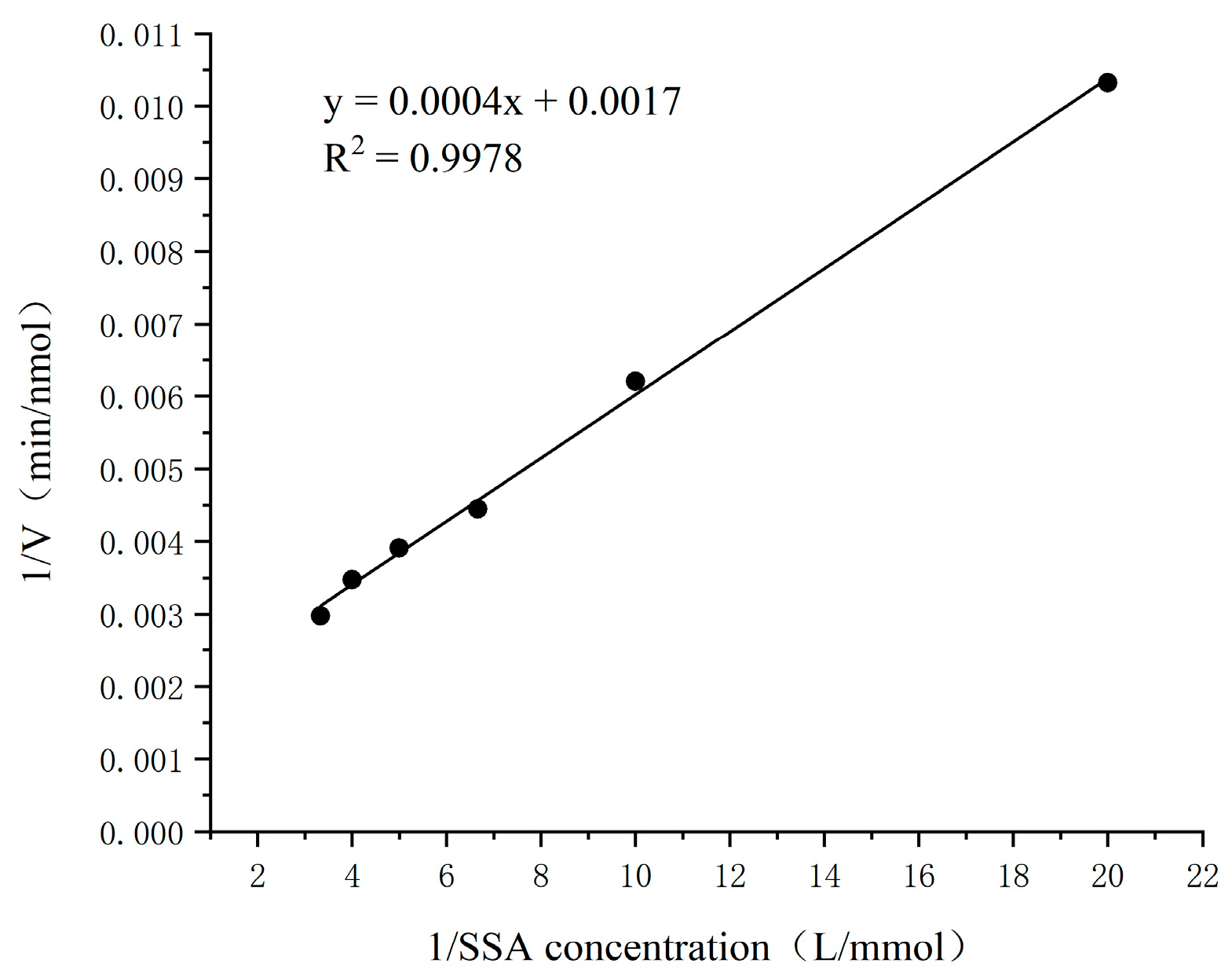
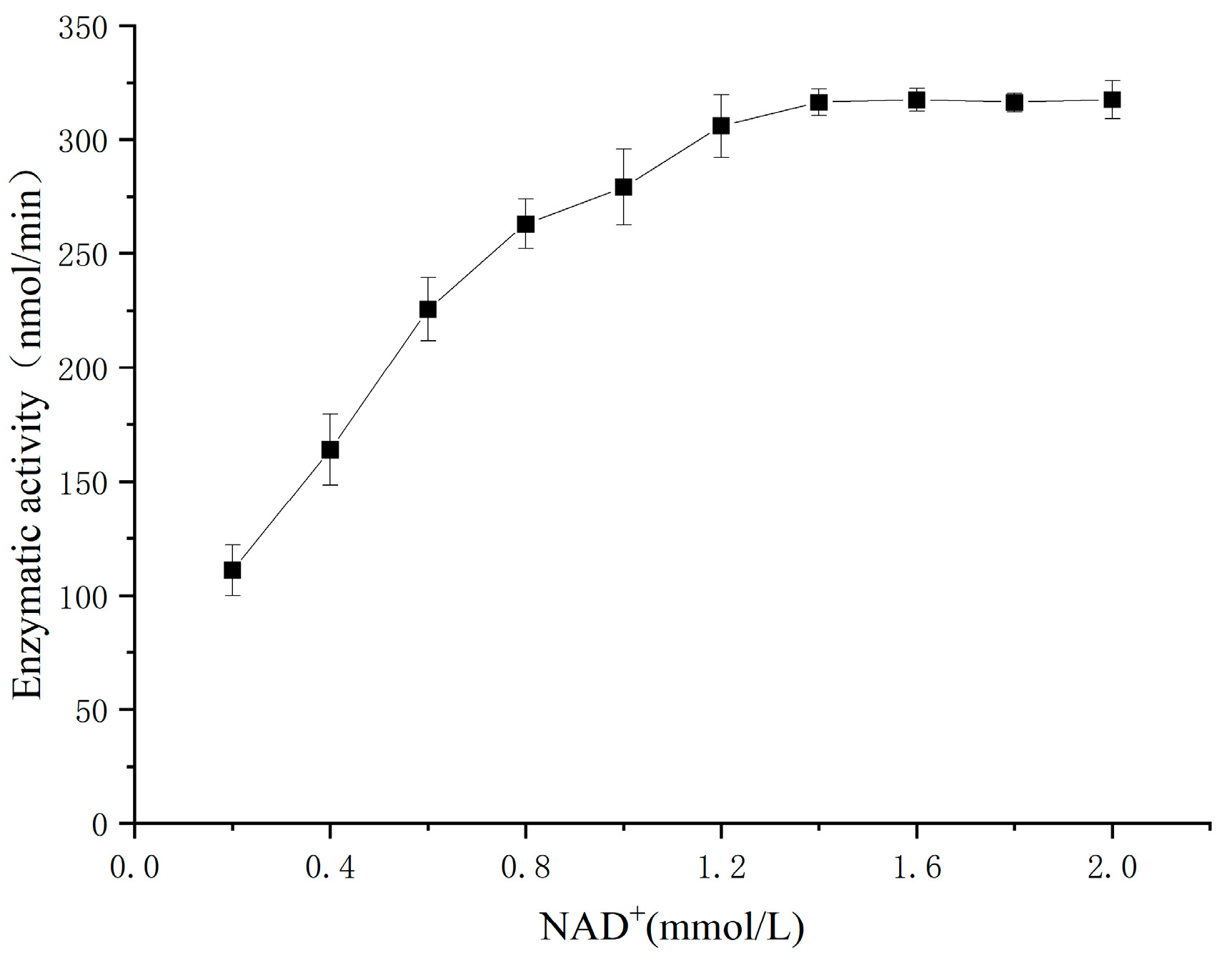
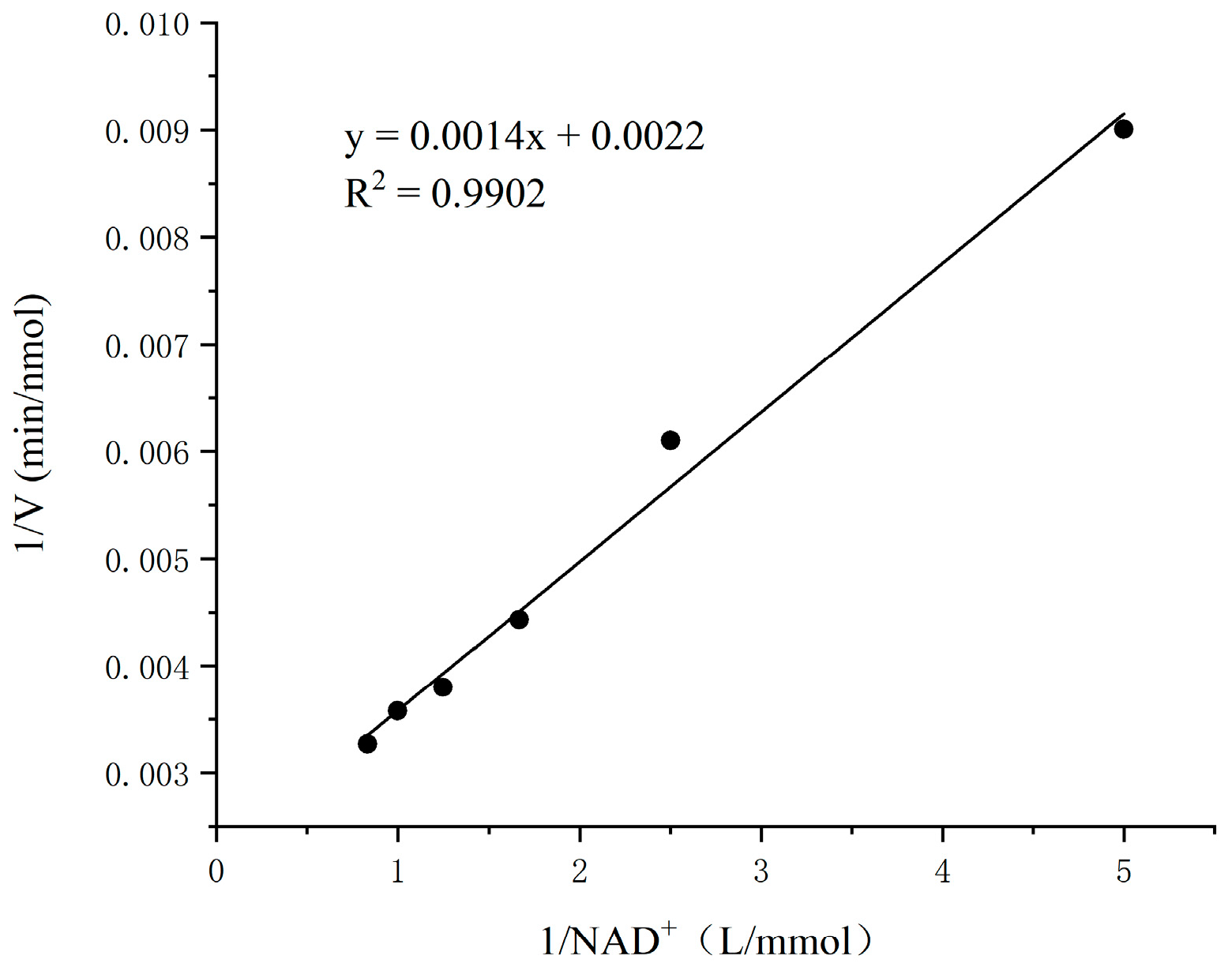
3.9. Enzymatic Kinetic Parameters of SSADH on Substrate SSA and Coenzyme NAD+ in Germinated Tartary Buckwheat
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, D.-T.; Wang, J.; Li, J.; Hu, J.-L.; Yan, H.; Zhao, J.; Zou, L.; Hu, Y.-C. Physicochemical properties and biological functions of soluble dietary fibers isolated from common and Tartary buckwheat sprouts. LWT 2023, 183, 114944. [Google Scholar] [CrossRef]
- Liu, M.; Ma, Z.; Zheng, T.; Sun, W.; Zhang, Y.; Jin, W.; Zhan, J.; Cai, Y.; Tang, Y.; Wu, Q.; et al. Insights into the correlation between Physiological changes in and seed development of tartary buckwheat (Fagopyrum tataricum Gaertn.). BMC Genom. 2018, 19, 648. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, X.; Ding, X. Composition and antioxidative properties of the flavonoid-rich fractions from tartary buckwheat grains. Food Sci. Biotechnol. 2010, 19, 711–716. [Google Scholar] [CrossRef]
- Zhou, X.; Hao, T.; Zhou, Y.; Tang, W.; Xiao, Y.; Meng, X.; Fang, X. Relationships between antioxidant compounds and antioxidant activities of tartary buckwheat during germination. J. Food Sci. Technol. 2015, 52, 2458–2463. [Google Scholar] [CrossRef] [PubMed]
- Bao, T.; Wang, Y.; Li, Y.; Gowd, V.; Niu, X.; Yang, H.; Chen, L.; Chen, W.; Sun, C. Antioxidant and antidiabetic properties of tartary buckwheat rice flavonoids after in vitro digestion. J. Zhejiang Univ. Sci. B 2016, 17, 941–951. [Google Scholar] [CrossRef]
- Zhao, J.L.; Zou, L.; Zhong, L.Y.; Peng, L.X.; Ying, P.L.; Tan, M.L.; Zhao, G. Effects of Polysaccharide Elicitors from Endophytic Bionectria pityrodes Fat6 on the Growth and Flavonoid Production in Tartary Buckwheat Sprout Cultures. Cereal Res. Commun. 2015, 43, 661–671. [Google Scholar] [CrossRef]
- Hao, J.; Wu, T.; Li, H.; Wang, W.; Liu, H. Dual effects of slightly acidic electrolyzed water (SAEW) treatment on the accumulation of γ-aminobutyric acid (GABA) and rutin in germinated buckwheat. Food Chem. 2016, 201, 87–93. [Google Scholar] [CrossRef]
- Almuhayawi, M.S.; Hassan, A.H.A.; Abdel-Mawgoud, M.; Khamis, G.; Selim, S.; Al Jaouni, S.K.; AbdElgawad, H. Laser light as a promising approach to improve the nutritional value, antioxidant capacity and anti-inflammatory activity of flavonoid-rich buckwheat sprouts. Food Chem. 2021, 345, 128788. [Google Scholar] [CrossRef]
- Li, L.; Chen, J.; Lu, Y.; Ren, B.; Huang, X.; Yu, L.; Zeng, F.; Wang, Q.; Wang, X.; Lu, L. Physiological and proteomic analyses of γ-aminobutyric acid (GABA)-treated tubers reveals that StPOD42 promotes sprouting in potato. J. Plant Physiol. 2022, 278, 153826. [Google Scholar] [CrossRef]
- Ni, Y.; Shi, F.; Wang, N. Specific γ-aminobutyric acid decomposition by gabP and gabT under neutral pH in recombinant Corynebacterium glutamicum. Biotechnol. Lett. 2015, 37, 2219–2227. [Google Scholar] [CrossRef]
- Wu, X.; Jia, Q.; Ji, S.; Gong, B.; Gao, H. Gamma-aminobutyric acid (GABA) alleviates salt damage in tomato by modulating Na+ uptake, the GAD gene, amino acid synthesis and reactive oxygen species metabolism. BMC Plant Biol. 2020, 20, 465. [Google Scholar] [CrossRef] [PubMed]
- Brocker, C.; Vasiliou, M.; Carpenter, S.; Carpenter, C.; Zhang, Y.; Wang, X.; Kotchoni, S.O.; Wood, A.J.; Kirch, H.-H.; Kopečný, D.; et al. Aldehyde dehydrogenase (ALDH) superfamily in plants: Gene nomenclature and comparative genomics. Planta 2013, 237, 189–210. [Google Scholar] [CrossRef]
- Gibson, K.M.; Gupta, M.; Pearl, P.L.; Tuchman, M.; Vezina, L.G.; Snead, O.C.; Smit, L.M.E.; Jakobs, C. Significant behavioral disturbances in succinic semialdehyde dehydrogenase (SSADH) deficiency (Gamma-Hydroxybutyric aciduria). Biol. Psychiatry 2003, 54, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Okada, K. Succinic semialdehyde dehydrogenase is involved in the robust patterning of Arabidopsis leaves along the adaxial-abaxial axis. Plant Cell Physiol. 2011, 52, 1340–1353. [Google Scholar]
- Zhang, J.Y.; Duan, Z.; Jahufer, Z.; An, S.J.; Wang, Y.R. Stress-inducible expression of a Cleistogenes songorica ALDH gene enhanced drought tolerance in transgenic Arabidopsis thaliana. Plant Omics 2014, 7, 438–444. [Google Scholar]
- Sharafi, Y.; Aghdam, M.S.; Luo, Z.; Jannatizadeh, A.; Razavi, F.; Fard, J.R.; Farmani, B. Melatonin treatment promotes endogenous melatonin accumulation and triggers GABA shunt pathway activity in tomato fruits during cold storage. Sci. Hortic. 2019, 254, 222–227. [Google Scholar] [CrossRef]
- Han, S.; Liu, H.; Han, Y.; He, Y.; Nan, Y.; Qu, W.; Rao, J. Effects of calcium treatment on malate metabolism and γ-aminobutyric acid (GABA) pathway in postharvest apple fruit. Food Chem. 2021, 334, 127479. [Google Scholar] [CrossRef] [PubMed]
- Frank, L.; Anke, H.; Hillel, F.; Linda, B.; Nicolas, B.; Markus, G. Mutants of GABA Transaminase (POP2) Suppress the Severe Phenotype of succinic semialdehyde dehydrogenase (ssadh) Mutants in Arabidopsis. PLoS ONE 2008, 3, e3383. [Google Scholar]
- Renault, H.; Roussel, V.; El Amrani, A.; Arzel, M.; Renault, D.; Bouchereau, A.; Deleu, C. The Arabidopsis pop2-1mutant reveals the involvement of GABA transaminase in salt stress tolerance. BMC Plant Biol. 2010, 10, 20. [Google Scholar] [CrossRef]
- Lin, L.Y.; Peng, C.C.; Yang, Y.L.; Peng, R.Y. Optimization of bioactive compounds in buckwheat sprouts and their effect on blood cholesterol in hamsters. J. Agric. Food Chem. 2008, 56, 1216–1223. [Google Scholar] [CrossRef]
- Martínez-Villaluenga, C.; Kuo, Y.H.; Lambein, F.; Frías, J.; Vidal-Valverde, C. Kinetics of free protein amino acids, free non-protein amino acids and trigonelline in soybean (Glycine max L.) and lupin (Lupinus angustifolius L.) sprouts. Eur. Food Res. Technol. 2006, 224, 177–186. [Google Scholar] [CrossRef]
- Wu, Y.; He, S.; Pan, T.; Miao, X.; Xiang, J.; Ye, Y.; Cao, X.; Sun, H. Enhancement of γ-aminobutyric acid and relevant metabolites in brown glutinous rice (Oryza sativa L.) through salt stress and low-frequency ultrasound treatments at pre-germination stage. Food Chem. 2023, 410, 135362. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-G.; Hu, Q.-P. Changes in γ-aminobutyric acid content and related enzyme activities in Jindou 25 soybean (Glycine max L.) seeds during germination. LWT—Food Sci. Technol. 2014, 55, 341–346. [Google Scholar] [CrossRef]
- Song, H.Y.; Yu, R.C. Optimization of Culture Conditions for Accumulating γ-Aminobutyric Acid (GABA) in Germinated Tartary Buckwheat under Salt Stress by Response Surface Methodology. Food Sci. 2015, 36, 96–100. [Google Scholar]
- Guo, Y.-X.; Wang, D.-X.; Ye, H.; Gu, Z.-X.; Yang, R.-Q. Abscisic Acid Promotes γ-Aminobutyric Acid Accumulation in Soybean Germinating Under Hypoxia-NaCl Stress. Curr. Top. Nutraceutical Res. 2020, 19, 283–287. [Google Scholar] [CrossRef]
- Guo, Y.; Zhu, Y.; Chen, C.; Chen, X. Effects of Aeration Treatment on γ-Aminobutyric Acid Accumulation in Germinated Tartary Buckwheat (Fagopyrum tataricum). J. Chem. 2016, 2016, 1–9. [Google Scholar] [CrossRef]
- Wen, W.; Si, G.; Lin, I.; Ming, W.; Gui, L. The Determination of Protein Content in Polysaccharides from Stanuntonia chineensis with Coomassie Brilliant Blue Method. Food Res. Dev. 2008, 1, 115–117. [Google Scholar]
- Bang, M.-H.; Choi, S.Y.; Jang, T.-O.; Kim, S.K.; Kwon, O.-S.; Kang, T.-C.; Won, M.; Park, J.; Baek, N. Phytol, SSADH inhibitory diterpenoid of Lactuca sativa. Arch. Pharmacal Res. 2002, 25, 643–646. [Google Scholar] [CrossRef]
- Mu, X.; Shen, L.; Wu, X. Enzymological Progress on the Study of γ-Aminobutyrate Metabolism (Review). J. China Agric. Univ. 1996, 29–33. Available online: http://en.cnki.com.cn/Article_en/CJFDTOTAL-NYDX199601008.htm (accessed on 14 December 2023).
- Yang, R.; Chen, H.; Gu, Z. Factors influencing diamine oxidase activity and γ-aminobutyric acid content of fava bean (Vicia faba L.) during germination. J. Agric. Food Chem. 2011, 59, 11616–11620. [Google Scholar] [CrossRef]
- Teisseire, H.; Guy, V. Copper-induced changes in antioxidant enzymes activities in fronds of duckweed (Lemna minor). Plant Sci. 2000, 153, 65–72. [Google Scholar] [CrossRef]
- Park, S.A.; Park, Y.S.; Lee, K.S. Kinetic characterization and molecular modeling of NAD(P)(+)-dependent succinic semialdehyde dehydrogenase from Bacillus subtilis as an ortholog YneI. J. Microbiol. Biotechnol. 2014, 24, 954–958. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, M.; Rothacker, B.; Ilg, T. Saturation transfer difference NMR studies on substrates and inhibitors of succinic semialdehyde dehydrogenases. Biochem. Biophys. Res. Commun. 2008, 372, 400–406. [Google Scholar] [CrossRef] [PubMed]





| Variable | Number | Experimental Level | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| temperature (°C) | A | 25 | 30 | 35 |
| pH value | B | 8.1 | 8.6 | 9.1 |
| SSA potency (mmol/L) | C | 0.1 | 0.2 | 0.3 |
| Purification Procedure | Total Protein (mg) | Total Activity (nmol·min−1) | Specific Activity (nmol·min−1·mg−1) | Purification Multiple | Live Enzyme Recovery Rate (%) |
|---|---|---|---|---|---|
| Tartary buckwheat SSADH extract | 28.56 | 756.56 × 103 | 26,491.60 | 1 | 100 |
| (NH4)2SO4 fractional precipitation of crude enzyme solution | 5.72 | 482.36 × 103 | 84,328.67 | 3.18 | 63.76 |
| Number | Factors and Levels | Enzymatic Activity | |||
|---|---|---|---|---|---|
| A | B | C | Actual Value | Predicted Value | |
| 1 | −1 | −1 | 0 | 124.26 | 135.78 |
| 2 | 1 | −1 | 0 | 136.00 | 136.91 |
| 3 | −1 | 1 | 0 | 130.33 | 129.42 |
| 4 | 1 | 1 | 0 | 220.96 | 209.44 |
| 5 | −1 | 0 | −1 | 94.87 | 86.11 |
| 6 | 1 | 0 | −1 | 109.08 | 110.93 |
| 7 | −1 | 0 | 1 | 225.36 | 223.51 |
| 8 | 1 | 0 | 1 | 271.07 | 279.83 |
| 9 | 0 | −1 | −1 | 109.08 | 106.32 |
| 10 | 0 | 1 | −1 | 135.41 | 145.08 |
| 11 | 0 | −1 | 1 | 274.81 | 265.14 |
| 12 | 0 | 1 | 1 | 289.80 | 292.56 |
| 13 | 0 | 0 | 0 | 302.34 | 313.69 |
| 14 | 0 | 0 | 0 | 318.56 | 313.69 |
| 15 | 0 | 0 | 0 | 320.15 | 313.69 |
| 16 | 0 | 0 | 0 | 330.26 | 313.69 |
| 17 | 0 | 0 | 0 | 297.12 | 313.69 |
| Source of Variance | Quadratic Sum | Free Degree | Mean Square | F-Value | p-Value | Conspicuousness |
|---|---|---|---|---|---|---|
| model | 1.253 × 105 | 9 | 13,922.42 | 70.98 | <0.0001 | ** |
| A—temperature | 3292.26 | 1 | 3292.26 | 16.78 | 0.0046 | ** |
| B-pH | 2189.57 | 1 | 2189.57 | 11.16 | 0.0124 | * |
| C—SSA potency | 46,909.85 | 1 | 46,909.85 | 239.16 | <0.0001 | ** |
| AB | 1555.91 | 1 | 1555.91 | 7.93 | 0.0259 | * |
| AC | 248.06 | 1 | 248.06 | 1.26 | 0.2978 | |
| BC | 32.15 | 1 | 32.15 | 0.16 | 0.6977 | |
| A2 | 37,195.70 | 1 | 37,195.70 | 189.63 | <0.0001 | ** |
| B2 | 18,793.58 | 1 | 18,793.58 | 95.81 | <0.0001 | ** |
| C2 | 8376.07 | 1 | 8376.07 | 42.70 | 0.0003 | ** |
| residual | 1373.03 | 7 | 196.15 | |||
| lack of fit | 629.63 | 3 | 209.88 | 1.13 | 0.4373 | |
| pure error | 743.40 | 4 | 185.85 | |||
| total variation | 1.267 × 105 | 16 | ||||
| R2 = 0.9892 | R2adj = 0.9752 | RSN = 21.187 | C.V = 6.45% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Liu, J.; Li, N.; Guo, Y.; Ye, H.; Li, Z.; Wang, D.; Guo, Y. The Optimization of Assay Conditions and Characterization of the Succinic Semialdehyde Dehydrogenase Enzyme of Germinated Tartary Buckwheat. Foods 2024, 13, 17. https://doi.org/10.3390/foods13010017
Yang Y, Liu J, Li N, Guo Y, Ye H, Li Z, Wang D, Guo Y. The Optimization of Assay Conditions and Characterization of the Succinic Semialdehyde Dehydrogenase Enzyme of Germinated Tartary Buckwheat. Foods. 2024; 13(1):17. https://doi.org/10.3390/foods13010017
Chicago/Turabian StyleYang, Yuchan, Jiashang Liu, Nan Li, Yu Guo, Hua Ye, Zhanming Li, Dongxu Wang, and Yuanxin Guo. 2024. "The Optimization of Assay Conditions and Characterization of the Succinic Semialdehyde Dehydrogenase Enzyme of Germinated Tartary Buckwheat" Foods 13, no. 1: 17. https://doi.org/10.3390/foods13010017







