Characterization of a Novel Starch Isolated from the Rhizome of Colombian Turmeric (Curcuma longa L.) Cultivars
Abstract
:1. Introduction
2. Materials and Methods
2.1. Brief Overview
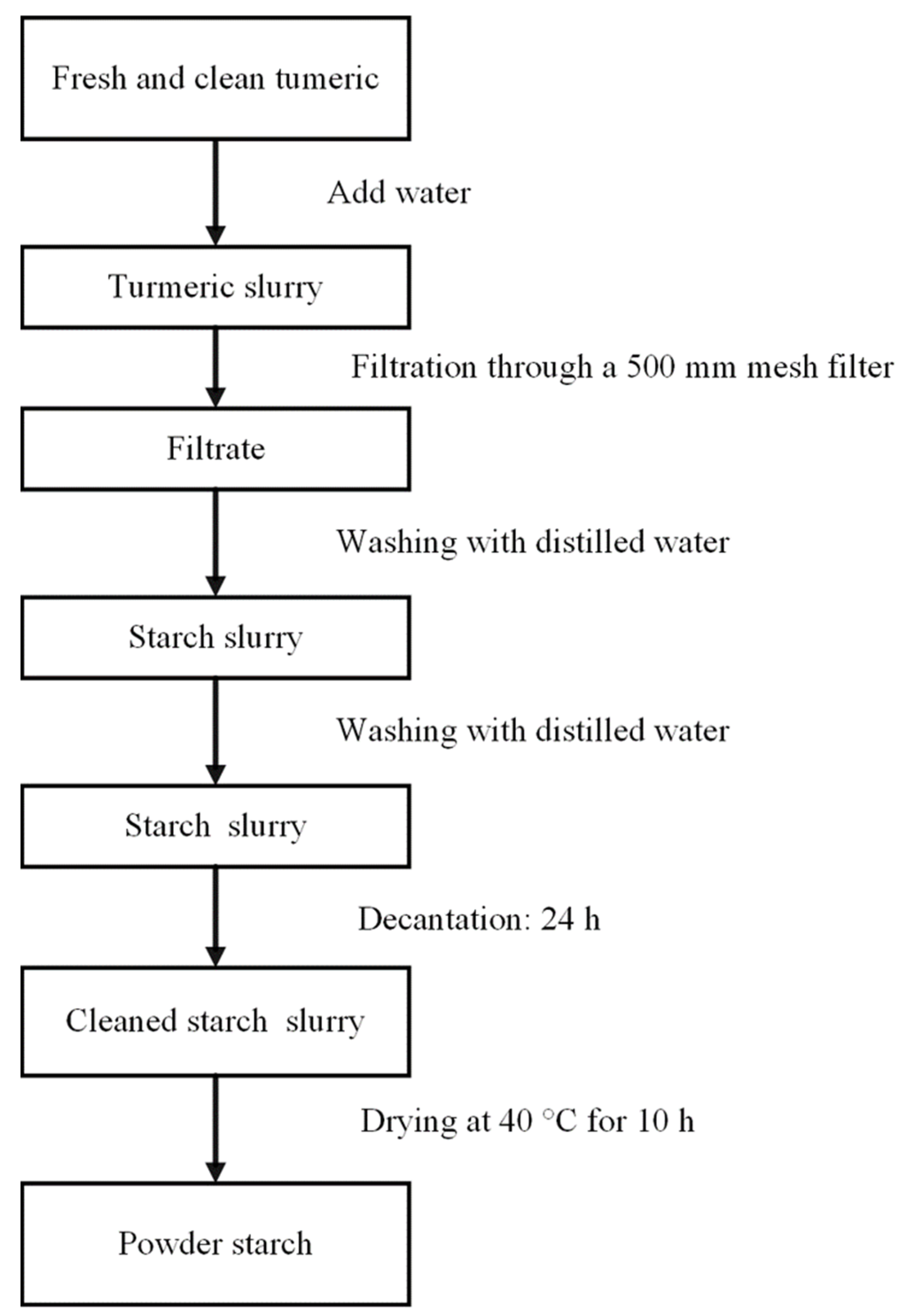
2.2. Isolation of Colombian Turmeric (Curcuma longa L.) Starch
2.3. Characterization of Colombian Turmeric (Curcuma longa L.) Starch
2.3.1. Morphological Analysis
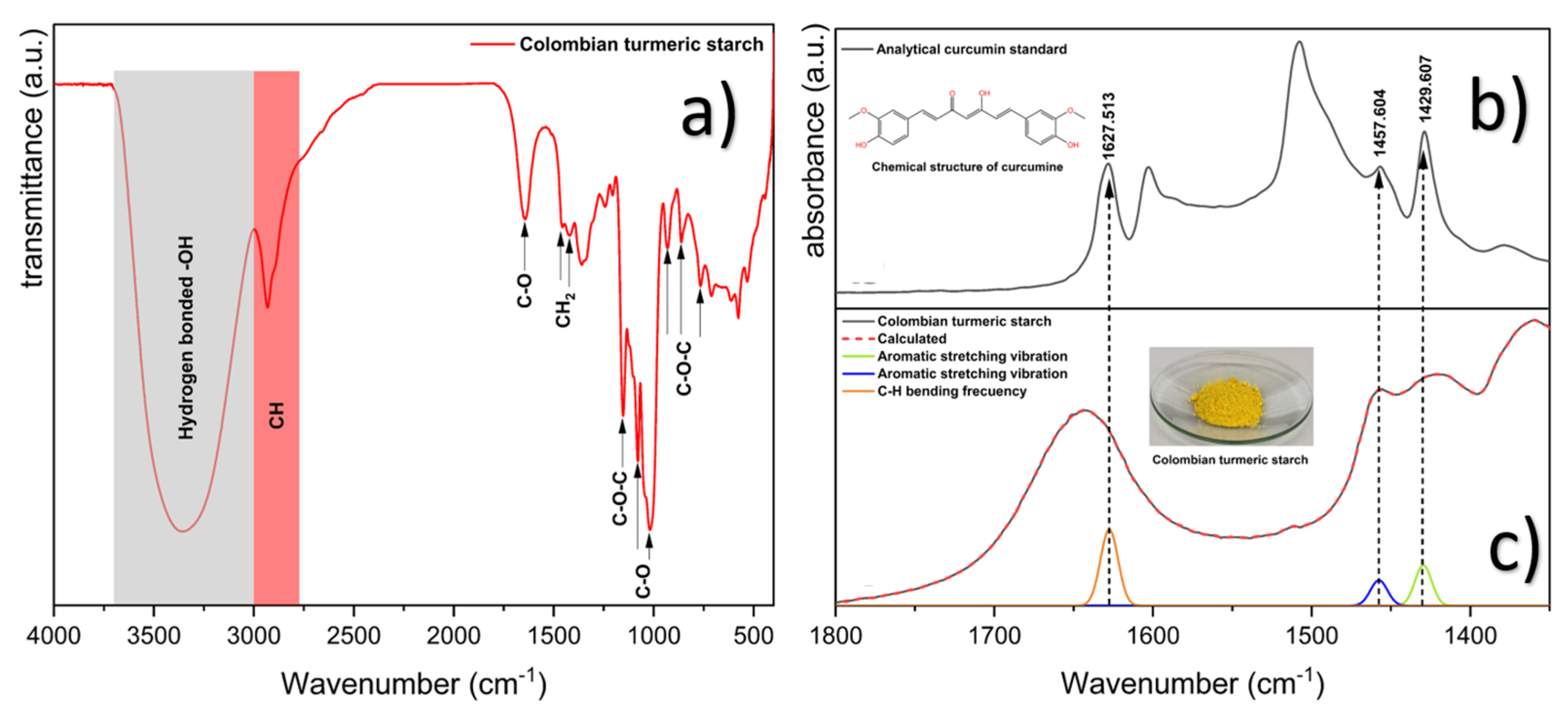
2.3.2. Chemical Analysis
- Remove the crystal effect by employing the ATR correction advanced algorithm. This correction eliminates the distortion of relative band intensity caused by the dependence of dp on wavelength, the shift of the bands to a lower wavenumber induced by refractive index dispersion, and the deviation from Beer’s Law, provoked by non-polarization effects [32].
- Nine-point smoothing.
- Automatic baseline correction.
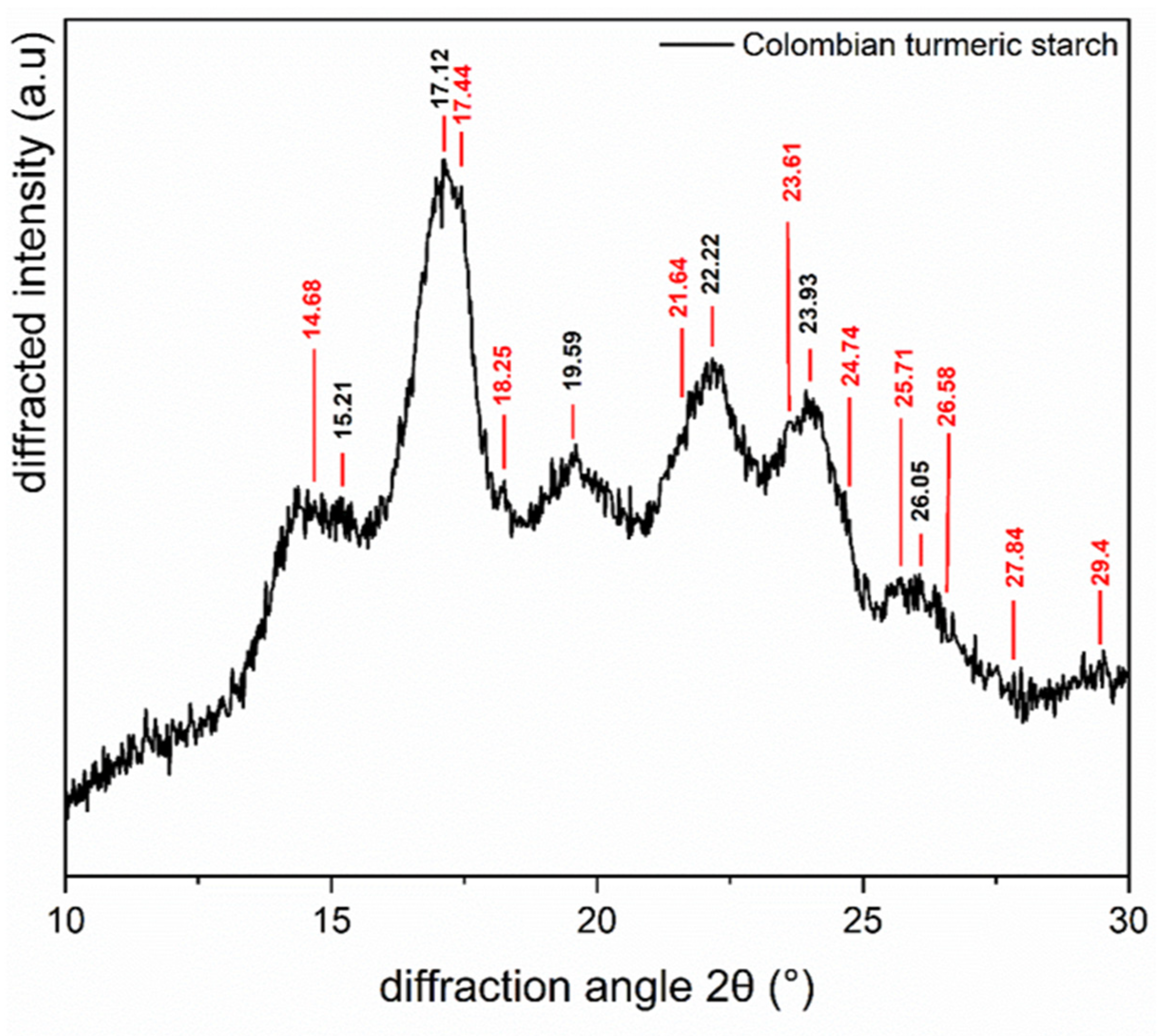
2.3.3. X-ray Diffraction Analysis
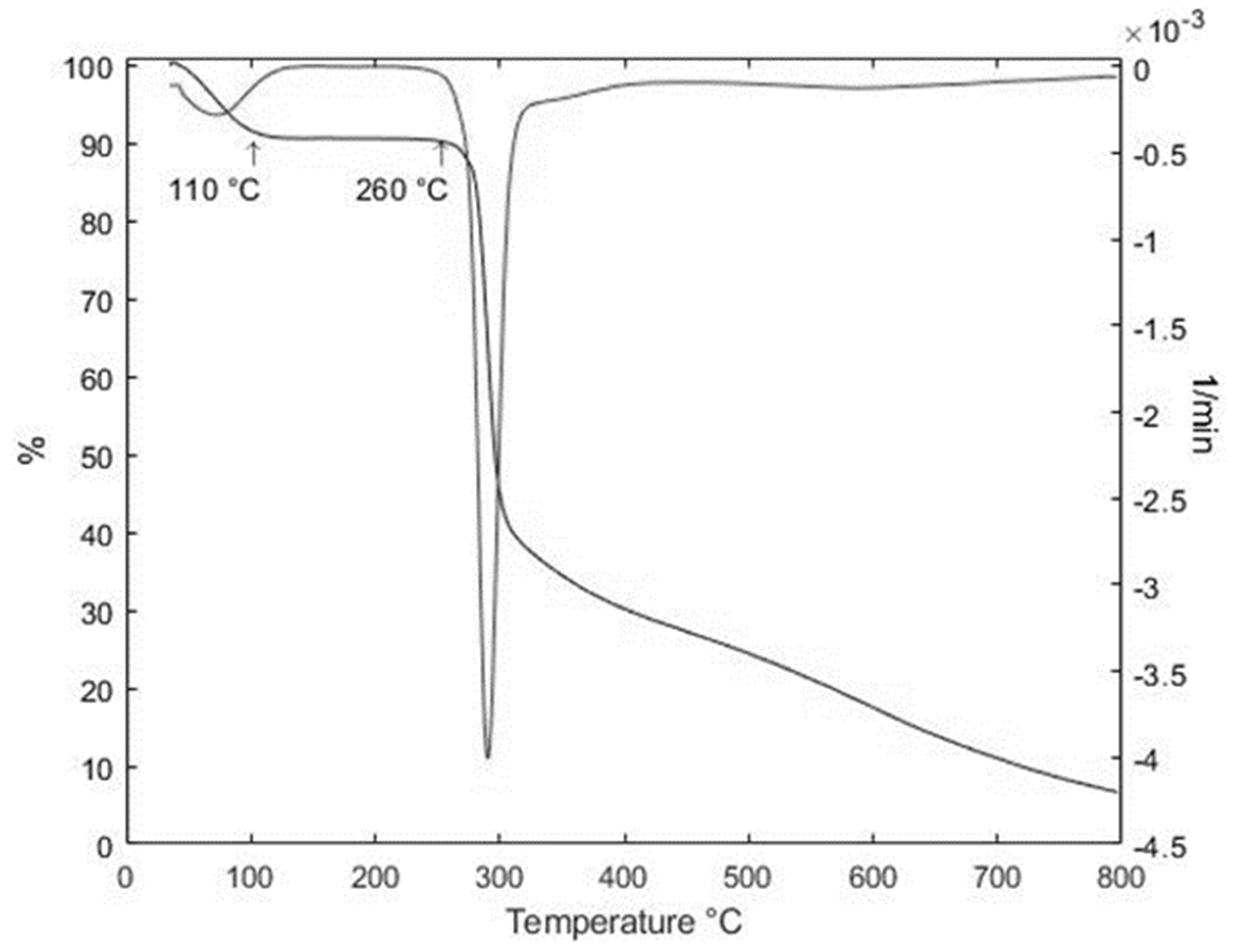
2.3.4. Thermal Degradation Analysis
2.3.5. Evaluation of Functional Characteristics
2.3.6. Colorimetric Evaluation
| Reference | Zingiberaceae Plant Named in the Publication by the Author | Reduction of Size | Isolation Solvent | Yield of the Isolation Process (%) | Starch Granulate Size (μm) | Swelling Power (SP) (g Water/g Starch) | Origin of the Cultivar |
|---|---|---|---|---|---|---|---|
| Maniglia et al., 2022 [36] | Turmeric dye extraction residue | Milling | Water | 30.00 ± 3.00 | NR | Around 4.5 (At 65 °C) | Brazil |
| Sodium hydroxide | 31.00 ± 1.00 | Around 6.0 (At 65 °C) | |||||
| Ascorbic acid | 24.00 ± 1.00 | Around 6.0 (At 65 °C) | |||||
| Naidu et al., 2022 [37] | Curcuma angustifolia Roxb. | Milling | Water | NR | 2.92–6.42 | 2.4% (At 30 °C) | India |
| 12.1 (At 80 °C) | |||||||
| Nakkala et al., 2022 [38] | Curcuma longa L. | Milling | Water | NR | NR | 6.24 ± 0.31 (At 70 °C) | India |
| Arini et al., 2021 [39] | Red ginger | Milling | Water | NR | NR | 9.09 (At 50 °C) | Indonesia |
| Elephant ginger | 9.30 (At 50 °C) | ||||||
| Emprit ginger | 10.31 (At 50 °C) | ||||||
| Curcuma | 10.53 (At 50 °C) | ||||||
| Oluba et al., 2021 [40] | Turmeric | Milling | Sodium metabisulphite solution | NR | NR | Swelling capacity 75.1 ± 10.6 (%) (At 50 °C) | Nigeria |
| Anu et al., 2020 [41] | Curcuma zanthorrhiza Roxb.; common name: false turmeric | Milling | Water | 10.4 ± 3.7 | 8.70–39.20 | 26.16 mL/g (At 90 °C) | India |
| Tejavathi et al., 2020 [6] | Curcuma karnatakensis | Milling | Ammonia solution (0.03 M) | Sample A: 76.4 ± 0.3 | 1.00–10.00 | 5.06 ± 0.07 (At 60 °C) | India |
| Sample B: 75.0 ± 0.4 | 5.03 ± 0.04 (At 60 °C) | ||||||
| Bento et al., 2019 [42] | Hedychium coronarium J. Koenig. Common name: white garland lily, butterfly lily, Napoleon, narcissus, Olympia, or white ginger | Milling | Water | 22.30 ± 0.30 | 12.00–38.00 | 2.22 (At 55 °C) | Brazil |
| Maniglia and Tapia-Blácido, 2019 [43] | Curcuma longa L. | Ball milling and cryogenic milling | Sodium hydroxide | NR | NR | NR | Brazil |
| Bleaching using NaClO | |||||||
| Bleaching using peroxide hydrogen | |||||||
| Das and Kumar, 2019 [44] | Kaempferia galanga Linn. | Milling | Water | NR | NR | 3.62 ± 0.01 | India |
| Silva et al., 2018 [45] | Turmeric–residues after curcuminoids-extract | Milling | Supercritical fluid extraction | NR | NR | NR | Brazil |
| Franklin et al., 2017 [46] | Commercial Curcuma angustifolia | Milling | Commercial sample | NR | 6.3–31.7 | NR | India |
| Jamir and Seshagirirao, 2017 [47] | Curcuma aeruginosa Roxb. | Milling | Water | NR | 6–25 | India | |
| Curcuma amada Roxb. | 10–30 | ||||||
| Curcuma aromatica Salisb. | 5–28 | ||||||
| Curcuma caesia Roxb. | 8–30 | ||||||
| Kaempferia parviflora Wall. ex Baker | 2–15 | ||||||
| Zingiber montanum (J. Koenig) Link ex A. Diet. | 5–20 | ||||||
| Mao et al., 2017 [48] | Curcuma phaeocaulis Val | Milling | Water | 51.28 | NR | Around 2.5 | China |
| Curcuma kwangsiensis | 56.88 | Around 2.5 | |||||
| Curcuma wenyujin | 54.94 | Around 2.5 | |||||
| Curcuma longa L. | 50.56 | Around 2.5 | |||||
| Santana et al., 2017 [49] | Turmeric | Milling | Supercritical fluid extraction using carbon dioxide as the solvent | 3.33 | NR | NR | Brazil |
| Van Hung and Vo, 2017 [50] | Curcuma longa | Milling | Water | NR | Smaller granules: <20 Larger granlues: 20–50 | NR | Vietnam |
| Curcuma caesia | Smaller granules: <20 Larger granlues: 20–50 | NR | |||||
| Huang et al., 2015 [51] | Curcuma longa | Homogenized with ice-cold sodium metabisulfite solution | Sodium metabisulfite solution | NR | 18.6 ± 0.1 | Around 2.5 | China |
| Patel et al., 2015 [52] | Curcuma angustifolia Roxb. Commonly known as Tikhur | Milling | Water | NR | NR | NR | India |
| Maniglia et al., 2015 [27] | Curcuma longa L. | Milling | Water | NR | 10.00–30.00 | NR | Brazil |
| Hansdah et al., 2015 [53] | Curcuma leucorrhiza | Milling | Water | NR | 30.00–50.00 | NR | India |
| Das et al., 2015 [54] | Curcuma angustifolia Roxb., known as Indian Palo | Milling | Water | 12.5 | Smaller granules: 5.39–7.78 | NR | India |
| Das et al., 2015 [55] | Curcuma angustifolia Roxb. known as Indian Palo | Milling | Water | 12.5 | Smaller granules: 5.39–7.78 | 2.61 ± 0.01 | India |
| Larger granules: 25.45–41.56 | |||||||
| Sajitha and Sasikumar, 2015 [56] | Curcuma amada Roxb. | Milling | Ammonium oxalate (1 wt%) | 48.48 ± 0.31 | 16–48 | 4.48 ± 0.04 | India |
| Curcuma aromatica Salisb | 45.90 ± 0.10 | 9–60 | 3.96 ± 0.05 | ||||
| Curcuma caesia Roxb. | 45.24 ± 0.25 | 10–39 | 3.74 ± 0.04 | ||||
| Curcuma xanthorrhiza Roxb. | 46.11 ± 0.18 | 9–47 | 4.07 ± 0.01 | ||||
| Xia et al., 2013 [57] | Curcuma phaeocaulis Val. | Milling | Ethanol (95%) | NR | Smaller granulates: 3.00–5.00 | NR | |
| Larger granulates: 15.00–20.00 | China | ||||||
| Rani et al., 2012 [58] | Curcuma angustifolia Roxb. | Lab mixer | Ammonium oxalate (1 wt%) | 37.64 | 3.32–32.55 | NR | India |
| Lab mixer | Ammonia (0.03 M) | 38.46 | |||||
| Kuttigounder et al., 2011 [59] | Curcuma longa L. | Lab milling | Water | 56 | Smaller granules: 3.00–20.00 | NR | India |
| Larger granules: 20.00–48.00 | |||||||
| Rajeevkumar et al., 2010 [60] | Curcuma angustifolia | Milling | Water | 27.5 | 9.86 | 11.29 | India |
| Ascheri et al., 2010 [61] | Hedychium coronarium | Milling | Water | NR | 11.80–52.73 | NR | Brazil |
| Policegoudra and Aradhya, 2008 [62] | Curcuma amada Roxb. | Milling | Water | NR | Smaller granules: 3.00–20.00 | NR | India |
| Larger granules: 20.00–48.00 | |||||||
| Ibezim et al., 2008 [63] | Zingiber officinale | Milling | Water | NR | NR | NR | Nigeria |
| Ranjini and Vijayan, 2006 [64] | Curcuma aeruginosa | Milling | Water | NR | NR | NR | India |
| Braga et al., 2006 [26] | Curcuma longa L. | Milling | Sodium hydroxide (0.25 wt%) - Supercritical fluid extraction | NR | 10.00–33.00 | 2.11 ± 0.04 | Brazil |
| Moreschi et al., 2006 [65] | Curcuma longa L. | Milling | Subcritical fluid extraction with water and CO2 | NR | 10.00–33.00 | NR | Brazil |
| Jyothi et al., 2003 [66] | Curcuma zedoaria | Milling | Water | NR | Smaller granules: 3.00–30.00 | Swelling volume (mL/g) 14.8 ± 1.2 | India |
| Larger granules: 35.00–60.00 | |||||||
| Curcuma malabarica | Smaller granules: 9.00–30.00 | Swelling volume (mL/g) 22.3 ± 0.5 | |||||
| Larger granules: 30.00–45.00 | |||||||
| Leonel et al., 2003 [25] | Curcuma longa L. | Milling | Water | NR | 20–25 | NR | Brazil |
| Curcuma zedoaria | 20–30 |
3. Results and Discussion
3.1. Brief Overview
3.2. Colombian Turmeric (Curcuma longa L.) Starch
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burrell, M.M. Starch: The need for improved quality or quantity—An overview. J. Exp. Bot. 2003, 54, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Starch Europe. The European Starch Industry. A Crucial Link between Farm and Fork. 2023. Available online: https://starch.eu/the-european-starch-industry/ (accessed on 4 December 2023).
- Starch: Global Strategic Business Report. Report. January 2023 Region: Global Industry Analysts, Inc. Available online: https://www.researchandmarkets.com/reports/2832332/starch-global-strategic-business-report?utm_source=GNOM&utm_medium=PressRelease&utm_code=czgx4c&utm_campaign=1825573+-+Starch+Global+Market+to+Reach+199.8+Million+Metric+Tons+by+2030%3a+Use+of+Starch+as+a+Fat+Replacer+Drives+Growth&utm_exec=jamu273prd-global?w=12 (accessed on 4 December 2023).
- Omoregie Egharevba, H. Chemical properties of starch and its application in the food industry. In Chemical Properties of Starch, 1st ed.; Emeje, M., Ed.; IntechOpen: London, UK, 2019; Volume 1, pp. 1–27. [Google Scholar]
- Zhu, J.; Bai, Y.; Gilbert, R.G. Effects of the molecular structure of starch in foods on human health. Foods 2023, 12, 2263. [Google Scholar] [CrossRef] [PubMed]
- Tejavathi, D.H.; Sujatha, B.S.; Karigar, C.S. Physicochemical properties of starch obtained from Curcuma karnatakensis—A new botanical source for high amylose content. Heliyon 2020, 6, e03169. [Google Scholar] [CrossRef] [PubMed]
- Falua, K.J.; Pokharel, A.; Babaei-Ghazvini, A.; Ai, Y.; Acharya, B. Valorization of starch to biobased materials: A review. Polymers 2022, 14, 2215. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yuan, Y.; Yue, T. The application of starch-based ingredients in flavor encapsulation. Starch 2015, 67, 225–236. [Google Scholar] [CrossRef]
- Szymońska, J.; Molenda, M.; Wieczorek, J. Study of quantitative interactions of potato and corn starch granules with ions in diluted solutions of heavy metal salts. Carbohydr. Polym. 2015, 134, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Dufresne, A.; Vignon, M.R. Improvement of starch film performances using cellulose microfibrils. Macromolecules 1998, 31, 2693–2696. [Google Scholar] [CrossRef]
- Montoya, U.; Zuluaga, R.; Castro, C.; Vélez, L.; Gañán, P. Starch and starch/bacterial nanocellulose films as alternatives for the management of minimally processed mangoes. Starch 2019, 71, 1800120. [Google Scholar] [CrossRef]
- Maniglia, B.C.; Domingos, J.R.; de Paula, R.L.; Tapia-Blácido, D.R. Development of bioactive edible film from turmeric dye solvent extraction residue. LWT 2014, 56, 269–277. [Google Scholar] [CrossRef]
- Istiqomah, A.; Rahmi Utami, M.; Firdaus, M.; Suryanti, V.; Kusumaningsih, T. Antibacterial chitosan-Dioscorea alata starch film enriched with essential oils optimally prepared by following response surface methodology. Food Biosci. 2022, 46, 101603. [Google Scholar] [CrossRef]
- Huang, J.; Wu, W.; Niu, B.; Fang, X.; Chen, H.; Wang, Y.; Gao, H. Characterization of Zizania latifolia polysaccharide-corn starch composite films and their application in the postharvest preservation of strawberries. LWT 2023, 173, 114332. [Google Scholar] [CrossRef]
- Ottum, E.; Baker, S.E.; Bredeweg, E.L. Production of Biofuels from Biomass by Fungi. In Encyclopedia of Mycology; Elsevier: Amsterdam, The Netherlands, 2021; pp. 555–576. [Google Scholar]
- Lee, B.-H.; Lee, Y.-T. Physicochemical and structural properties of different colored sweet potato starches. Starch 2017, 69, 1600001. [Google Scholar] [CrossRef]
- Horstmann, S.; Lynch, K.; Arendt, E. Starch characteristics linked to gluten-free products. Foods 2017, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Alolga, R.N.; Wang, F.; Zhang, X.; Li, J.; Tran, L.-S.P.; Yin, X. Bioactive compounds from the Zingiberaceae family with known antioxidant activities for possible therapeutic uses. Antioxidants 2022, 11, 1281. [Google Scholar] [CrossRef]
- Serpa Guerra, A.; Gómez Hoyos, C.; Velásquez-Cock, J.A.; Vélez Acosta, L.; Gañán Rojo, P.; Velásquez Giraldo, A.M.; Zuluaga Gallego, R. The nanotech potential of turmeric (Curcuma longa L.) in food technology: A review. Crit. Rev. Food Sci. 2020, 60, 1842–1854. [Google Scholar] [CrossRef]
- OEC. Turmeric (Curcuma). Available online: https://oec.world/en/profile/hs/turmeric-curcuma (accessed on 8 November 2023).
- Consumer Goods & FMCG Food & Nutrition. 2023. Available online: https://www.statista.com/statistics/798287/main-turmeric-export-countries-worldwide/#:~:text=Leading%20global%20exporting%20countries%20of%20turmeric%202022&text=India%20is%20y%20far%20the,exporter%20of%20turmeric%2C%20the%20Myanmar (accessed on 8 November 2023).
- Food and Agriculture Organization of the United Nations (FAO). Turmeric Post-Harvest Operations. 2004. Available online: http://www.fao.org/3/aau994e.pdf (accessed on 8 November 2023).
- United Nations Office on Drugs and Crime. Informe Ejecutivo: Encuentro Nacional de Desarrollo Alternativo. 2013. Available online: https://www.unodc.org/documents/colombia/2014/Agosto/EncuentroDA/ENDA_2013_ESPANOL.pdf (accessed on 8 November 2023).
- Agronet. Reporte: Área, Producción y Rendimiento Nacional por Cultivo. Available online: https://agronet.gov.co/estadistica/Paginas/home.aspx?cod=1 (accessed on 8 November 2023).
- Leonel, M.; Sarmento, S.B.S.; Cereda, M.P. New starches for the food industry: Curcuma longa and Curcuma zedoaria. Carbohydr. Polym. 2003, 54, 385–388. [Google Scholar] [CrossRef]
- Braga, M.E.M.; Moreschi, S.R.M.; Meireles, M.A.A. Effects of supercritical fluid extraction on Curcuma longa L. and Zingiber officinale R. starches. Carbohydr. Polym. 2006, 63, 340–346. [Google Scholar] [CrossRef]
- Maniglia, B.C.; De Paula, R.L.; Domingos, J.R.; Tapia-Blácido, D.R. Turmeric dye extraction residue for use in bioactive film production: Optimization of turmeric film plasticized with glycerol. LWT 2015, 64, 1187–1195. [Google Scholar] [CrossRef]
- Gañán, P.; Barajas, J.; Zuluaga, R.; Castro, C.; Marín, D.; Tercjak, A.; Builes, D.H. The evolution and future trends of unsaturated polyester biocomposites: A bibliometric analysis. Polymers 2023, 15, 2970. [Google Scholar] [CrossRef]
- Bello-Pérez, L.; Agama-Acevedo, E.; Sánchez-Hernández, L.; Paredes-López, O. Isolation and partial characterization of banana starches. J. Agric. Food Chem. 1999, 47, 854–857. [Google Scholar] [CrossRef]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef] [PubMed]
- Arzapalo Quinto, D.; Huamán Cóndor, K.; Quispe Solano, M.; Espinoza Silva, C. Extracción y caracterización del almidón de tres variedades de quinua (Chenopodium quinoa Willd) Negra collana, Pasankalla roja y Blanca junín. Rev. Soc. Química Perú 2015, 81, 44–54. [Google Scholar] [CrossRef]
- Nunn, S.; Nishikida, K. Advanced ATR Correction Algorithm, 1st ed.; Thermo Fisher Scientific: Waltham, MA, USA, 2008; pp. 1–4. [Google Scholar]
- Bradley, M. Curve Fitting in Raman and IR Spectroscopy: Basic Theory of Line Shapes and Applications, 1st ed.; Thermo Fisher Scientific: Waltham, MA, USA, 2007; pp. 1–4. [Google Scholar]
- Anderson, R.A.; Conway, H.F.; Peplinski, A.J. Gelatinization of corn grits by roll and extrusion cooking. Starch 1970, 22, 130–135. [Google Scholar] [CrossRef]
- Sappi Fine Paper North America. Defining and Communicating Color: The CIELAB System. 2013. Available online: https://cdn-s3.sappi.com/s3fs-public/sappietc/Defining%20and%20Communicating%20Color.pdf (accessed on 6 March 2023).
- Maniglia, B.C.; Garcia Silveira, T.M.; Tapia-Blácido, D.R. Starch isolation from turmeric dye extraction residue and its application in active film production. Int. J. Biol. Macromol. 2022, 202, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Naidu, M.M.; Shilpa, H.N.; Maheshwari, S.U.; Sruthi, P. Studies on physico-chemical structural and functional properties of tikhur (Curcuma angustifolia Roxb) starch. Trends Carbohydr. Res. 2022, 14, 45–54. [Google Scholar]
- Nakkala, K.; Godiyal, S.; Ettaboina, S.K.; Laddha, K.S. Chemical modifications of turmeric starch by oxidation, phosphorylation, and succinylation. Starch 2022, 74, 2200053. [Google Scholar] [CrossRef]
- Arini, T.; Yusraini, E.; Lubis, Z. Functional characteristics of starch from red ginger, elephant ginger, emprit ginger and curcuma. IOP Conf. Ser. Earth Environ. Sci. 2021, 782, 032096. [Google Scholar] [CrossRef]
- Oluba, O.M.; Osayame, E.; Shoyombo, A.O. Production and characterization of keratin-starch bio-composite film from chicken feather waste and turmeric starch. Biocatal. Agric. Biotechnol. 2021, 33, 101996. [Google Scholar] [CrossRef]
- Anu, S.; Dan, M.; Suja, S.R. Wild relative of turmeric, Curcuma zanthorrhiza Roxb.—A source of edible starch. Indian J. Tradit. Knowl. 2020, 19, 519–524. [Google Scholar]
- Bento, J.A.C.; Ferreira, K.C.; de Oliveira, A.L.M.; Lião, L.M.; Caliari, M.; Júnior, M.S.S. Extraction, characterization and technological properties of white garland-lily starch. Int. J. Biol. Macromol. 2019, 135, 422–428. [Google Scholar] [CrossRef]
- Maniglia, B.C.; Tapia-Blácido, D.R. Structural modification of fiber and starch in turmeric residue by chemical and mechanical treatment for production of biodegradable films. Int. J. Biol. Macromol. 2019, 126, 507–516. [Google Scholar] [CrossRef]
- Das, D.; Kumar, K.J. Enhancing resilient property of Kaempferia galanga rhizome starch by succinylation. Int. J. Biol. Macromol. 2019, 124, 1033–1039. [Google Scholar] [CrossRef]
- Silva, E.K.; Martelli-Tosi, M.; Vardanega, R.; Nogueira, G.C.; Zabot, G.L.; Meireles, M.A.A. Technological characterization of biomass obtained from the turmeric and annatto processing by using green technologies. J. Clean. Prod. 2018, 189, 231–239. [Google Scholar] [CrossRef]
- Franklin, M.E.E.; Pushpadass, H.A.; Kumar, B.; Kulkarni, S.; Muthurayappa, M.; Kandasamy, R.; Venkatachalam, P.; Vellingiri, P. Physicochemical, thermal, pasting, and microstructural characterization of commercial Curcuma angustifolia starch. Food Hydrocoll. 2017, 67, 27–36. [Google Scholar] [CrossRef]
- Jamir, K.; Seshagirirao, K. Isolation, characterization and comparative study of starches from selected Zingiberaceae species, a non-conventional source. Food Hydrocoll. 2017, 72, 247–253. [Google Scholar] [CrossRef]
- Mao, X.; Huang, H.; Li, X.; Wang, T.; Chen, X.; Gao, W. Physicochemical characterization, digestibility and anticonstipation activity of some high-resistant untraditional starches from Zingiberaceae plants. Int. J. Food Sci. Technol. 2017, 52, 617–625. [Google Scholar] [CrossRef]
- Santana, Á.L.; Zabot, G.L.; Osorio-Tobón, J.F.; Johner, J.C.F.; Coelho, A.S.; Schmiele, M.; Steel, C.J.; Meireles, M.A.A. Starch recovery from turmeric wastes using supercritical technology. J. Food Eng. 2017, 214, 266–276. [Google Scholar] [CrossRef]
- Van Hung, P.; Vo, T.N.D. Structure, physicochemical characteristics, and functional properties of starches isolated from yellow (Curcuma longa) and black (Curcuma caesia) turmeric rhizomes. Starch 2017, 69, 1600285. [Google Scholar] [CrossRef]
- Huang, J.; Zhao, L.; Man, J.; Wang, J.; Zhou, W.; Huai, H.; Wei, C. Comparison of physicochemical properties of B-type nontraditional starches from different sources. Int. J. Biol. Macromol. 2015, 78, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Tiwari, S.; Pisalkar, P.S.; Mishra, N.K.; Naik, R.K.; Khokhar, D. Indigenous processing of Tikhur (Curcuma angustifolia Roxb.) for the extraction of starch in Baster, Chhattisgarh. Indian J. Nat. Prod. Resour. 2015, 6, 213–220. [Google Scholar]
- Hansdah, R.; Prabhakar, P.K.; Srivastav, P.P.; Mishra, H.N. Physico-chemical characterization of lesser known Palo (Curcuma leucorrhiza) starch. Int. Food Res. J. 2015, 22, 1368–1373. [Google Scholar]
- Das, D.; Jha, S.; Kumar, K.J. Effect of carboxymethylation on physicochemical and release characteristics of Indian Palo starch. Int. J. Biol. Macromol. 2015, 77, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Jha, S.; Kumar, K.J. Isolation and release characteristics of starch from the rhizome of Indian Palo. Int. J. Biol. Macromol. 2015, 72, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Sajitha, P.K.; Sasikumar, B. Qualitative and quantitative variation in starch from four species of Curcuma. Cytologia 2015, 80, 45–50. [Google Scholar] [CrossRef]
- Xia, Y.; Gao, W.; Wang, H.; Jiang, Q.; Li, X.; Huang, L.; Xiao, P. Characterization of tradition Chinese medicine (TCM) starch for potential cosmetics industry application. Starch 2013, 65, 367–373. [Google Scholar] [CrossRef]
- Rani, A.; Chawhaan, P.H. Extraction and scanning electron microscopic studies of Curcuma angustifolia Roxb. Starch 2012, 3, 407–410. [Google Scholar]
- Kuttigounder, D.; Rao Lingamallu, J.; Bhattacharya, S. Turmeric powder and starch: Selected physical, physicochemical, and microstructural properties. J. Food Sci. 2011, 76, CI1284–CI1291. [Google Scholar] [CrossRef]
- Rajeevkumar, P.; Rajeev, R.E.K.H.A.; Anilkumar, N. Studies on Curcuma angustifolia starch as a pharmaceutical excipient. Int. J. PharmTech Res. 2010, 2, 2456–2460. [Google Scholar]
- Ascheri, D.P.R.; de Souza Moura, W.; Ascheri, J.L.R.; de Carvalho, C.W.P. Caracterização física e físico-química de rizomas e amido do lírio-do-brejo (Hedychium coronarium). Pesqui. Agropecu. 2010, 40, 159–166. [Google Scholar]
- Policegoudra, R.S.; Aradhya, S.M. Structure and biochemical properties of starch from an unconventional source—Mango ginger (Curcuma amada Roxb.) rhizome. Food Hydrocoll. 2008, 22, 513–519. [Google Scholar] [CrossRef]
- Ibezim, E.C.; Ofoefule, S.I.; Omeje, E.O.; Onyishi, V.I.; Odoh, U.E. The role of ginger starch as a binder in acetaminophen tablets. J. Sci. Res. Essays 2008, 3, 46–50. [Google Scholar]
- Ranjini, C.E.; Vijayan, K.K. A high amylose starch isolated from the tubers of Curcuma aeruginosa. Indian J. Chem.—B Org. Med. 2006, 45B, 2773–2775. [Google Scholar]
- Moreschi, S.R.M.; Leal, J.C.; Braga, M.E.M.; Meireles, M.A.A. Ginger and turmeric starches hydrolysis using subcritical water+ CO2: The effect of the SFE pre-treatment. Braz. J. Chem. Eng. 2006, 23, 235–242. [Google Scholar] [CrossRef]
- Jyothi, A.N.; Moorthy, S.N.; Vimala, B. Physicochemical and functional properties of starch from two species of Curcuma. Int. J. Food Prop. 2003, 6, 135–145. [Google Scholar] [CrossRef]
- Svegmark, K.; Hermansson, A.M. Microstructure and rheological properties of composites of potato starches granules and amylose: A comparison of observed and predicted structure. Food Struct. 1993, 12, 181–193. [Google Scholar]
- Hernández-Medina, M.; Torruco-Uco, J.G.; Chel-Guerrero, L.; Betancur-Ancona, D. Caracterización fisicoquímica de almidones de tubérculos cultivados en Yucatán, México. Food Sci. Technol. 2008, 28, 718–726. [Google Scholar] [CrossRef]
- Liu, J.; Chen, J.; Dong, N.; Ming, J.; Zhao, G. Determination of degree of substitution of carboxymethyl starch by Fourier transform mid-infrared spectroscopy coupled with partial least squares. Food Chem. 2012, 132, 2224–2230. [Google Scholar] [CrossRef]
- Hong, J.; Chen, R.; Zeng, X.-A.; Han, Z. Effect of pulsed electric fields assisted acetylation on morphological, structural and functional characteristics of potato starch. Food Chem. 2016, 192, 15–24. [Google Scholar] [CrossRef]
- Pozo, C.; Rodríguez-Llamazares, S.; Bouza, R.; Barral, L.; Castaño, J.; Müller, N.; Restrepo, I. Study of the structural order of native starch granules using combined FTIR and XRD analysis. J. Polym. Res. 2018, 25, 266. [Google Scholar] [CrossRef]
- Horitsu, K. Physicochemical Properties: On Infrared Absorption Spectra of Native Starch and Modified Starch using Film. Bull. Agric. Chem. Soc. Jpn. 1960, 24, 44–51. [Google Scholar] [CrossRef]
- Van Soest, J.J.; Tournois, H.; de Wit, D.; Vliegenthart, J.F. Short-range structure in (partially) crystalline potato starch determined with attenuated total reflectance Fourier-transform IR spectroscopy. Carbohydr. Res. 1995, 279, 201–214. [Google Scholar] [CrossRef]
- Paluch, M.; Ostrowska, J.; Tyński, P.; Sadurski, W.; Konkol, M. Structural and thermal properties of starch plasticized with glycerol/urea mixture. J. Polym. Environ. 2022, 30, 728–740. [Google Scholar] [CrossRef]
- Hettiarachchi, S.S.; Dunuweera, S.P.; Dunuweera, A.N.; Rajapakse, R.M.G. Synthesis of curcumin nanoparticles from raw turmeric rhizome. ACS Omega. 2021, 6, 8246–8252. [Google Scholar] [CrossRef] [PubMed]
- Priyanka, S.K. Influence of operating parameters on supercritical fluid extraction of essential oil from turmeric root. J. Clean. Prod. 2018, 188, 816–824. [Google Scholar] [CrossRef]
- Arik Kibar, E.A.; Us, F. Evaluation of structural properties of cellulose ether-corn starch based biodegradable films. Int. J. Polym. Mater. 2014, 63, 342–351. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, Q.; Feng, N.; Wang, J.-R.; Wang, S.-J.; He, Z.-H. Characterization of A- and B-type starch granules in Chinese wheat cultivars. J. Integr. Agric. 2016, 15, 2203–2214. [Google Scholar] [CrossRef]
- Paranthaman, R.; Moses, J.A.; Anandharamakrishnan, C. Powder X-ray diffraction conditions for screening curcumin in turmeric powder. J. Food Meas. Charact. 2022, 16, 1105–1113. [Google Scholar] [CrossRef]
- Pandey, K.U.; Dalvi, S.V. Understanding stability relationships among three curcumin polymorphs. Adv. Powder Technol. 2019, 30, 266–276. [Google Scholar] [CrossRef]
- Montoya-Escobar, N.; Ospina-Acero, D.; Velásquez-Cock, J.A.; Gómez-Hoyos, C.; Serpa Guerra, A.; Gañan Rojo, P.F.; Vélez Acosta, L.M.; Escobar, J.P.; Correa-Hincapié, N.; Triana-Chávez, O.; et al. Use of Fourier series in X-ray diffraction (XRD). Analysis and Fourier-Transform Infrared Spectroscopy (FTIR) for estimation of crystallinity in cellulose from different sources. Polymers 2022, 14, 5199. [Google Scholar] [CrossRef]
- An, D.; Li, H.; Li, D.; Zhang, D.; Huang, Y.; Obadi, M.; Xu, B. The relation between wheat starch properties and noodle springiness: From the view of microstructure quantitative analysis of gluten-based network. Food Chem. 2022, 393, 133396. [Google Scholar] [CrossRef]
- Jia, R.; Cui, C.; Gao, L.; Qin, Y.; Ji, N.; Dai, l.; Wang, Y.; Xiong, L.; Shi, R.; Sun, Q. A review of starch swelling behavior: Its mechanism, determination methods, influencing factors, and influence on food quality. Carbohydr. Polym. 2023, 321, 121260. [Google Scholar] [CrossRef] [PubMed]
- Algar, A.F.C.; Umali, A.B.; Tayobong, R.R.P. Physicochemical and functional properties of starch from Philippine edible Canna (Canna indica L.) rhizomes. J. Microbiol. Biotechnol. Food Sci. 2019, 9, 34–37. [Google Scholar] [CrossRef]



| Variable | Units | Result |
|---|---|---|
| Chemical composition | ||
| Carbohydrates | g/100 g | 83.69 |
| Ashes | g/100 g | 0.82 |
| Fat | g/100 g | 0.05 |
| Protein | g/100 g | 0.44 |
| Moisture | g/100 g | 15.00 |
| Physical characteristics | ||
| Swelling power (SP) | g water/g starch | 3.52 ± 0.30 |
| Solubility | wt% | 2.41 ± 0.10 |
| Water retention capacity (WRC) | g gel/g starch | 3.44 ± 0.30 |
| Color evaluation | ||
| L* | 67.66 ± 0.49 | |
| a* | 21.25 ± 0.19 | |
| b* (WRC) | 74.48 ± 0.47 | |
| C* | 77.45 ± 0.44 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Argel-Pérez, S.; Gañán-Rojo, P.; Cuartas-Marulanda, D.; Gómez-Hoyos, C.; Velázquez-Cock, J.; Vélez-Acosta, L.; Zuluaga, R.; Serpa-Guerra, A. Characterization of a Novel Starch Isolated from the Rhizome of Colombian Turmeric (Curcuma longa L.) Cultivars. Foods 2024, 13, 7. https://doi.org/10.3390/foods13010007
Argel-Pérez S, Gañán-Rojo P, Cuartas-Marulanda D, Gómez-Hoyos C, Velázquez-Cock J, Vélez-Acosta L, Zuluaga R, Serpa-Guerra A. Characterization of a Novel Starch Isolated from the Rhizome of Colombian Turmeric (Curcuma longa L.) Cultivars. Foods. 2024; 13(1):7. https://doi.org/10.3390/foods13010007
Chicago/Turabian StyleArgel-Pérez, Shaydier, Piedad Gañán-Rojo, Diego Cuartas-Marulanda, Catalina Gómez-Hoyos, Jorge Velázquez-Cock, Lina Vélez-Acosta, Robin Zuluaga, and Angélica Serpa-Guerra. 2024. "Characterization of a Novel Starch Isolated from the Rhizome of Colombian Turmeric (Curcuma longa L.) Cultivars" Foods 13, no. 1: 7. https://doi.org/10.3390/foods13010007
APA StyleArgel-Pérez, S., Gañán-Rojo, P., Cuartas-Marulanda, D., Gómez-Hoyos, C., Velázquez-Cock, J., Vélez-Acosta, L., Zuluaga, R., & Serpa-Guerra, A. (2024). Characterization of a Novel Starch Isolated from the Rhizome of Colombian Turmeric (Curcuma longa L.) Cultivars. Foods, 13(1), 7. https://doi.org/10.3390/foods13010007







