Hypoglycemic Effect of Edible Fungi Polysaccharides Depends on Their Metabolites from the Fermentation of Human Fecal Microbiota
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Preparation of Crude Polysaccharide
2.3. In Vitro Fermentation of Fecal Inocula
2.4. Hypoglycemic Effect of Fermentation Supernatant on IR-HepG2 Cells
2.4.1. Cell Culture and Viability
2.4.2. Establishment of Insulin Resistance Model
2.4.3. Glucose Consumption Measurement
2.4.4. Determination of Glycogen Content, Activity of Hexokinase (HK), Phosphoenolpyruvate Carboxylase Kinase (PEPCK)
2.4.5. Quantitative Reverse Transcription Polymerase Chain Reaction
2.5. 16S rDNA Sequencing and Bioinformatics Analysis
2.6. Non-Targeted Metabolomics Analysis of Metabolite
2.7. Statistical Analysis
3. Results
3.1. Effects of Fermentation Supernatants on Glucose Uptake in Insulin Resistance (IR) Cell Model
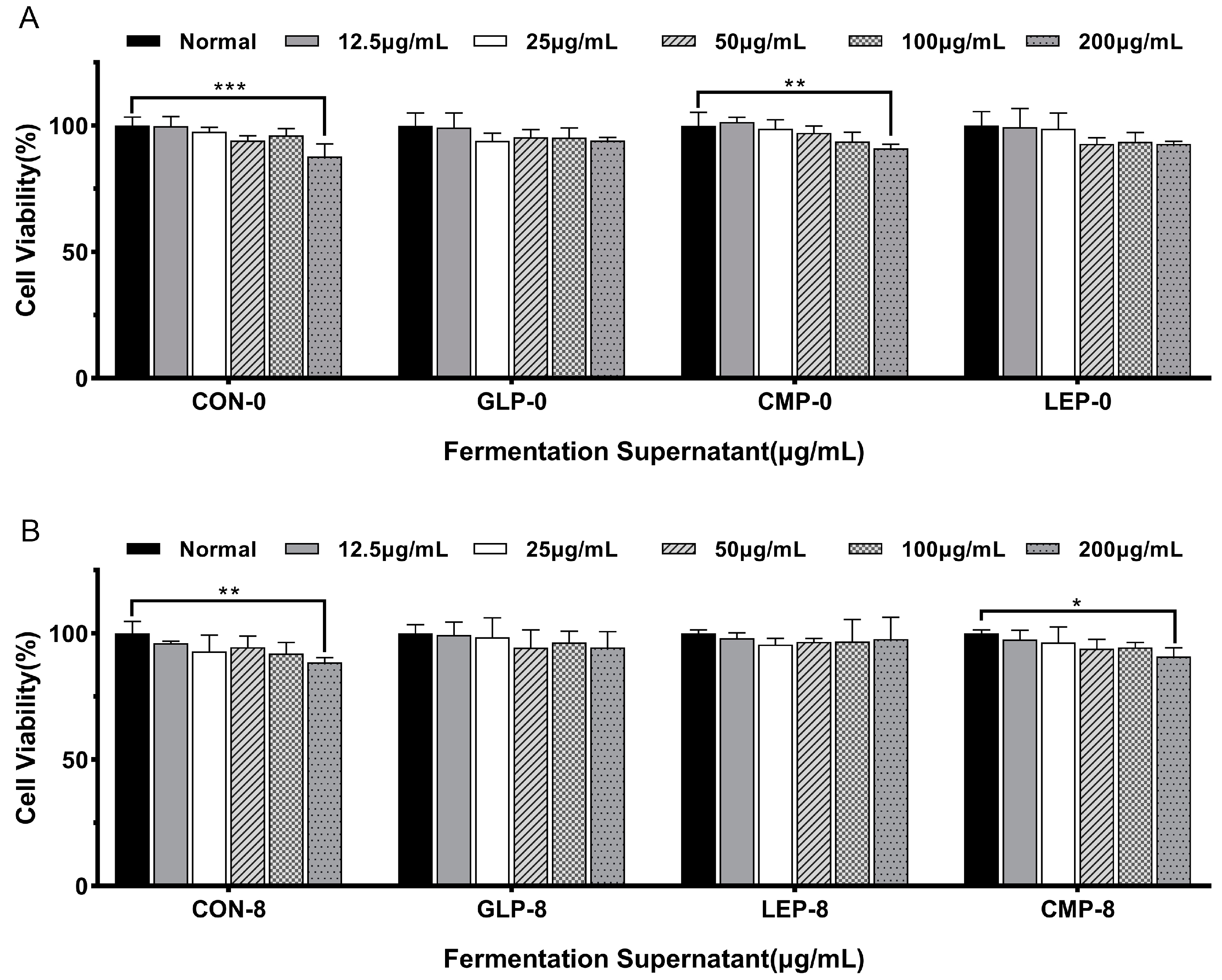
3.1.1. Effect of Fermentation Supernatants on Cell Viability
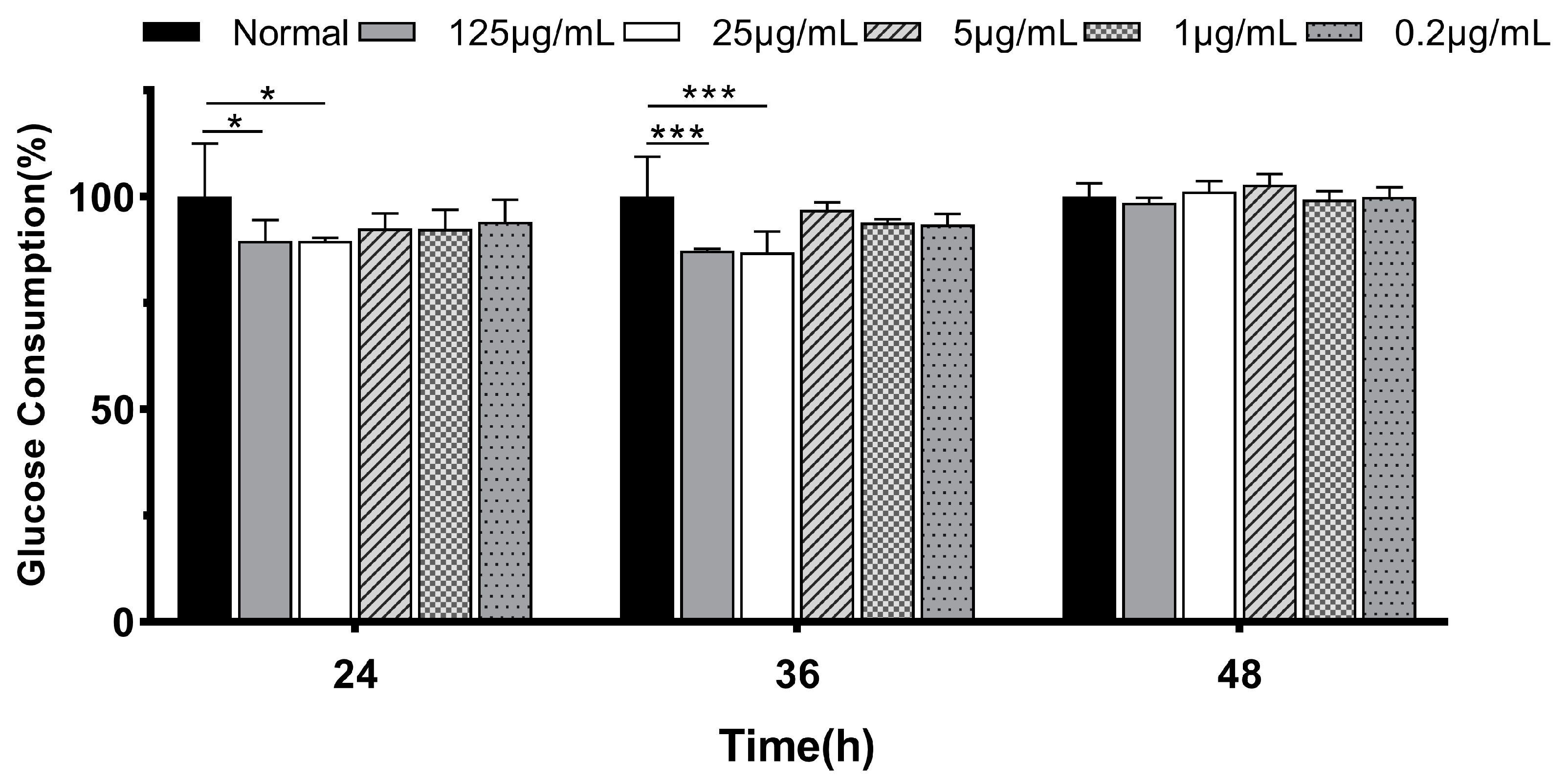
3.1.2. Glucose Consumption in IR-HepG2
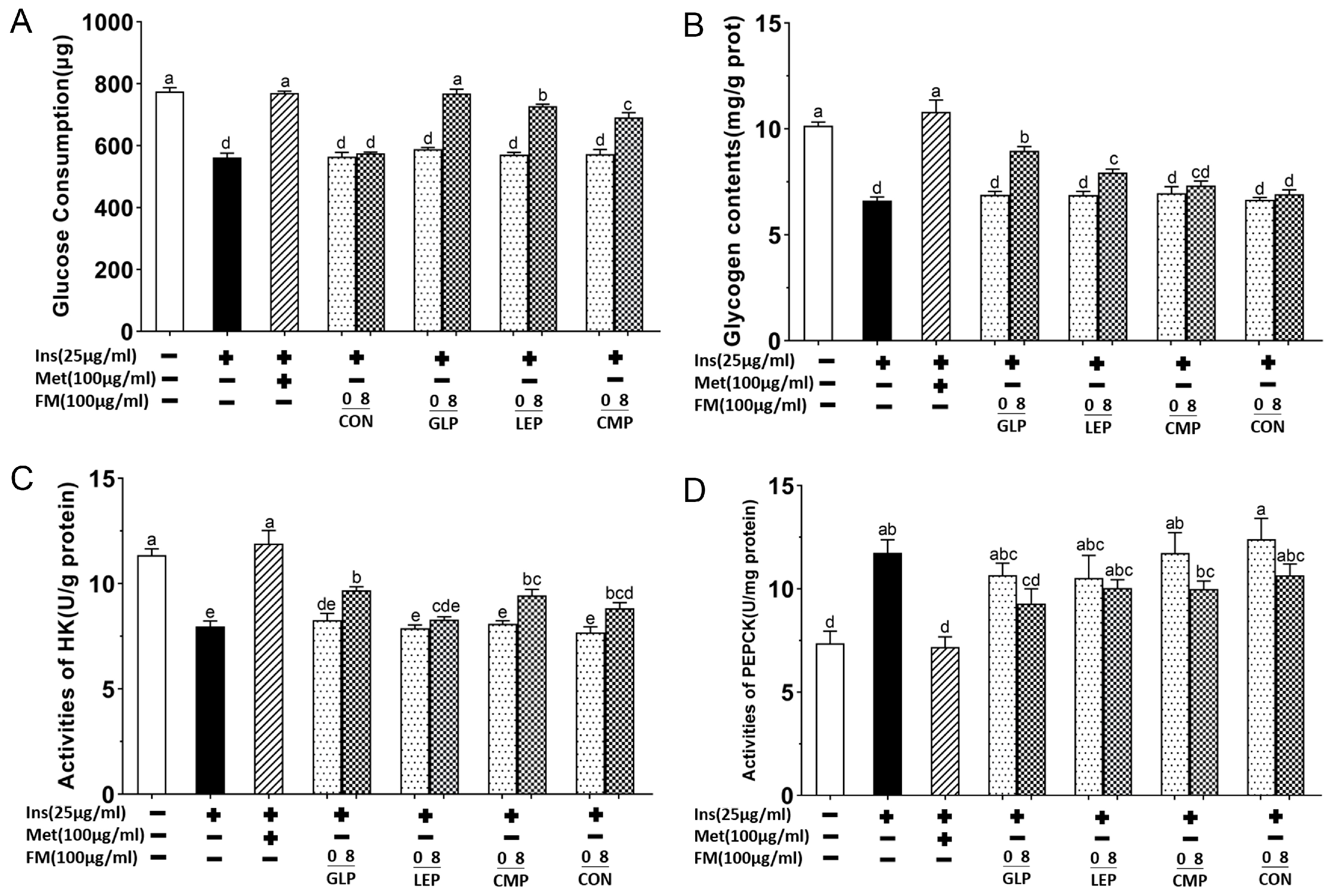
3.1.3. Glycogen Content, Activities of HK, and PEPCK in IR-HepG2 Cell Model
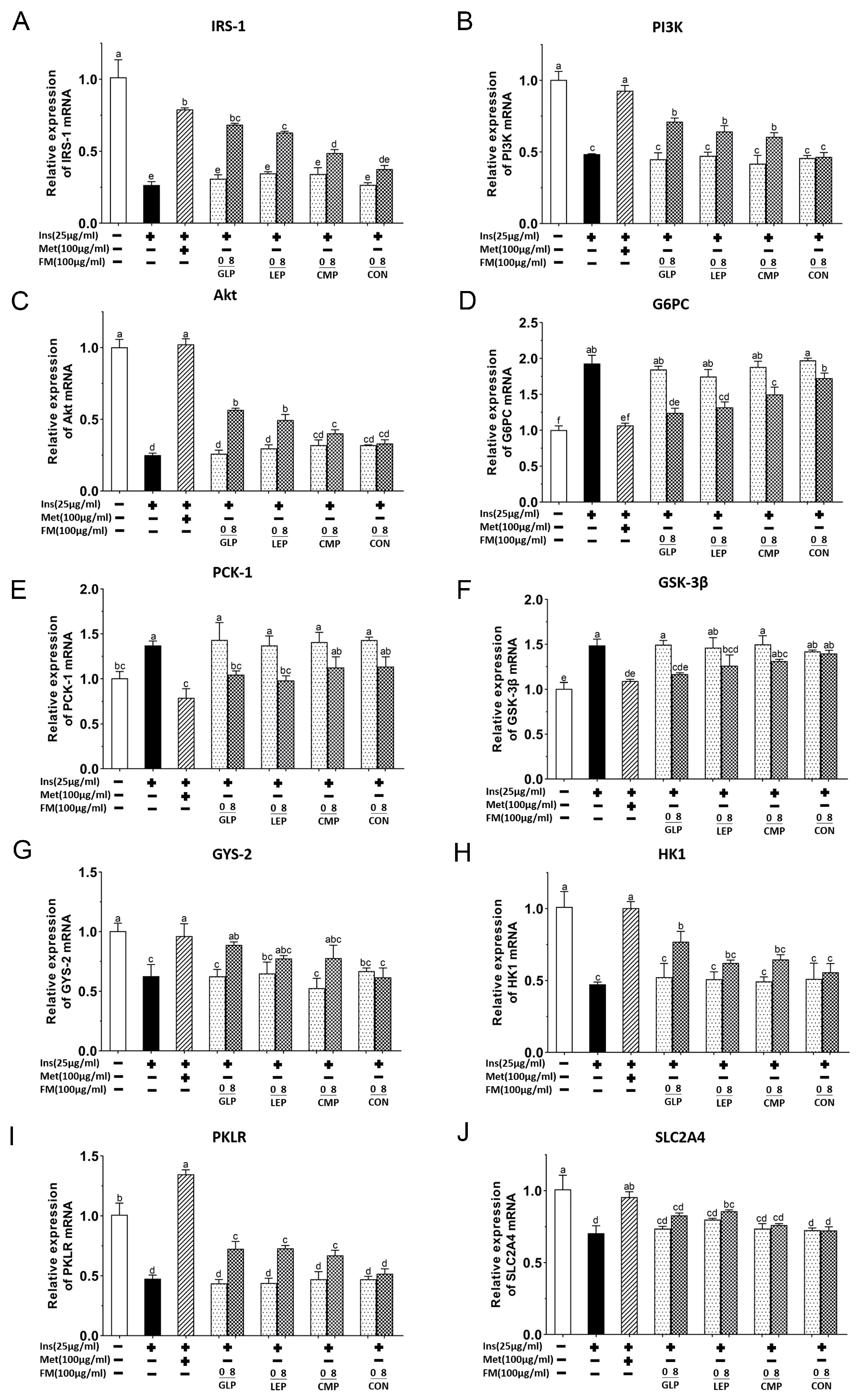
3.1.4. Effect of Fermentation Supernatant on mRNA Expression of IRS-1/PI3K/Akt Signaling Pathway
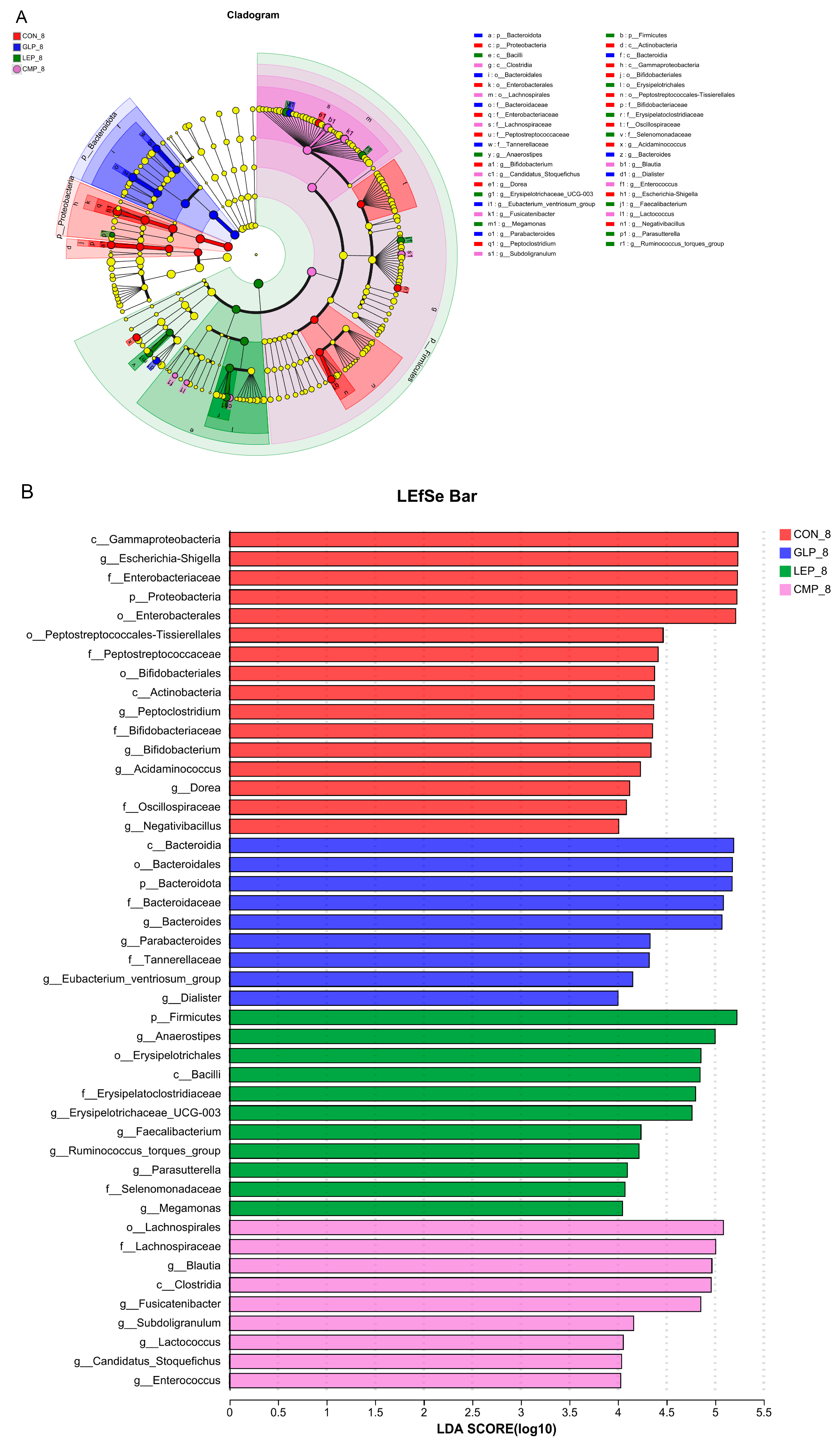
3.2. Effects of Polysaccharides on Fecal Microbiota Composition
3.2.1. Diversity Analysis of Fermentation Supernatant Microbiota
3.2.2. Composition of Fermentation Supernatant Microbiota
3.2.3. Linear Discriminant Analysis Effect Size
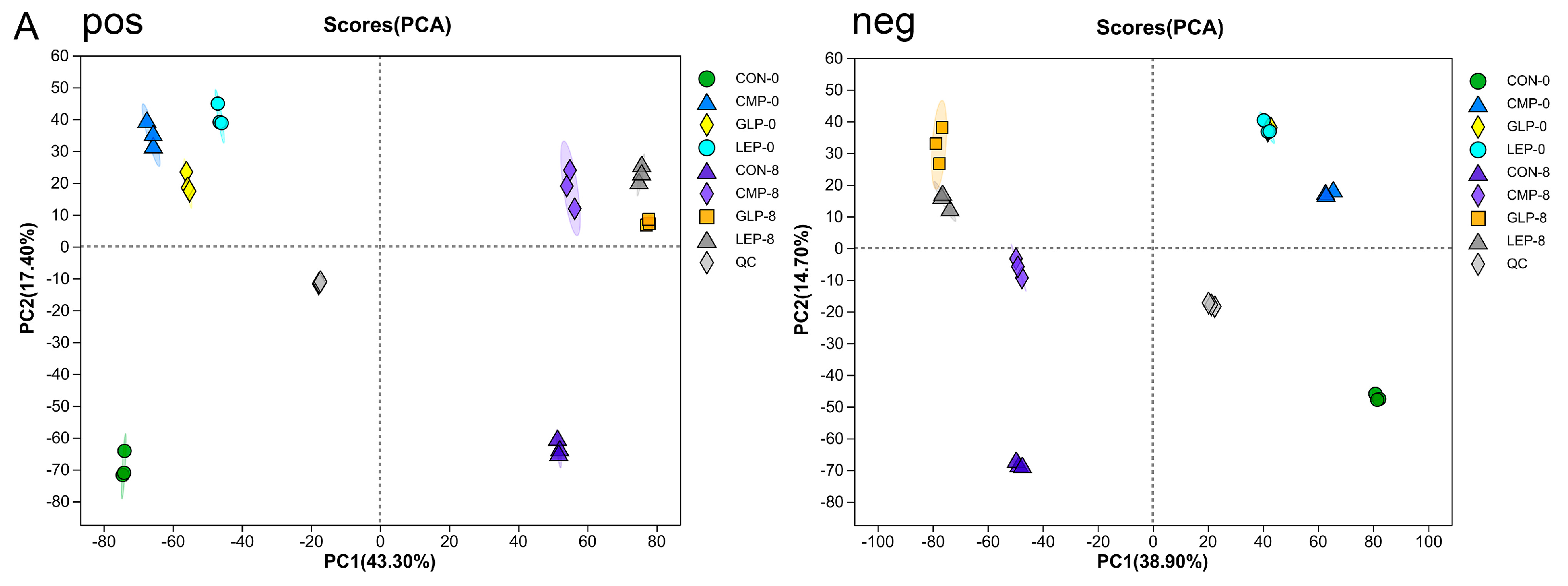
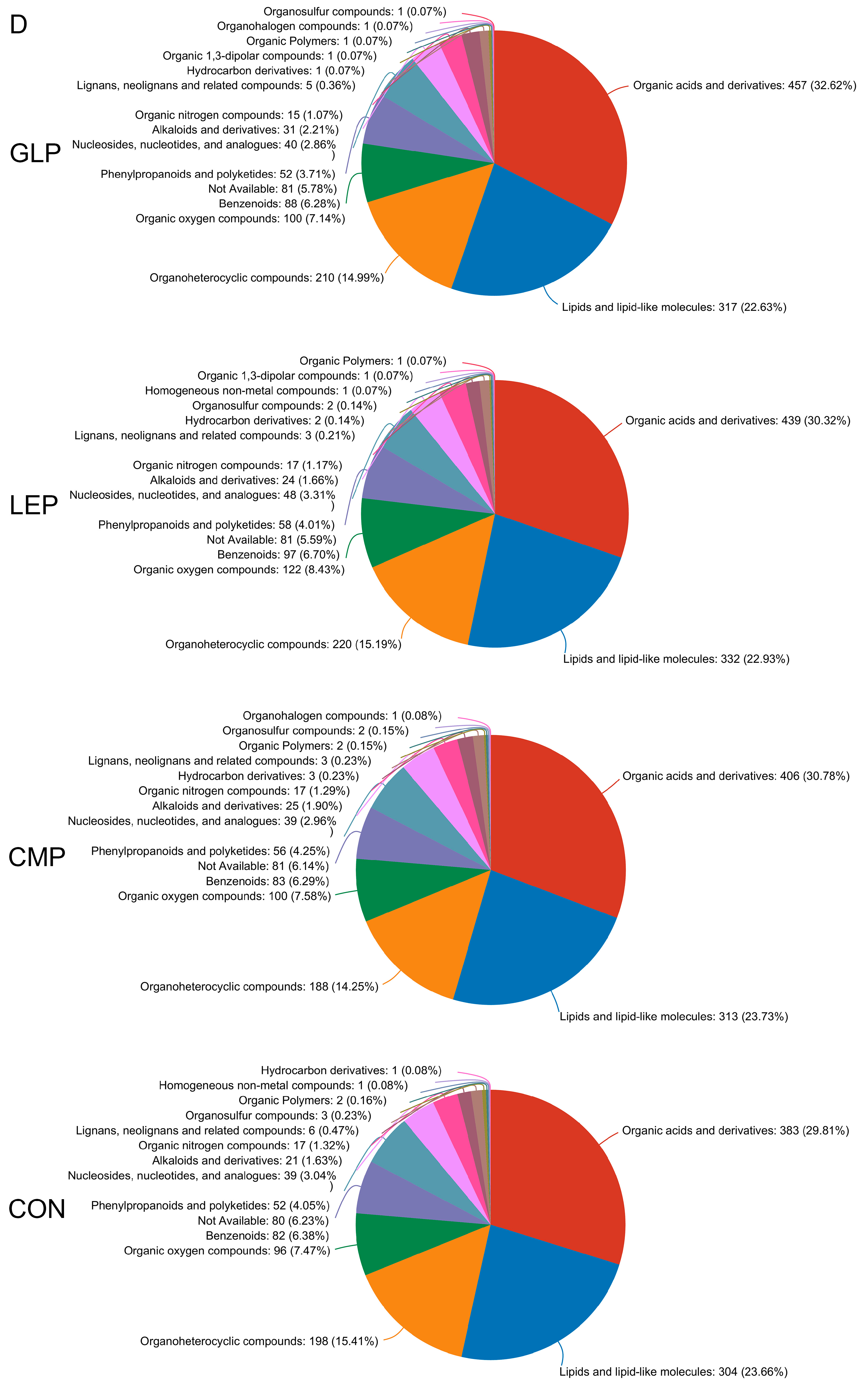
3.3. Metabolite Profiles between Different Fermentation Groups
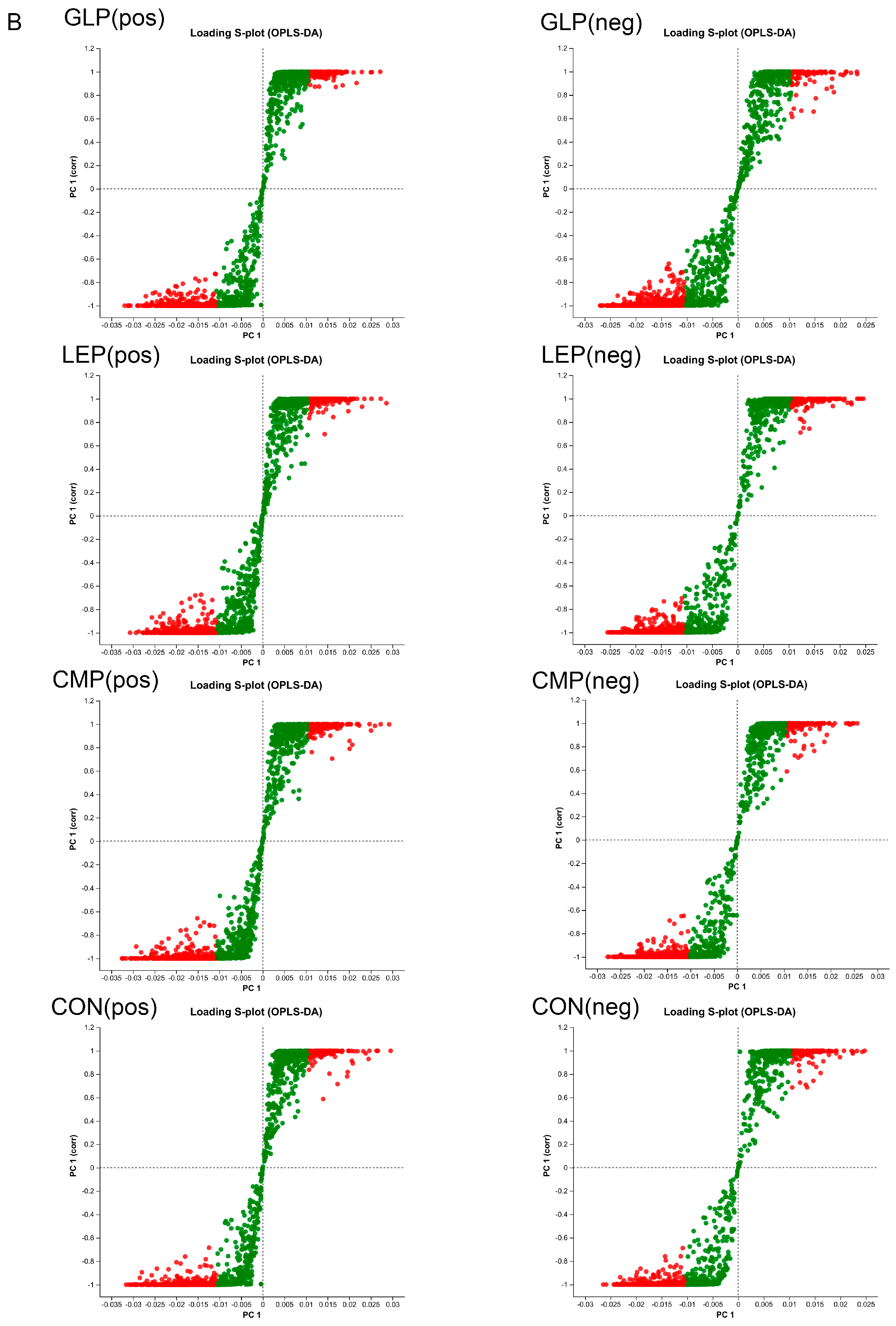
3.3.1. Effect of Polysaccharides on Metabolites
3.3.2. Screening of Differential Metabolites
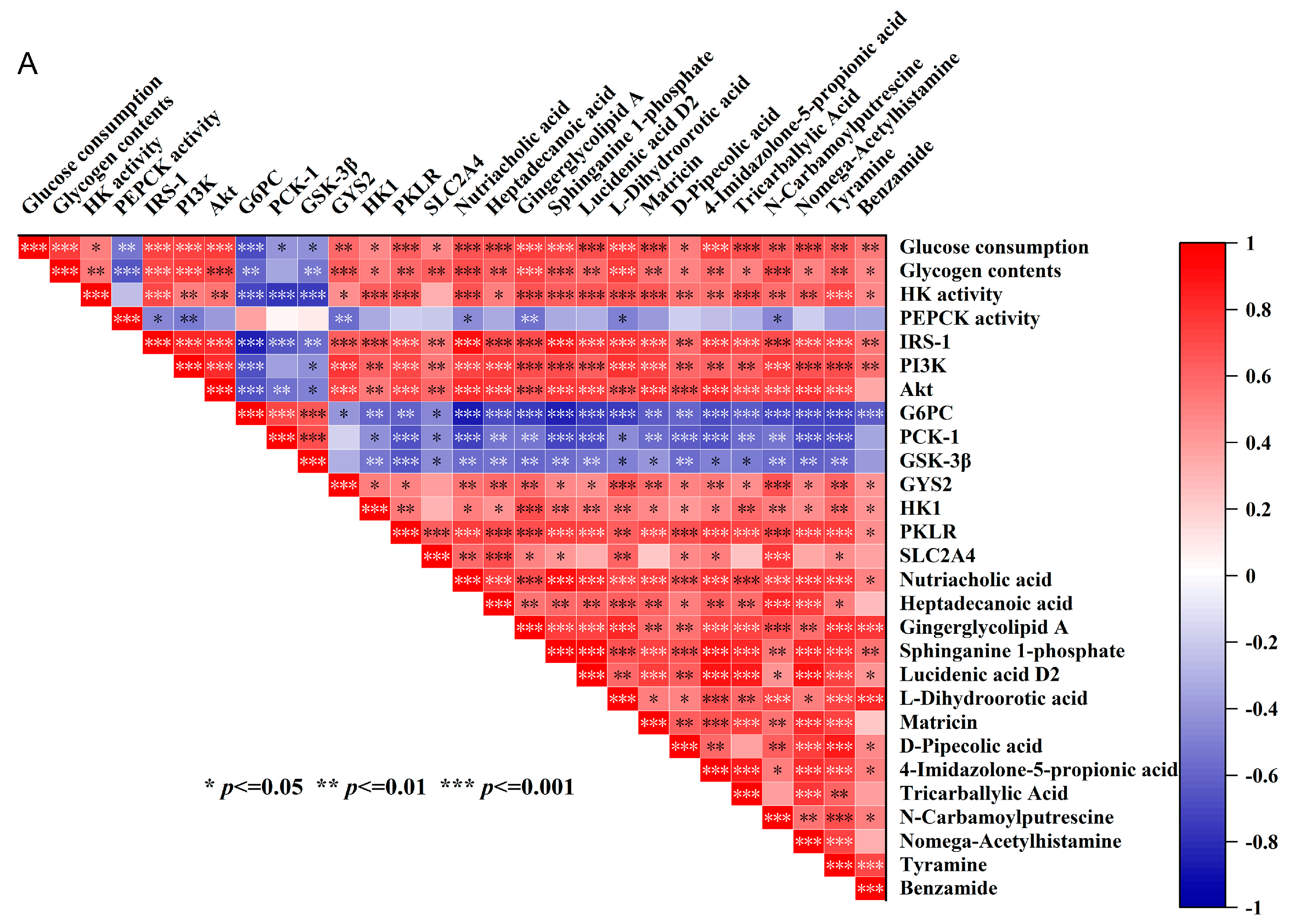
3.3.3. Specific Gut Bacteria and Metabolites May Contribute to the Hypoglycemic Effects of IR-HepG2 Cells
3.4. Poria Cocos Alkaline-Soluble Polysaccharides’ Interventions Verified the Hypoglycemic Effect of Key Bacteria and Metabolites
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, Regional and Country-Level Diabetes Prevalence Estimates for 2021 and Projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Akhter, M.S.; Uppal, P. Toxicity of Metformin and Hypoglycemic Therapies. Adv. Chronic Kidney Dis. 2020, 27, 18–30. [Google Scholar] [CrossRef] [PubMed]
- O’Hearn, M.; Lara-Castor, L.; Cudhea, F.; Miller, V.; Reedy, J.; Shi, P.; Zhang, J.; Wong, J.B.; Economos, C.D.; Micha, R.; et al. Incident Type 2 Diabetes Attributable to Suboptimal Diet in 184 Countries. Nat. Med. 2023, 29, 982–995. [Google Scholar] [CrossRef]
- Das, A.; Chen, C.-M.; Mu, S.-C.; Yang, S.-H.; Ju, Y.-M.; Li, S.-C. Medicinal Components in Edible Mushrooms on Diabetes Mellitus Treatment. Pharmaceutics 2022, 14, 436. [Google Scholar] [CrossRef]
- Ahmad, R.; Riaz, M.; Khan, A.; Aljamea, A.; Algheryafi, M.; Sewaket, D.; Alqathama, A. Ganoderma lucidum (Reishi) an Edible Mushroom; a Comprehensive and Critical Review of Its Nutritional, Cosmeceutical, Mycochemical, Pharmacological, Clinical, and Toxicological Properties. Phytother. Res. 2021, 35, 6030–6062. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-J.; Sheu, B.-S. Metabolic Interaction of Helicobacter Pylori Infection and Gut Microbiota. Microorganisms 2016, 4, 15. [Google Scholar] [CrossRef]
- Yang, S.; Qu, Y.; Zhang, H.; Xue, Z.; Liu, T.; Yang, L.; Sun, L.; Zhou, Y.; Fan, Y. Hypoglycemic Effects of Polysaccharides from Gomphidiaceae Rutilus Fruiting Bodies and Their Mechanisms. Food Funct. 2020, 11, 424–434. [Google Scholar] [CrossRef]
- Thomson, P.; Medina, D.A.; Ortúzar, V.; Gotteland, M.; Garrido, D. Anti-Inflammatory Effect of Microbial Consortia during the Utilization of Dietary Polysaccharides. Food Res. Int. 2018, 109, 14–23. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, H.; Xiao, X.; Hu, L.; Xin, F.; Yu, X. Inulin-Type Fructan Improves Diabetic Phenotype and Gut Microbiota Profiles in Rats. PeerJ 2018, 6, e4446. [Google Scholar] [CrossRef]
- Fu, X.; Cao, C.; Ren, B.; Zhang, B.; Huang, Q.; Li, C. Structural Characterization and in vitro Fermentation of a Novel Polysaccharide from Sargassum Thunbergii and Its Impact on Gut Microbiota. Carbohydr. Polym. 2018, 183, 230–239. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, Q.; Jia, L.; He, H.; Zhang, T.; Jia, W.; Zhu, L.; Qi, W.; Wang, N. Effects of in vitro Fermentation of Atractylodes Chinensis (DC.) Koidz. Polysaccharide on Fecal Microbiota and Metabolites in Patients with Type 2 Diabetes Mellitus. Int. J. Biol. Macromol. 2023, 253, 126860. [Google Scholar] [CrossRef] [PubMed]
- Birkeland, E.; Gharagozlian, S.; Birkeland, K.I.; Valeur, J.; Måge, I.; Rud, I.; Aas, A.-M. Prebiotic Effect of Inulin-Type Fructans on Faecal Microbiota and Short-Chain Fatty Acids in Type 2 Diabetes: A Randomised Controlled Trial. Eur. J. Nutr. 2020, 59, 3325–3338. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, H.; Ma, J.; Xu, T.; Zhou, X.; Jia, S.; Zha, J.; Xue, D.; Tao, W.; Xiong, Q.; et al. A Green and Efficient Deproteination Method for Polysaccharide from Meretrix Meretrix Linnaeus by Copper Ion Chelating Aerogel Adsorption. J. Clean. Prod. 2020, 252, 119842. [Google Scholar] [CrossRef]
- Zhao, M.; Guan, Z.; Tang, N.; Cheng, Y. The Differences between the Water- and Alkaline-Soluble Poria Cocos Polysaccharide: A Review. Int. J. Biol. Macromol. 2023, 235, 123925. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Hu, Q.; Liu, J.; Luo, J.; Liu, L.; Peng, X. Metabolites of Gut Microbiota Fermenting Poria Cocos Polysaccharide Alleviates Chronic Nonbacterial Prostatitis in Rats. Int. J. Biol. Macromol. 2022, 209, 1593–1604. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Ren, G.; Zhang, B.; Ma, F.; Fan, J.; Qiu, Z. Screening and Identification of a Novel Antidiabetic Peptide from Collagen Hydrolysates of Chinese Giant Salamander Skin: Network Pharmacology, Inhibition Kinetics and Protection of IR-HepG2 Cells. Food Funct. 2022, 13, 3329–3342. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Maruyama, F.; Kato, H.; Toyoda, A.; Dozono, A.; Ohtsubo, Y.; Nagata, Y.; Fujiyama, A.; Tsuda, M.; Kurokawa, K. Design and Experimental Application of a Novel Non-Degenerate Universal Primer Set That Amplifies Prokaryotic 16S rRNA Genes with a Low Possibility to Amplify Eukaryotic rRNA Genes. DNA Res. 2014, 21, 217–227. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, L.; Luo, J.; Peng, X. Anti-Aging Potency Correlates with Metabolites from in vitro Fermentation of Edible Fungal Polysaccharides Using Human Fecal Intestinal Microflora. Food Funct. 2022, 13, 11592–11603. [Google Scholar] [CrossRef]
- Zheng, M.; Wang, L.; Sun, Y.; Pi, X.; Zhang, W.; Gao, P.; Lu, S.; Liu, W. Hypoglycemic Effect of the Phellinus Baumii Extract with α-Glucosidase-Inhibited Activity and Its Modulation to Gut Microbiota in Diabetic Patients. Biomed. Pharmacother. 2023, 158, 114130. [Google Scholar] [CrossRef]
- Yan, F.; Zhang, J.; Zhang, L.; Zheng, X. Mulberry Anthocyanin Extract Regulates Glucose Metabolism by Promotion of Glycogen Synthesis and Reduction of Gluconeogenesis in Human HepG2 Cells. Food Funct. 2016, 7, 425–433. [Google Scholar] [CrossRef]
- Olson, A.L. Regulation of GLUT4 and Insulin-Dependent Glucose Flux. ISRN Mol. Biol. 2012, 2012, 856987. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; Xiao, C.; Yong, T.; Zhang, Y.; Hu, H.; Xie, T.; Liu, R.; Huang, L.; Li, X.; Xie, Y.; et al. A Polysaccharide Isolated from Ganoderma Lucidum Ameliorates Hyperglycemia through Modulating Gut Microbiota in Type 2 Diabetic Mice. Int. J. Biol. Macromol. 2022, 197, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.; Wang, X.; Liu, M.; Wang, M.; Wang, S.; Guo, Y.; Chang, X.; Yang, W.; Chen, X.; Chen, F. Hypoglycemic Effect of a Novel Polysaccharide from Lentinus Edodes on STZ-Induced Diabetic Mice via Metabolomics Study and Nrf2/HO-1 Pathway. Food Funct. 2022, 13, 3036–3049. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Yu, X.; Li, T.; Zhu, Z. Structure and Hypoglycemic Activity of a Novel Exopolysaccharide of Cordyceps Militaris. Int. J. Biol. Macromol. 2021, 166, 496–508. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.-H.; Li, X.-F.; Wang, M.-N.; Zha, X.-Q.; Yang, X.-F.; Liu, Z.-J.; Luo, Y.-B.; Luo, J.-P. Comparison of Hypoglycemic and Antioxidative Effects of Polysaccharides from Four Different Dendrobium Species. Int. J. Biol. Macromol. 2014, 64, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.-J.; Liu, D.; Wan, Y.-J.; Huang, X.-J.; Nie, S.-P. Comparison of Hypoglycemic Effects of Polysaccharides from Four Legume Species. Food Hydrocoll. 2019, 90, 299–304. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, D.; Qin, L.; Yang, X.; Gao, C. Comparative Investigation for Hypoglycemic Effects of Polysaccharides from Four Substitutes of Lonicera Japonica in Chinese Medicine. Int. J. Biol. Macromol. 2018, 109, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Julia, P.J.; Munoz-Munoz, J.; van Sinderen, D. A Comprehensive Review on the Impact of β-Glucan Metabolism by Bacteroides and Bifidobacterium Species as Members of the Gut Microbiota. Int. J. Biol. Macromol. 2021, 181, 877–889. [Google Scholar] [CrossRef]
- Marchetti, L.; Truzzi, E.; Frosi, I.; Papetti, A.; Cappellozza, S.; Saviane, A.; Pellati, F.; Bertelli, D. In vitro Bioactivity Evaluation of Mulberry Leaf Extracts as Nutraceuticals for the Management of Diabetes Mellitus. Food Funct. 2022, 13, 4344–4359. [Google Scholar] [CrossRef]
- Yalcinkaya, M.; von Eckardstein, A. Apolipoprotein M and Sphingosine-1-Phosphate: A Potentially Antidiabetic Tandem Carried by HDL. Diabetes 2020, 69, 859–861. [Google Scholar] [CrossRef]
- Ramadan, M.; Goeters, S.; Watzer, B.; Krause, E.; Lohmann, K.; Bauer, R.; Hempel, B.; Imming, P. Chamazulene Carboxylic Acid and Matricin: A Natural Profen and Its Natural Prodrug, Identified through Similarity to Synthetic Drug Substances. J. Nat. Prod. 2006, 69, 1041–1045. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, H.; Arya, S.; Ghole, V.; Apte, K.; Shelke, D.; Chaskar, M. Hypoglycemic and Anticataract Activity of Crude Exopolysaccharides of Medicinal Mushroom Phellinus Badius on Streptozotocin-Induced Diabetic Rats and Goat Eye Lenses Respectively. Bioact. Carbohydr. Diet. Fibre 2020, 24, 100241. [Google Scholar] [CrossRef]
- Ashbrook, M.J.; McDonough, K.L.; Pituch, J.J.; Christopherson, P.L.; Cornell, T.T.; Selewski, D.T.; Shanley, T.P.; Blatt, N.B. Citrate Modulates Lipopolysaccharide-Induced Monocyte Inflammatory Responses. Clin. Exp. Immunol. 2015, 180, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Zarei, I.; Koistinen, V.M.; Kokla, M.; Klåvus, A.; Babu, A.F.; Lehtonen, M.; Auriola, S.; Hanhineva, K. Tissue-Wide Metabolomics Reveals Wide Impact of Gut Microbiota on Mice Metabolite Composition. Sci. Rep. 2022, 12, 15018. [Google Scholar] [CrossRef]
- Wang, W.; Fan, L.; Ge, F.; Li, Z.; Zhu, J.; Yin, K.; Xia, J.; Xue, M. Effects of Danggui Buxue Decoction on Host Gut Microbiota and Metabolism in GK Rats with Type 2 Diabetes. Front. Microbiol. 2022, 13, 1029409. [Google Scholar] [CrossRef]
- Visentin, V.; Bour, S.; Boucher, J.; Prévot, D.; Valet, P.; Ordener, C.; Parini, A.; Carpéné, C. Glucose Handling in Streptozotocin-Induced Diabetic Rats Is Improved by Tyramine but Not by the Amine Oxidase Inhibitor Semicarbazide. Eur. J. Pharmacol. 2005, 522, 139–146. [Google Scholar] [CrossRef]
- Zhang, L.; Li, H.; Zhu, Q.; Liu, J.; Chen, L.; Leng, Y.; Jiang, H.; Liu, H. Benzamide Derivatives as Dual-Action Hypoglycemic Agents That Inhibit Glycogen Phosphorylase and Activate Glucokinase. Bioorganic Med. Chem. 2009, 17, 7301–7312. [Google Scholar] [CrossRef]
- Zhao, L.; Lou, H.; Peng, Y.; Chen, S.; Zhang, Y.; Li, X. Comprehensive Relationships between Gut Microbiome and Faecal Metabolome in Individuals with Type 2 Diabetes and Its Complications. Endocrine 2019, 66, 526–537. [Google Scholar] [CrossRef]
- Huang, X.; Qiu, Y.; Gao, Y.; Zhou, R.; Hu, Q.; He, Z.; Lv, Y.; Wang, X.; Chen, W.; Deng, Y.; et al. Gut Microbiota Mediate Melatonin Signalling in Association with Type 2 Diabetes. Diabetologia 2022, 65, 1627–1641. [Google Scholar] [CrossRef]
- Ganesan, K.; Chung, S.K.; Vanamala, J.; Xu, B. Causal Relationship between Diet-Induced Gut Microbiota Changes and Diabetes: A Novel Strategy to Transplant Faecalibacterium Prausnitzii in Preventing Diabetes. Int. J. Mol. Sci. 2018, 19, 3720. [Google Scholar] [CrossRef]
- Wei, X.; Tao, J.; Xiao, S.; Jiang, S.; Shang, E.; Zhu, Z.; Qian, D.; Duan, J. Xiexin Tang Improves the Symptom of Type 2 Diabetic Rats by Modulation of the Gut Microbiota. Sci. Rep. 2018, 8, 3685. [Google Scholar] [CrossRef]
- Zafar, H.; Saier, M.H. Gut Bacteroides Species in Health and Disease. Gut Microbes 2021, 13, 1848158. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, S.; Li, S.; Zhang, Q.; Cai, Y.; Li, P.; Li, H.; Shen, B.; Liao, Q.; Hong, Y.; et al. Indoleacrylic Acid Produced by Parabacteroides Distasonis Alleviates Type 2 Diabetes via Activation of AhR to Repair Intestinal Barrier. BMC Biol. 2023, 21, 90. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.P.N.; Mannerås-Holm, L.; Puschmann, R.; Wu, H.; Troise, A.D.; Nijsse, B.; Boeren, S.; Bäckhed, F.; Fiedler, D.; deVos, W.M. Conversion of Dietary Inositol into Propionate and Acetate by Commensal Anaerostipes Associates with Host Health. Nat. Commun. 2021, 12, 4798. [Google Scholar] [CrossRef] [PubMed]
- Kieler, I.N.; Shamzir Kamal, S.; Vitger, A.D.; Nielsen, D.S.; Lauridsen, C.; Bjornvad, C.R. Gut Microbiota Composition May Relate to Weight Loss Rate in Obese Pet Dogs. Vet. Med. Sci. 2017, 3, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; He, Z.; Hu, R.; Yan, J.; Zhang, Q.; Li, B.; Yuan, X.; Zhang, H.; He, J.; Wu, S. Dietary β-Carotene on Postpartum Uterine Recovery in Mice: Crosstalk Between Gut Microbiota and Inflammation. Front. Immunol. 2021, 12, 744425. [Google Scholar] [CrossRef]
- Prudente, S.; Morini, E.; Trischitta, V. Insulin Signaling Regulating Genes: Effect on T2DM and Cardiovascular Risk. Nat. Rev. Endocrinol. 2009, 5, 682–693. [Google Scholar] [CrossRef]
- Agbu, P.; Carthew, R.W. MicroRNA-Mediated Regulation of Glucose and Lipid Metabolism. Nat. Rev. Mol. Cell Biol. 2021, 22, 425–438. [Google Scholar] [CrossRef]
- Alaaeldin, R.; Abdel-Rahman, I.A.M.; Hassan, H.A.; Youssef, N.; Allam, A.E.; Abdelwahab, S.F.; Zhao, Q.-L.; Fathy, M. Carpachromene Ameliorates Insulin Resistance in HepG2 Cells via Modulating IR/IRS1/PI3k/Akt/GSK3/FoxO1 Pathway. Molecules 2021, 26, 7629. [Google Scholar] [CrossRef]
- Daisy, P.; Balasubramanian, K.; Rajalakshmi, M.; Eliza, J.; Selvaraj, J. Insulin Mimetic Impact of Catechin Isolated from Cassia Fistula on the Glucose Oxidation and Molecular Mechanisms of Glucose Uptake on Streptozotocin-Induced Diabetic Wistar Rats. Phytomedicine 2010, 17, 28–36. [Google Scholar] [CrossRef]
- Wigger, D.; Schumacher, F.; Schneider-Schaulies, S.; Kleuser, B. Sphingosine 1-Phosphate Metabolism and Insulin Signaling. Cell. Signal. 2021, 82, 109959. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Lee, H.; Choi, B.W.; Ro, S.; Lee, D.; Na, J.E.; Hong, J.-H.; Lee, J.-S.; Kim, B.-W.; Ko, Y.-G. An MG53-IRS1-Interaction Disruptor Ameliorates Insulin Resistance. Exp. Mol. Med. 2018, 50, 1–12. [Google Scholar] [CrossRef] [PubMed]





| Gene | Sequence 5′-3′ |
|---|---|
| IRS1-F | GAACTCACTCGGCAGGCACATC |
| IRS1-R | TGGTGGGTAGGCAGGCATCATC |
| PI3K-F | GAAGCAGCAACCGAAACAAAGC |
| PI3K-R | ACCACTACAGAGCAGGCATAGC |
| Akt-F | AGGATGTGGACCAACGTGAGG |
| Akt-R | GCAGGCAGCGGATGATGAAG |
| G6PC-F | GTCTGTCTGTCACGAATCTACCTTG |
| G6PC-R | ATGCTGTGGATGTGGCTGAAAG |
| PCK1-F | CCAATGCCATCAAGACCATCCAG |
| PCK1-R | TCATCAATGCCTTCCCAGTAAACG |
| GSK3β-F | ACTTCACCACTCAAGAACTGTCAAG |
| GSK3β-R | TGTCCACGGTCTCCAGTATTAGC |
| GYS2-F | AACTAACATCACCACCAACGACAG |
| GYS2-R | CATCCTCCACTTCATCTTCCACATC |
| HK1-F | GCGGCTTGTGCTGCTCAG |
| HK1-R | TCTCCACCTGCGACACGAAG |
| PKLR-F | GAAGGACACGGCATCAAGATCATC |
| PKLR-R | CTCACCTCCAGGATTTCATCAAACC |
| SLC2A4-F | TCCAACAGATAGGCTCCGAAGATG |
| SLC2A4-R | CAAGCACCGCAGAGAACACAG |
| Group\Estimator | Shannon | Simpson | Ace | Chao |
|---|---|---|---|---|
| OR | 3.77 ± 0.05 ab | 0.04 ± 0.00 b | 326.96 ± 23.27 b | 319.18 ± 28.50 b |
| GLP-8 | 3.82 ± 0.28 a | 0.07 ± 0.03 b | 361.64 ± 17.00 a | 366.25 ± 22.29 a |
| LEP-8 | 3.55 ± 0.07 b | 0.07 ± 0.01 b | 335.18 ± 12.14 ab | 330.81 ± 9.72 b |
| CMP-8 | 3.14 ± 0.12 c | 0.11 ± 0.01 a | 323.69 ± 6.66 b | 316.00 ± 6.47 b |
| CON-8 | 3.22 ± 0.02 c | 0.13 ± 0.01 a | 342.45 ± 6.46 ab | 338.92 ± 8.85 ab |
| Metabolite | Metab ID | Mode | CAS ID | Formula |
|---|---|---|---|---|
| Nutriacholic acid | metab_10614 | neg | 4651-67-6 | C24H38O4 |
| N-Methyl-1-deoxynojirimycin | metab_2758 | pos | 69567-10-8 | C7H15NO4 |
| Heptadecanoic acid | metab_1745 | pos | 3546-17-6; 106182-29-0; 506-12-7 | C17H34O2 |
| Gingerglycolipid A | metab_6324 | pos | 145937-22-0 | C33H56O14 |
| Sphinganine 1-phosphate | metab_6251 | pos | 19794-97-9 | C18H40NO5P |
| Lucidenic acid D2 | metab_5169 | pos | 98665-16-8 | C29H38O8 |
| L-Dihydroorotic acid | metab_8809 | pos | 5988-19-2 | C5H6N2O4 |
| Cyclohexanecarboxylic acid | metab_16424 | neg | 98-89-5 | C7H12O2 |
| Matricin | metab_6723 | pos | 29041-35-8 | C17H22O5 |
| D-Pipecolic acid | metab_1245 | pos | 1723-00-8 | C6H11NO2 |
| Sparfloxacin | metab_5420 | pos | 110871-86-8 | C19H22F2N4O3 |
| 4-Imidazolone-5-propionic acid | metab_1435 | pos | 17340-16-8 | C6H8N2O3 |
| Tricarballylic Acid | metab_14608 | neg | 99-14-9 | C6H8O6 |
| Gamma-Eudesmol rhamnoside | metab_14104 | neg | 349112-31-8 | C21H36O5 |
| Ergocornine | metab_7383 | pos | 564-36-3 | C31H39N5O5 |
| N-Carbamoylputrescine | metab_1423 | pos | 6851-51-0 | C5H13N3O |
| Nomega-Acetylhistamine | metab_8547 | pos | 673-49-4 | C7H11N3O |
| Lycorine | metab_5677 | pos | 476-28-8 | C16H17NO4 |
| N6,N6,N6-Trimethyl-L-lysine | metab_5628 | pos | 19253-88-4 | C9H20N2O2 |
| Huperzine b | metab_15873 | neg | 103548-82-9 | C16H20N2O |
| Tyramine | metab_16366 | neg | 51-67-2 | C8H11NO |
| Benzamide | metab_17548 | neg | 55-21-0 | C7H7NO |
| Metabolite | Metab ID | Significant | Regulate | m/z | PCP-8 Mean | PCP-8 SD | PCP-0 Mean | PCP-0 SD |
|---|---|---|---|---|---|---|---|---|
| Nutriacholic acid | metab_10614 | no | up | 389.2708 | 7.554 | 0.01353 | 7.077 | 0.01193 |
| Heptadecanoic acid | metab_1745 | no | down | 288.2891 | 5.621 | 0.02393 | 5.69 | 0.009962 |
| Gingerglycolipid A | metab_6324 | yes | up | 641.3563 | 4.808 | 0.22 | 3.899 | 0.3994 |
| Sphinganine 1-phosphate | metab_6251 | yes | up | 420.228 | 6.113 | 0.04017 | 5.333 | 0.04451 |
| Lucidenic acid D2 | metab_5169 | no | up | 537.2494 | 4.789 | 0.05297 | 4.451 | 0.005533 |
| L-Dihydroorotic acid | metab_8809 | no | up | 200.0681 | 4.333 | 0.07629 | 4.14 | 0.05721 |
| Matricin | metab_6723 | yes | up | 630.3285 | 5.583 | 0.1096 | 4.54 | 0.232 |
| D-Pipecolic acid | metab_1245 | no | up | 152.0679 | 5.621 | 0.03066 | 5.447 | 0.02621 |
| 4-Imidazolone-5-propionic acid | metab_1435 | no | up | 157.0606 | 4.297 | 0.1564 | 3.973 | 0.005532 |
| Tricarballylic Acid | metab_14608 | yes | up | 175.0246 | 4.863 | 0.02584 | 3.753 | 0.1764 |
| N-Carbamoylputrescine | metab_1423 | yes | up | 173.1395 | 4.792 | 0.05204 | 3.882 | 0.1446 |
| Nomega-Acetylhistamine | metab_8547 | yes | up | 154.0973 | 4.092 | 0.07222 | 3.285 | 0.03266 |
| Tyramine | metab_16366 | yes | up | 136.0764 | 4.381 | 0.1467 | 3.39 | 0.08128 |
| Benzamide | metab_17548 | yes | up | 166.0507 | 3.711 | 0.08852 | 2.713 | 0.1963 |
| Species Name | Significant | Regulate | PCP-8 Mean | PCP-8 Sd | CON-8 Mean | CON-8 Sd |
|---|---|---|---|---|---|---|
| Bifidobacterium | no | up | 7.044 | 2.573 | 6.118 | 0.8949 |
| Peptoclostridium | no | up | 7.231 | 0.5436 | 4.616 | 1.311 |
| norank_f__Ruminococcaceae | yes | up | 1.721 | 0.06132 | 1.035 | 0.1088 |
| Dorea | yes | up | 4.877 | 0.5175 | 3.409 | 0.4135 |
| Blautia | no | down | 9.106 | 1.041 | 9.34 | 0.6807 |
| Escherichia-Shigella | yes | down | 12.64 | 3.18 | 34.18 | 2.083 |
| Acidaminococcus | yes | down | 0.4363 | 0.07902 | 3.766 | 1.309 |
| Negativibacillus | yes | down | 1.514 | 0.1336 | 2.164 | 0.1598 |
| Faecalibacterium | yes | up | 1.186 | 0.2241 | 0.4235 | 0.1428 |
| Coprococcus | yes | up | 0.3538 | 0.05773 | 0.1825 | 0.02315 |
| Bacteroides | yes | up | 1.963 | 0.1521 | 0.2867 | 0.2351 |
| Eubacterium_ventriosum_group | yes | up | 1.088 | 0.2302 | 0.03385 | 0.02429 |
| Anaerostipes | yes | up | 1.445 | 0.07625 | 0.6959 | 0.05073 |
| Erysipelotrichaceae_UCG-003 | no | up | 0.1617 | 0.0277 | 0.1197 | 0.03493 |
| Parabacteroides | no | up | 1.465 | 0.949 | 0.1544 | 0.1301 |
| Agathobacter | no | up | 2.295 | 0.5811 | 0.9188 | 0.08288 |
| Candidatus_Stoquefichus | yes | up | 0.09542 | 0.00634 | 0.003066 | 0.002658 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, R.; Luo, J.; Liu, L.; Peng, X. Hypoglycemic Effect of Edible Fungi Polysaccharides Depends on Their Metabolites from the Fermentation of Human Fecal Microbiota. Foods 2024, 13, 97. https://doi.org/10.3390/foods13010097
Yu R, Luo J, Liu L, Peng X. Hypoglycemic Effect of Edible Fungi Polysaccharides Depends on Their Metabolites from the Fermentation of Human Fecal Microbiota. Foods. 2024; 13(1):97. https://doi.org/10.3390/foods13010097
Chicago/Turabian StyleYu, Rongxuan, Jianming Luo, Liu Liu, and Xichun Peng. 2024. "Hypoglycemic Effect of Edible Fungi Polysaccharides Depends on Their Metabolites from the Fermentation of Human Fecal Microbiota" Foods 13, no. 1: 97. https://doi.org/10.3390/foods13010097
APA StyleYu, R., Luo, J., Liu, L., & Peng, X. (2024). Hypoglycemic Effect of Edible Fungi Polysaccharides Depends on Their Metabolites from the Fermentation of Human Fecal Microbiota. Foods, 13(1), 97. https://doi.org/10.3390/foods13010097








