Biological Activity of Lactic Acid Bacteria Exopolysaccharides and Their Applications in the Food and Pharmaceutical Industries
Abstract
:1. Introduction
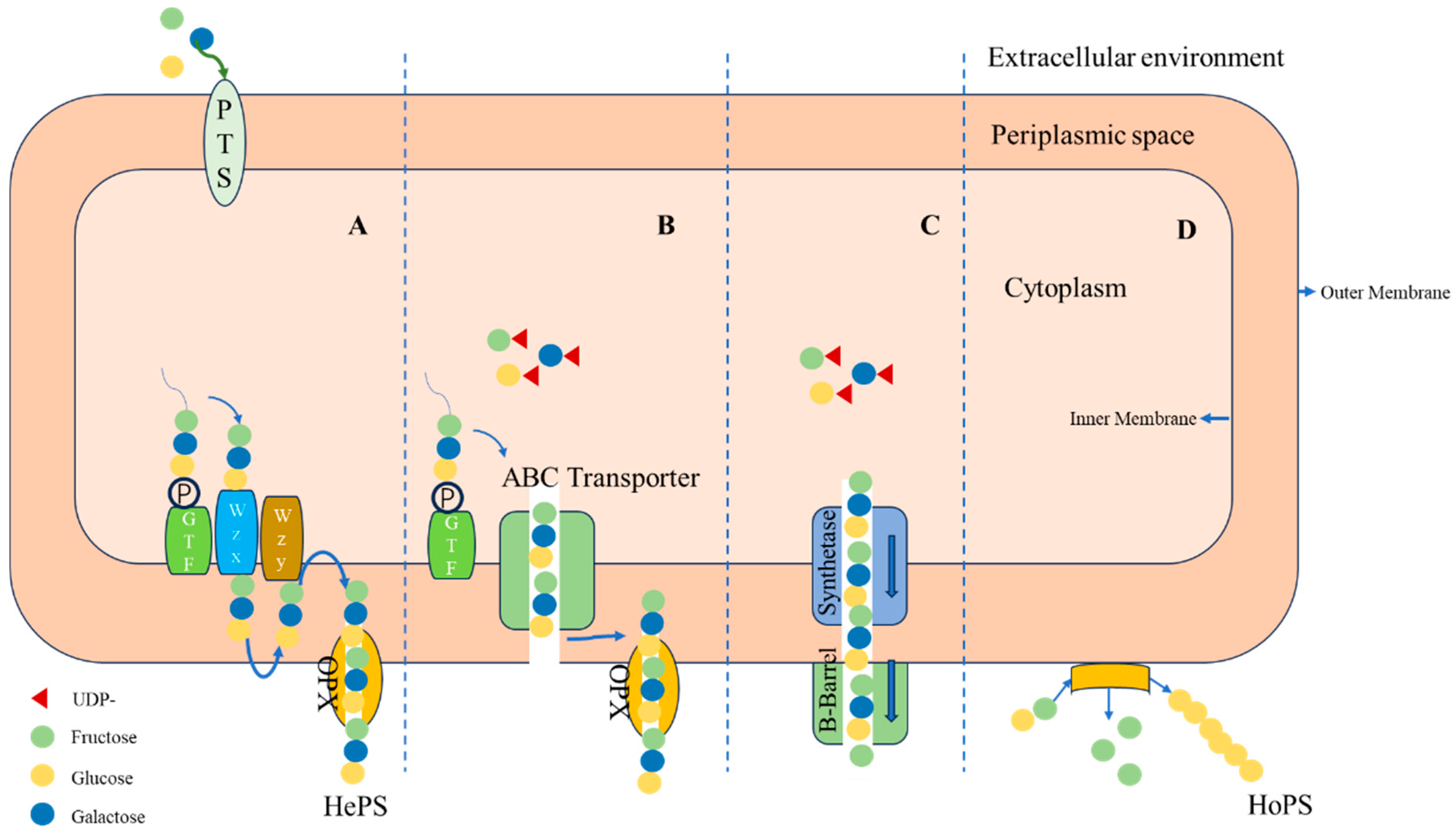
2. Biosynthesis of EPS Produced by LAB
3. The Biological Activity of EPS Produced by LAB
3.1. Antioxidant
3.2. Immunoregulation
3.3. Antitumor Activity
3.4. Regulating Gut Microbiota
3.5. Anti-Biofilm
3.6. Hypoglycemic
4. Application of EPS Produced by LAB
4.1. The Application of EPS Produced by LAB in the Food Industry
4.1.1. Fermented Dairy Products
4.1.2. Plant-Based Dairy Products
4.1.3. Cheese
4.1.4. Bakery Products
4.1.5. Meat Products
4.2. Application of EPS Produced by LAB in the Pharmaceutical Industry
4.2.1. Drug Delivery
4.2.2. Biopolymer Delivery
4.2.3. Gene Delivery
4.2.4. Diagnosis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, Y.-W.; Liong, M.-T.; Tsai, Y.-C. New perspectives of Lactobacillus plantarum as a probiotic: The gut-heart-brain axis. J. Microbiol. 2018, 56, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Hakim, B.N.A.; Xuan, N.J.; Oslan, S.N.H. A Comprehensive Review of Bioactive Compounds from Lactic Acid Bacteria: Potential Functions as Functional Food in Dietetics and the Food Industry. Foods 2023, 12, 2850. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Castillo, V.; Marcial, G.; Albarracin, L.; Tomokiyo, M.; Clua, P.; Takahashi, H.; Kitazawa, H.; Garcia-Cancino, A.; Villena, J. The Exopolysaccharide of Lactobacillus fermentum UCO-979C Is Partially Involved in Its Immunomodulatory Effect and Its Ability to Improve the Resistance against Helicobacter pylori Infection. Microorganisms 2020, 8, 479. [Google Scholar] [CrossRef] [PubMed]
- Cescutti, P. Bacterial capsular polysaccharides and exopolysaccharides. In Microbial Glycobiology; Elsevier: Amsterdam, The Netherlands, 2010; pp. 93–108. [Google Scholar]
- Silva, L.A.; Pereira Lopes Neto, J.H.; Cardarelli, H.R. Exopolysaccharides produced by Lactobacillus plantarum: Technological properties, biological activity, and potential application in the food industry. Ann. Microbiol. 2019, 69, 321–328. [Google Scholar] [CrossRef]
- Saadat, Y.R.; Khosroushahi, A.Y.; Gargari, B.P. A comprehensive review of anticancer, immunomodulatory and health beneficial effects of the lactic acid bacteria exopolysaccharides. Carbohydr. Polym. 2019, 217, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Saravanakumar, K.; Sathiyaseelan, A.; Han, K.-S.; Lee, J.; Wang, M.-H. Polysaccharides of Weissella cibaria Act as a Prebiotic to Enhance the Probiotic Potential of Lactobacillus rhamnosus. Appl. Biochem. Biotechnol. 2023, 195, 3928–3940. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Dey, P. Bacterial exopolysaccharides as emerging bioactive macromolecules: From fundamentals to applications. Res. Microbiol. 2023, 174, 104024. [Google Scholar] [CrossRef] [PubMed]
- Salazar, N.; Gueimonde, M.; de los Reyes-Gavilan, C.G.; Ruas-Madiedo, P. Exopolysaccharides Produced by Lactic Acid Bacteria and Bifidobacteria as Fermentable Substrates by the Intestinal Microbiota. Crit. Rev. Food Sci. Nutr. 2016, 56, 1440–1453. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Liu, S.; Liang, S.; Xiang, F.; Wang, X.; Lian, H.; Li, B.; Liu, F. Exopolysaccharides of lactic acid bacteria: Structure, biological activity, structure-activity relationship, and application in the food industry: A review. Int. J. Biol. Macromol. 2024, 257, 128733. [Google Scholar] [CrossRef]
- Prado, M.R.M.; Boller, C.; Zibetti, R.G.M.; de Souza, D.; Pedroso, L.L.; Soccol, C.R. Anti-inflammatory and angiogenic activity of polysaccharide extract obtained from Tibetan kefir. Microvasc. Res. 2016, 108, 29–33. [Google Scholar] [CrossRef]
- Sharma, K.; Sharma, N.; Handa, S.; Pathania, S. Purification and characterization of novel exopolysaccharides produced from Lactobacillus paraplantarum KM1 isolated from human milk and its cytotoxicity. J. Genet. Eng. Biotechnol. 2020, 18, 56. [Google Scholar] [CrossRef] [PubMed]
- Vasanthakumari, D.S.; Harikumar, S.; Beena, D.J.; Pandey, A.; Nampoothiri, K.M. Physicochemical Characterization of an Exopolysaccharide Produced by a Newly Isolated Weissella cibaria. Appl. Biochem. Biotechnol. 2015, 176, 440–453. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, O.N.; Sasmal, S.; Kataria, A.K.; Devi, I. Application of microbial extracellular carbohydrate polymeric substances in food and allied industries. 3 Biotech 2020, 10, 221. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Dong, S.; Qu, X. New progress in the identifying regulatory factors of exopolysaccharide synthesis in lactic acid bacteria. World J. Microb. Biot. 2023, 39, 301. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.S.; Han, X.P.; Ye, M.Z.; Li, Y.; Wang, X.; Zhong, Q.P. Exopolysaccharides synthesized by lactic acid bacteria: Biosynthesis pathway, structure-function relationship, structural modification and applicability. Crit. Rev. Food Sci. Nutr. 2023, 63, 7043–7064. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.-T.; Nguyen, T.-T.; Bui, D.-C.; Hong, P.-T.; Hoang, Q.-K.; Nguyen, H.-T. Exopolysaccharide production by lactic acid bacteria: The manipulation of environmental stresses for industrial applications. AIMS Microbiol. 2020, 6, 451–469. [Google Scholar] [CrossRef]
- Donot, F.; Fontana, A.; Baccou, J.C.; Schorr-Galindo, S. Microbial exopolysaccharides: Main examples of synthesis, excretion, genetics and extraction. Carbohydr. Polym. 2012, 87, 951–962. [Google Scholar] [CrossRef]
- Schmid, J.; Sieber, V.; Rehm, B. Bacterial exopolysaccharides: Biosynthesis pathways and engineering strategies. Front. Microbiol. 2015, 6, 147519. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Fan, C.; Liu, S.; Zang, Z.; Jiao, L.; Zhang, L. Chemical composition and antitumor activity of polysaccharide from Inonotus obliquus. J. Med. Plants Res. 2011, 5, 1251–1260. [Google Scholar]
- Mozzi, F.; Vaningelgem, F.; Hebert, E.M.; Van der Meulen, R.; Foulquie Moreno, M.R.; Font de Valdez, G.; De Vuyst, L. Diversity of heteropolysaccharide-producing lactic acid bacterium strains and their biopolymers. Appl. Environ. Microbiol. 2006, 72, 4431–4435. [Google Scholar] [CrossRef]
- Daba, G.M.; Elnahas, M.O.; Elkhateeb, W.A. Contributions of exopolysaccharides from lactic acid bacteria as biotechnological tools in food, pharmaceutical, and medical applications. Int. J. Biol. Macromol. 2021, 173, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Lynch, K.M.; Coffey, A.; Arendt, E.K. Exopolysaccharide producing lactic acid bacteria: Their techno-functional role and potential application in gluten-free bread products. Food Res. Int. 2018, 110, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhang, J. Bacterial exopolysaccharides: Chemical structures, gene clusters and genetic engineering. Int. J. Biol. Macromol. 2021, 173, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Ale, E.C.; Rojas, M.F.; Reinheimer, J.A.; Binetti, A.G. Lactobacillus fermentum: Could EPS production ability be responsible for functional properties? Food Microbiol. 2020, 90, 103465. [Google Scholar] [CrossRef] [PubMed]
- Rajoka, M.S.R.; Wu, Y.; Mehwish, H.M.; Bansal, M.; Zhao, L. Lactobacillus exopolysaccharides: New perspectives on engineering strategies, physiochemical functions, and immunomodulatory effects on host health. Trends Food Sci. Technol. 2020, 103, 36–48. [Google Scholar] [CrossRef]
- Zhao, X.; Liang, Q.; Song, X.; Zhang, Y. Whole genome sequence of Lactiplantibacillus plantarum MC5 and comparative analysis of eps gene clusters. Front. Microbiol. 2023, 14, 1146566. [Google Scholar] [CrossRef] [PubMed]
- Deo, D.; Davray, D.; Kulkarni, R. A Diverse Repertoire of Exopolysaccharide Biosynthesis Gene Clusters in Lactobacillus Revealed by Comparative Analysis in 106 Sequenced Genomes. Microorganisms 2019, 7, 444. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [PubMed]
- Sahiner, M.; Yilmaz, A.S.; Gungor, B.; Ayoubi, Y.; Sahiner, N. Therapeutic and Nutraceutical Effects of Polyphenolics from Natural Sources. Molecules 2022, 27, 6225. [Google Scholar] [CrossRef]
- Trabelsi, I.; Ktari, N.; Ben Slima, S.; Triki, M.; Bardaa, S.; Mnif, H.; Ben Salah, R. Evaluation of dermal wound healing activity and in vitro antibacterial and antioxidant activities of a new exopolysaccharide produced by Lactobacillus sp.Ca6. Int. J. Biol. Macromol. 2017, 103, 194–201. [Google Scholar] [CrossRef]
- Amiri, S.; Rezaei Mokarram, R.; Sowti Khiabani, M.; Rezazadeh Bari, M.; Alizadeh Khaledabad, M. Exopolysaccharides production by Lactobacillus acidophilus LA5 and Bifidobacterium animalis subsp. lactis BB12: Optimization of fermentation variables and characterization of structure and bioactivities. Int. J. Biol. Macromol. 2019, 123, 752–765. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Han, S.; Zhou, J.; Xu, Q.; Dong, M.; Fan, X.; Rui, X.; Chen, X.; Zhang, Q.; Li, W. Preparation, characterization and antioxidant activities of derivatives of exopolysaccharide from Lactobacillus helveticus MB2-1. Int. J. Biol. Macromol. 2020, 145, 1008–1017. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Dong, L.; Jia, K.; Zhan, H.; Zhang, Z.; Shah, N.P.; Tao, X.; Wei, H. Sulfonation of Lactobacillus plantarum WLPL04 exopolysaccharide amplifies its antioxidant activities in vitro and in a Caco-2 cell model. J. Dairy Sci. 2019, 102, 5922–5932. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Cui, Y.; Qu, X. Exopolysaccharides of lactic acid bacteria: Structure, bioactivity and associations: A review. Carbohydr. Polym. 2019, 207, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Mei, X. Antioxidant activity of an exopolysaccharide purified from Lactococcus lactis subsp lactis 12. Carbohydr. Polym. 2010, 80, 908–914. [Google Scholar] [CrossRef]
- Yang, X.; Ren, Y.; Zhang, L.; Wang, Z.; Li, L. Structural characteristics and antioxidant properties of exopolysaccharides isolated from soybean protein gel induced by lactic acid bacteria. LWT-Food Sci. Technol. 2021, 150, 111811. [Google Scholar] [CrossRef]
- Yue, Y.; Wang, Y.; Han, Y.; Zhang, Y.; Cao, T.; Huo, G.; Li, B. Genome Analysis of Bifidobacterium Bifidum E3, Structural Characteristics, and Antioxidant Properties of Exopolysaccharides. Foods 2023, 12, 2988. [Google Scholar] [CrossRef] [PubMed]
- Tarannum, N.; Hossain, T.J.; Ali, F.; Das, T.; Dhar, K.; Nafiz, I.H. Antioxidant, antimicrobial and emulsification properties of exopolysaccharides from lactic acid bacteria of bovine milk: Insights from biochemical and genomic analysis. LWT 2023, 186, 115263. [Google Scholar] [CrossRef]
- Jurášková, D.; Ribeiro, S.C.; Silva, C.C.G. Exopolysaccharides Produced by Lactic Acid Bacteria: From Biosynthesis to Health-Promoting Properties. Foods 2022, 11, 156. [Google Scholar] [CrossRef]
- Li, W.; Ji, J.; Chen, X.; Jiang, M.; Rui, X.; Dong, M. Structural elucidation and antioxidant activities of exopolysaccharides from Lactobacillus helveticus MB2-1. Carbohydr. Polym. 2014, 102, 351–359. [Google Scholar] [CrossRef]
- Ding, J.; Chen, P.; Qi, C. Circadian rhythm regulation in the immune system. Immunology 2024, 171, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Wienecke, L.M.; Cohen, S.; Bauersachs, J.; Mebazaa, A.; Chousterman, B.G. Immunity and inflammation: The neglected key players in congenital heart disease? Heart Fail. Rev. 2022, 27, 1957–1971. [Google Scholar] [CrossRef] [PubMed]
- Angelin, J.; Kavitha, M. Exopolysaccharides from probiotic bacteria and their health potential. Int. J. Biol. Macromol. 2020, 162, 853–865. [Google Scholar] [CrossRef] [PubMed]
- You, X.; Li, Z.; Ma, K.; Zhang, C.; Chen, X.; Wang, G.; Yang, L.; Dong, M.; Rui, X.; Zhang, Q.; et al. Structural characterization and immunomodulatory activity of an exopolysaccharide produced by Lactobacillus helveticus LZ-R-5. Carbohydr. Polym. 2020, 235, 115977. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Hao, X.; Aziz, T.; Zhang, J.; Yang, Z. Exopolysaccharides from Lactobacillus plantarum YW11 improve immune response and ameliorate inflammatory bowel disease symptoms. Acta Biochim. Pol. 2020, 67, 485–493. [Google Scholar]
- Ciszek-Lenda, M.; Nowak, B.; Sróttek, M.; Gamian, A.; Marcinkiewicz, J. Immunoregulatory potential of exopolysaccharide from Lactobacillus rhamnosus KL37: Effects on the production of inflammatory mediators by mouse macrophages. Int. J. Exp. Pathol. 2011, 92, 382–391. [Google Scholar] [CrossRef]
- Wang, X.; Tian, J.; Zhang, X.; Tang, N.; Rui, X.; Zhang, Q.; Dong, M.; Li, W. Characterization and Immunological Activity of Exopolysaccharide from Lacticaseibacillus paracasei GL1 Isolated from Tibetan Kefir Grains. Foods 2022, 11, 3330. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Han, J.; Zhu, S.; Wang, Y.; Zhang, W.; Wu, Z. Structural elucidation of the exopolysaccharide from Streptococcus thermophilus XJ53 and the effect of its molecular weight on immune activity. Int. J. Biol. Macromol. 2023, 230, 123177. [Google Scholar] [CrossRef] [PubMed]
- Wallimann, A.; Hildebrand, M.; Groeger, D.; Stanic, B.; Akdis, C.A.; Zeiter, S.; Richards, R.G.; Moriarty, T.F.; O’Mahony, L.; Thompson, K. An Exopolysaccharide Produced by Bifidobacterium longum 35624® Inhibits Osteoclast Formation via a TLR2-Dependent Mechanism. Calcif. Tissue Int. 2021, 108, 654–666. [Google Scholar] [CrossRef]
- Liu, S.; Li, H.; Dong, Y.; Zhang, D. Case Report: Tumor-to-tumor metastasis with prostate cancer metastatic to lung cancer: The first reported case. Front. Oncol. 2023, 13, 1238331. [Google Scholar] [CrossRef]
- Fukurnura, D.; Kloepper, J.; Amoozgar, Z.; Duda, D.G.; Jain, R.K. Enhancing cancer immunotherapy using antiangiogenics: Opportunities and challenges. Nat. Rev. Clin. Oncol. 2018, 15, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, Y.; Ye, L.; Wang, C. The anti-cancer effects and mechanisms of lactic acid bacteria exopolysaccharides in vitro: A review. Carbohydr. Polym. 2021, 253, 117308. [Google Scholar] [CrossRef] [PubMed]
- Mojibi, P.; Tafvizi, F.; Bikhof Torbati, M. Cell-bound Exopolysaccharide Extract from Indigenous Probiotic Bacteria Induce Apoptosis in HT-29 cell-line. Iran. J. Pathol. 2019, 14, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Shiomi, M.; Sasaki, K.; Murofushi, M.; Aibara, K. Antitumor activity in mice of orally administered polysaccharide from Kefir grain. Jpn. J. Med. Sci. Biol. 1982, 35, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Kitazawa, H.; Yamaguchi, T.; Miura, M.; Saito, T.; Itoh, T. B-cell mitogen produced by slime-forming, encapsulated Lactococcus lactis ssp. cremoris isolated from ropy sour milk, viili. J. Dairy Sci. 1993, 76, 1514–1519. [Google Scholar] [CrossRef] [PubMed]
- Fujiike, A.Y.; Lee, C.; Rodrigues, F.S.T.; Oliveira, L.C.B.; Barbosa-Dekker, A.M.; Dekker, R.F.H.; Cólus, I.M.S.; Serpeloni, J.M. Anticancer effects of carboxymethylated (1→3)(1→6)-β-D-glucan (botryosphaeran) on multicellular tumor spheroids of MCF-7 cells as a model of breast cancer. J. Toxicol. Environ. Health Part A 2022, 85, 521–537. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Jiao, X.; Zhao, J.; Liao, X.; Wei, Y.; Li, Q. Antitumor mechanisms of an exopolysaccharide from Lactobacillus fermentum on HT-29 cells and HT-29 tumor-bearing mice. Int. J. Biol. Macromol. 2022, 209, 552–562. [Google Scholar] [CrossRef] [PubMed]
- Caggianiello, G.; Kleerebezem, M.; Spano, G. Exopolysaccharides produced by lactic acid bacteria: From health-promoting benefits to stress tolerance mechanisms. Appl. Microbiol. Biotechnol. 2016, 100, 3877–3886. [Google Scholar] [PubMed]
- Bengoa, A.A.; Dardis, C.; Gagliarini, N.; Garrote, G.L.; Abraham, A.G. Exopolysaccharides From Lactobacillus paracasei Isolated From Kefir as Potential Bioactive Compounds for Microbiota Modulation. Front. Microbiol. 2020, 11, 583254. [Google Scholar] [CrossRef]
- Kuang, J.-H.; Huang, Y.-Y.; Hu, J.-S.; Yu, J.-J.; Zhou, Q.-Y.; Liu, D.-M. Exopolysaccharides from Bacillus amyloliquefaciens DMBA-K4 ameliorate dextran sodium sulfate-induced colitis via gut microbiota modulation. J. Funct. Foods 2020, 75, 104212. [Google Scholar]
- Li, B.; Chen, H.; Cao, L.; Hu, Y.; Chen, D.; Yin, Y. Effects of an Escherichia coli exopolysaccharide on human and mouse gut microbiota in vitro. Int. J. Biol. Macromol. 2020, 150, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Tarique, M.; Ali, A.H.; Kizhakkayil, J.; Gan, R.-Y.; Liu, S.-Q.; Kamal-Eldin, A.; Ayyash, M. Investigating the biological activities and prebiotic potential of exopolysaccharides Produced by Lactobacillus delbrueckii and Lacticaseibacillus rhamnosus: Implications for gut microbiota modulation and rheological properties in fermented milk. Food Hydrocoll. Health 2023, 4, 100162. [Google Scholar] [CrossRef]
- Martinez, B.; Rodriguez, A.; Kulakauskas, S.; Chapot-Chartier, M.-P. Cell wall homeostasis in lactic acid bacteria: Threats and defences. FEMS Microbiol. Rev. 2020, 44, 538–564. [Google Scholar] [CrossRef] [PubMed]
- Kavanaugh, J.S.; Flack, C.E.; Lister, J.; Ricker, E.B.; Ibberson, C.B.; Jenul, C.; Moormeier, D.E.; Delmain, E.A.; Bayles, K.W.; Horswill, A.R. Identification of Extracellular DNA-Binding Proteins in the Biofilm Matrix. Mbio 2019, 10, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Fulaz, S.; Vitale, S.; Quinn, L.; Casey, E. Nanoparticle-Biofilm Interactions: The Role of the EPS Matrix. Trends Microbiol. 2019, 27, 915–926. [Google Scholar] [CrossRef] [PubMed]
- Mgomi, F.C.; Yang, Y.-R.; Cheng, G.; Yang, Z.-Q. Lactic acid bacteria biofilms and their antimicrobial potential against pathogenic microorganisms. Biofilm 2023, 5, 100118. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Sun, M.; Feng, L.; Liang, X.; Song, X.; Mu, G.; Tuo, Y.; Jiang, S.; Qian, F. Antibiofilm Activity of Lactobacillus plantarum 12 Exopolysaccharides against Shigella flexneri. Appl. Environ. Microbiol. 2020, 86, e00694-20. [Google Scholar] [CrossRef] [PubMed]
- Mahdhi, A.; Leban, N.; Chakroun, I.; Chaouch, M.A.; Hafsa, J.; Fdhila, K.; Mahdouani, K.; Majdoub, H. Extracellular polysaccharide derived from potential probiotic strain with antioxidant and antibacterial activities as a prebiotic agent to control pathogenic bacterial biofilm formation. Microb. Pathog. 2017, 109, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Sarikaya, H.; Aslim, B.; Yuksekdag, Z. Assessment of anti-biofilm activity and bifidogenic growth stimulator (BGS) effect of lyophilized exopolysaccharides (l-EPSs) from Lactobacilli strains. Int. J. Food Prop. 2017, 20, 362–371. [Google Scholar] [CrossRef]
- Ahmed, S.; Saeed, S.; Shubrook, J.H. Masqueraders: How to identify atypical diabetes in primary care. J. Osteopath. Med. 2021, 121, 899–904. [Google Scholar] [CrossRef]
- Bradley, D. The Intriguing Intersection of Type 2 Diabetes, Obesity-Related Insulin Resistance, and Osteoarthritis Comment. J. Clin. Endocrinol. Metab. 2021, 106, E2370–E2372. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, H.; Ashrafizadeh, M.; Oroojan, A.A. Type 2 Diabetes Mellitus and Osteoarthritis: The Role of Glucose Transporters. Clin. Rev. Bone Miner. Metab. 2020, 18, 1–17. [Google Scholar] [CrossRef]
- Yan, J.; Peng, D.; Jiang, F.; Zhang, R.; Hu, C.; Jia, W. Impaired pancreatic β-cell compensatory function in Chinese patients with type 2 diabetes and high genetic risk: A 9-year prospective cohort study. Lancet Diabetes Endocrinol. 2016, 4, S32. [Google Scholar] [CrossRef]
- Wang, G.; Li, X.; Zhao, J.; Zhang, H.; Chen, W. Lactobacillus casei CCFM419 attenuates type 2 diabetes via a gut microbiota dependent mechanism (vol 8, pg 3155, 2017). Food Funct. 2017, 8, 3814. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liang, X.; Lv, Y.; Yi, H.; Chen, Y.; Bai, L.; Zhou, H.; Liu, T.; Li, R.; Zhang, L. Evaluation of probiotics for improving and regulation metabolism relevant to type 2 diabetes in vitro. J. Funct. Foods 2020, 64, 103664. [Google Scholar] [CrossRef]
- Huang, Z.; Lin, F.; Zhu, X.; Zhang, C.; Jiang, M.; Lu, Z. An exopolysaccharide from Lactobacillus plantarum H31 in pickled cabbage inhibits pancreas α-amylase and regulating metabolic markers in HepG2 cells by AMPK/PI3K/Akt pathway. Int. J. Biol. Macromol. 2020, 143, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Lanza, B.; Zago, M.; Di Marco, S.; Di Loreto, G.; Cellini, M.; Tidona, F.; Bonvini, B.; Bacceli, M.; Simone, N. Single and Multiple Inoculum of Lactiplantibacillus plantarum Strains in Table Olive Lab-Scale Fermentations. Fermentation 2020, 6, 126. [Google Scholar] [CrossRef]
- Li, P.; Bai, Y.; Yang, Y.; Li, M. Structural characterization of exopolysaccharide produced by Streptococcus thermophilus and rheology of mixed protein-polysaccharide systems. Food Biosci. 2023, 55, 102961. [Google Scholar] [CrossRef]
- Zhang, K.; Tang, H.; Farid, M.S.; Xiang, F.; Li, B. Effect of Lactobacillus helveticus exopolysaccharides molecular weight on yogurt gel properties and its internal mechanism. Int. J. Biol. Macromol. 2024, 262 Pt 1, 130006. [Google Scholar] [CrossRef]
- Angelov, A.; Georgieva, A.; Petkova, M.; Bartkiene, E.; Rocha, J.M.; Ognyanov, M.; Gotcheva, V. On the Molecular Selection of Exopolysaccharide-Producing Lactic Acid Bacteria from Indigenous Fermented Plant-Based Foods and Further Fine Chemical Characterization. Foods 2023, 12, 3346. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, S.; Tang, H.; Evivie, S.E.; Guo, Z.; Li, B. Effect of exopolysaccharides yield and addition concentration of Lactobacillus helveticus on the processing characteristics of fermented milk and its mechanism. Int. J. Biol. Macromol. 2024, 260, 129480. [Google Scholar] [CrossRef]
- Zannini, E.; Jeske, S.; Lynch, K.M.; Arendt, E.K. Development of novel quinoa-based yoghurt fermented with dextran producer Weissella cibaria MG1. Int. J. Food Microbiol. 2018, 268, 19–26. [Google Scholar] [CrossRef]
- Galle, S.; Schwab, C.; Arendt, E.K.; Gaenzle, M.G. Structural and rheological characterisation of heteropolysaccharides produced by lactic acid bacteria in wheat and sorghum sourdough. Food Microbiol. 2011, 28, 547–553. [Google Scholar] [CrossRef]
- Di Cagno, R.; De Pasquale, I.; De Angelis, M.; Buchin, S.; Rizzello, C.G.; Gobbetti, M. Use of microparticulated whey protein concentrate, exopolysaccharide-producing Streptococcus thermophilus, and adjunct cultures for making low-fat Italian Caciotta-type cheese. J. Dairy Sci. 2014, 97, 72–84. [Google Scholar] [CrossRef]
- Wang, L.; Gu, Y.; Zheng, X.; Zhang, Y.; Deng, K.; Wu, T.; Cheng, H. Analysis of physicochemical properties of exopolysaccharide from Leuconostoc mesenteroides strain XR1 and its application in fermented milk. LWT-Food Sci. Technol. 2021, 146, 111449. [Google Scholar] [CrossRef]
- Ge, Z.; Bao, X.; Li, Z.; Chen, X.; Li, W.; Rui, X.; Wu, J.; Zhang, Q.; Dong, M. In Situ exopolysaccharides produced by Lactobacillus helveticus MB2-1 and its effect on gel properties of Sayram ketteki yoghurt. Int. J. Biol. Macromol. 2022, 208, 314–323. [Google Scholar] [CrossRef]
- Cao, F.; Liang, M.; Liu, J.; Liu, Y.; Renye Jr, J.A.; Qi, P.X.; Ren, D. Characterization of an exopolysaccharide (EPS-3A) produced by Streptococcus thermophilus ZJUIDS-2-01 isolated from traditional yak yogurt. Int. J. Biol. Macromol. 2021, 192, 1331–1343. [Google Scholar] [CrossRef]
- Zhao, X.; Liang, Q. EPS-Producing Lactobacillus plantarum MC5 as a Compound Starter Improves Rheology, Texture, and Antioxidant Activity of Yogurt during Storage. Foods 2022, 11, 1660. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, X.; Pan, W.; Shen, X.; He, Y.; Yin, H.; Zhou, K.; Zou, L.; Chen, S.; Liu, S. Exopolysaccharides produced by yogurt-texture improving Lactobacillus plantarum RS20D and the immunoregulatory activity. Int. J. Biol. Macromol. 2019, 121, 342–349. [Google Scholar] [CrossRef]
- Ramos, I.M.; Sesena, S.; Poveda, J.M.; Palop, M.L. Screening of Lactic Acid Bacteria Strains to Improve the Properties of Non-fat Set Yogurt by in situ EPS Production. Food Bioprocess Technol. 2023, 16, 2541–2558. [Google Scholar] [CrossRef]
- Tiwari, S.; Kavitake, D.; Devi, P.B.; Shetty, P.H. Bacterial exopolysaccharides for improvement of technological, functional and rheological properties of yoghurt. Int. J. Biol. Macromol. 2021, 183, 1585–1595. [Google Scholar] [CrossRef]
- Prasanna, P.H.P.; Grandison, A.S.; Charalampopoulos, D. Screening human intestinal Bifidobacterium strains for growth, acidification, EPS production and viscosity potential in low-fat milk. Int. Dairy J. 2012, 23, 36–44. [Google Scholar] [CrossRef]
- Bai, Y.; Liu, N. Effect of Exopolysaccharide Produced by Lactobacillus casei HS4 on Microstructure and Rheological Properties of Fermented Milk. Food Sci. 2019, 40, 145–152. [Google Scholar]
- Bruls, M.; Foroutanparsa, S.; Olsthoorn, M.; Tas, R.P.; Voets, I.K. Investigating the impact of exopolysaccharides on yogurt network mechanics and syneresis through quantitative microstructural analysis. Food Hydrocoll. 2024, 150, 109629. [Google Scholar] [CrossRef]
- Gentes, M.-C.; St-Gelais, D.; Turgeon, S.L. Exopolysaccharide-milk protein interactions in a dairy model system simulating yoghurt conditions. Dairy Sci. Technol. 2013, 93, 255–271. [Google Scholar] [CrossRef]
- Buldo, P.; Benfeldt, C.; Folkenberg, D.M.; Jensen, H.B.; Amigo, J.M.; Sieuwerts, S.; Thygesen, K.; van den Berg, F.; Ipsen, R. The role of exopolysaccharide-producing cultures and whey protein ingredients in yoghurt. LWT-Food Sci. Technol. 2016, 72, 189–198. [Google Scholar] [CrossRef]
- Yang, X.; Ren, Y.; Li, L. The relationship between charge intensity and bioactivities/processing characteristics of exopolysaccharides from lactic acid bacteria. LWT-Food Sci. Technol. 2022, 153, 112345. [Google Scholar] [CrossRef]
- Kristo, E.; Miao, Z.; Corredig, M. The role of exopolysaccharide produced by Lactococcus lactis subsp cremoris in structure formation and recovery of acid milk gels. Int. Dairy J. 2011, 21, 656–662. [Google Scholar] [CrossRef]
- Ripari, V.; Ganzle, M.G.; Berardi, E. Evolution of sourdough microbiota in spontaneous sourdoughs started with different plant materials. Int. J. Food Microbiol. 2016, 232, 35–42. [Google Scholar] [CrossRef]
- Masia, C.; Geppel, A.; Jensen, P.E.; Buldo, P. Effect of Lactobacillus rhamnosus on Physicochemical Properties of Fermented Plant-Based Raw Materials. Foods 2021, 10, 1182. [Google Scholar] [CrossRef]
- Ripari, V. Techno-Functional Role of Exopolysaccharides in Cereal-Based, Yogurt-Like Beverages. Beverages 2019, 5, 16. [Google Scholar] [CrossRef]
- Russo, P.; de Chiara, M.L.V.; Capozzi, V.; Arena, M.P.; Amodio, M.L.; Rascon, A.; Teresa Duenas, M.; Lopez, P.; Spano, G. Lactobacillus plantarum strains for multifunctional oat-based foods. LWT-Food Sci. Technol. 2016, 68, 288–294. [Google Scholar] [CrossRef]
- Lorusso, A.; Coda, R.; Montemurro, M.; Rizzello, C.G. Use of Selected Lactic Acid Bacteria and Quinoa Flour for Manufacturing Novel Yogurt-Like Beverages. Foods 2018, 7, 51. [Google Scholar] [CrossRef]
- Ayyash, M.; Abu-Jdayil, B.; Hamed, F.; Shaker, R. Rheological, textural, microstructural and sensory impact of exopolysaccharide-producing Lactobacillus plantarum isolated from camel milk on low-fat akawi cheese. LWT-Food Sci. Technol. 2018, 87, 423–431. [Google Scholar] [CrossRef]
- Wang, X.; Xu, J.; Li, J.; Cheng, Y.; Liu, L.; Du, Z. Effect of regional arterial infusion combined with early enteral nutrition on severe acute pancreatitis. J. Int. Med. Res. 2019, 47, 6235–6243. [Google Scholar] [CrossRef]
- Yang, F.; Xie, J.; Wang, W.; Xie, Y.; Sun, H.; Jin, Y.; Xu, D.; Chen, B.; Andersson, R.; Zhou, M. Regional arterial infusion with lipoxin A4 attenuates experimental severe acute pancreatitis. PLoS ONE 2014, 9, e108525. [Google Scholar] [CrossRef]
- Patlan-Velázquez, L.-F.; González-Olivares, L.-G.; García-Garibay, M.; Alatorre-Santamaría, S.; Gómez-Ruiz, L.; Rodríguez-Serrano, G.; Cruz-Guerrero, A. Effect of biogenic exopolysaccharides in characteristics and stability of a novel Requeson-type cheese. J. Food Biosci. 2024, 59, 103896. [Google Scholar] [CrossRef]
- Surber, G.; Spiegel, T.; Bich Phuong, D.; Pombo, A.W.; Rohm, H.; Jaros, D. Cream cheese made with exopolysaccharide-producing Lactococcus lactis: Impact of strain and curd homogenization pressure on texture and syneresis. J. Food Eng. 2021, 308, 110664. [Google Scholar] [CrossRef]
- Trancoso-Reyes, N.; Gutiérrez-Méndez, N.; Sepulveda, D.R.; Hernández-Ochoa, L.R. Assessing the yield, microstructure, and texture properties of miniature Chihuahua-type cheese manufactured with a phospholipase A1 and exopolysaccharide-producing bacteria. J. Dairy Sci. 2014, 97, 598–608. [Google Scholar] [CrossRef]
- Abedfar, A.; Hosseininezhad, M.; Rafe, A. Effect of microbial exopolysaccharide on wheat bran sourdough: Rheological, thermal and microstructural characteristics. Int. J. Biol. Macromol. 2020, 154, 371–379. [Google Scholar] [CrossRef]
- Galle, S.; Arendt, E.K. Exopolysaccharides from sourdough lactic acid bacteria. Crit. Rev. Food Sci. Nutr. 2014, 54, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Lynch, K.M.; Zannini, E.; Coffey, A.; Arendt, E.K. Lactic Acid Bacteria Exopolysaccharides in Foods and Beverages: Isolation, Properties, Characterization, and Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 155–176. [Google Scholar] [CrossRef] [PubMed]
- Wolter, A.; Hager, A.S.; Zannini, E.; Galle, S.; Gänzle, M.G.; Waters, D.M.; Arendt, E.K. Evaluation of exopolysaccharide producing Weissella cibaria MG1 strain for the production of sourdough from various flours. Food Microbiol. 2014, 37, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Compaoré-Sérémé, D.; Sawadogo-Lingani, H.; Coda, R.; Katina, K.; Maina, N.H. Influence of dextran synthesized in situ on the rheological, technological and nutritional properties of whole grain pearl millet bread. Food Chem. 2019, 285, 221–230. [Google Scholar] [CrossRef]
- Korcz, E.; Varga, L. Exopolysaccharides from lactic acid bacteria: Techno-functional application in the food industry. Trends Food Sci. Technol. 2021, 110, 375–384. [Google Scholar] [CrossRef]
- Wang, Y.; Han, J.; Wang, D.; Gao, F.; Zhang, K.; Tian, J.; Jin, Y. Research Update on the Impact of Lactic Acid Bacteria on the Substance Metabolism, Flavor, and Quality Characteristics of Fermented Meat Products. Foods 2022, 11, 2090. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, L.; Liu, Q.; Wang, Y.; Chen, Q.; Kong, B. The potential correlation between bacterial diversity and the characteristic volatile flavour of traditional dry sausages from Northeast China. Food Microbiol. 2020, 91, 103505. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.T.; Dertli, E.; Toker, O.S.; Tatlisu, N.B.; Sagdic, O.; Arici, M. Effect of in situ exopolysaccharide production on physicochemical, rheological, sensory, and microstructural properties of the yogurt drink ayran: An optimization study based on fermentation kinetics. J. Dairy Sci. 2015, 98, 1604–1624. [Google Scholar] [CrossRef] [PubMed]
- Hilbig, J.; Gisder, J.; Prechtl, R.M.; Herrmann, K.; Weiss, J.; Loeffler, M. Influence of exopolysaccharide-producing lactic acid bacteria on the spreadability of fat-reduced raw fermented sausages (Teewurst). Food Hydrocoll. 2019, 93, 422–431. [Google Scholar] [CrossRef]
- Dertli, E.; Yilmaz, M.T.; Tatlisu, N.B.; Toker, O.S.; Cankurt, H.; Sagdic, O. Effects of in situ exopolysaccharide production and fermentation conditions on physicochemical, microbiological, textural and microstructural properties of Turkish-type fermented sausage (sucuk). Meat Sci. 2016, 121, 156–165. [Google Scholar] [CrossRef]
- Xu, H.; Li, Y.; Song, J.; Zhou, L.; Wu, K.; Lu, X.; Zhai, X.; Wan, Z.; Gao, J. Highly active probiotic hydrogels matrixed on bacterial EPS accelerate wound healing via maintaining stable skin microbiota and reducing inflammation. Bioact. Mater. 2024, 35, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Jiao, Y.; Wang, Y.; Zhou, C.; Zhang, Z. Polysaccharides-based nanoparticles as drug delivery systems. Adv. Drug Deliv. Rev. 2008, 60, 1650–1662. [Google Scholar] [CrossRef] [PubMed]
- Andrew, M.; Jayaraman, G. Molecular Characterization and Biocompatibility of Exopolysaccharide Produced by Moderately Halophilic Bacterium Virgibacillus dokdonensis from the Saltern of Kumta Coast. Polymers 2022, 14, 3986. [Google Scholar] [CrossRef] [PubMed]
- Mohd Nadzir, M.; Nurhayati, R.W.; Idris, F.N.; Nguyen, M.H. Biomedical Applications of Bacterial Exopolysaccharides: A Review. Polymers 2021, 13, 530. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Qamar, M.; Qamar, S.A.; Khan, M.I.; Bilal, M.; Iqbal, H.M.N. Insight of nanomedicine strategies for a targeted delivery of nanotherapeutic cues to cope with the resistant types of cancer stem cells. J. Drug Deliv. Sci. Technol. 2021, 64, 102681. [Google Scholar] [CrossRef]
- Brar, V.; Kaur, G. Biopolymers as Carriers for Nasal Drug Delivery. Polym.-Plast. Technol. Eng. 2014, 53, 1518–1531. [Google Scholar] [CrossRef]
- Pradeepa; Vidya, S.M.; Mutalik, S.; Bhat, K.U.; Huilgol, P.; Avadhani, K. Preparation of gold nanoparticles by novel bacterial exopolysaccharide for antibiotic delivery. Life Sci. 2016, 153, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Kalimuthu, A.K.; Pandian, S.R.K.; Pavadai, P.; Panneerselvam, T.; Kabilan, S.J.; Sankaranarayanan, M.; Ala, C.; Kunjiappan, S. Drug Delivery Applications of Exopolysaccharides from Endophytic Bacteria Pseudomonas otitidis from Tribulus terrestris L. J. Polym. Environ. 2023, 31, 3632–3649. [Google Scholar] [CrossRef]
- Sarmento, B.; Ferreira, D.; Veiga, F.; Ribeiro, A. Characterization of insulin-loaded alginate nanoparticles produced by ionotropic pre-gelation through DSC and FTIR studies. Carbohydr. Polym. 2006, 66, 1–7. [Google Scholar] [CrossRef]
- Moscovici, M. Present and future medical applications of microbial exopolysaccharides. Front. Microbiol. 2015, 6, 137616. [Google Scholar] [CrossRef]
- Ferreira Albani, S.M.; da Silva, M.R.; Fratelli, F.; Cardoso Junior, C.P.; Iourtov, D.; Cintra, F.d.O.; Takagi, M.; Cabrera-Crespo, J. Polysaccharide purification from Haemophilus influenzae type b through tangential microfiltration. Carbohydr. Polym. 2015, 116, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Raemdonck, K.; Naeye, B.; Hogset, A.; Demeester, J.; De Smedt, S.C. Prolonged gene silencing by combining siRNA nanogels and photochemical internalization. J. Control. Release 2010, 145, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Cai, J.; Borjihan, W.; Ganbold, T.; Rana, T.M.; Baigude, H. Preparation of novel curdlan nanoparticles for intracellular siRNA delivery. Carbohydr. Polym. 2015, 117, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.A.A.; Hsu, F.-T.; Hsieh, C.-L.; Shiau, C.-Y.; Chiang, C.-H.; Wei, Z.-H.; Chen, C.-Y.; Huang, H.-S. Erlotinib- Conjugated Iron Oxide Nanoparticles as a Smart Cancer-Targeted Theranostic Probe for MRI. Sci. Rep. 2016, 6, 36650. [Google Scholar] [CrossRef] [PubMed]
- Qiao, R.; Yang, C.; Gao, M. Superparamagnetic iron oxide nanoparticles: From preparations to in vivo MRI applications. J. Mater. Chem. 2009, 19, 6274–6293. [Google Scholar] [CrossRef]
- Prajapati, V.D.; Jani, G.K.; Khanda, S.M. Pullulan: An exopolysaccharide and its various applications. Carbohydr. Polym. 2013, 95, 540–549. [Google Scholar] [CrossRef]
- Kong, S.-H.; Noh, Y.-W.; Suh, Y.-S.; Park, H.S.; Lee, H.-J.; Kang, K.W.; Kim, H.C.; Lim, Y.T.; Yang, H.-K. Evaluation of the novel near-infrared fluorescence tracers pullulan polymer nanogel and indocyanine green/γ-glutamic acid complex for sentinel lymph node navigation surgery in large animal models. Gastric Cancer 2015, 18, 55–64. [Google Scholar] [CrossRef]

| Strain | Monosaccharide Composition | Application | Reference |
|---|---|---|---|
| Lactiplantibacillus plantarum B51 | - | Shorten the debittering time of olives and inhibit cell apoptosis | [78] |
| Lactiplantibacillus paraplantarum KM1 | Glucose, galactose, and mannose | Emulsifier | [12] |
| Streptococcus salivarius subsp. thermophilus | Glucose, galactose, and rhamnose | Increase viscosity | [79] |
| Lacticaseibacillus rhamnosus LH43 | Mannose, rhamnose, galacturonic acid, glucose, and galactose | Increase yogurt viscosity and improve texture | [80] |
| Lactiplantibacillus plantarum | - | Improve the rheological properties of plant-based milk | [81] |
| Lacticaseibacillus rhamnosus LH18 | Mannose, rhamnose, galacturonic acid, glucose, and galactose | Improve yogurt gel properties | [82] |
| Weissella cibaria MG1 | - | Improve the water-holding capacity of quinoa milk | [83] |
| Lactobacillus buchneri FUA3154 | Glucose, galactose, and rhamnose | Affect the rheological properties of sorghum dough | [84] |
| Streptococcus salivarius subsp. thermophilus | - | Increase the flavor and chewiness of cheese | [85] |
| Leuconostoc mesenteroides XR1 | Glucose and galactose | Improve the flocculation and thermal stability of yogurt | [86] |
| Lactobacillus helveticus MB2-1 | Mannose, rhamnose, glucuronic acid, glucose, galactose, arabinose, and fucose | Improve the viscosity, texture, and microstructure of fermented milk | [87] |
| Streptococcus salivarius subsp. Thermophilus ZJUIDS-2-01 | Glucose, galactose, N-acetyl-D-galactosamine, and rhamnose | Improve the emulsification and flocculation characteristics | [88] |
| Lactiplantibacillus plantarum MC5 | - | Increase apparent viscosity and elastic modulus, reduce dehydration shrinkage | [89] |
| Lactiplantibacillus plantarum RS20D | Glucose, galactose, and glucosamine | Improve texture and reduce dehydration shrinkage | [90] |
| Levilactobacillus brevis UCLM-Lb47, Leuconostoc mesenterides subsp. mesenteroides 6F6-12 and Leuconostoc mesenterides subsp. mesenteroides 2F6-9 | - | High water-holding capacity and oral viscosity value, improving texture | [91] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, S.; Wang, X.; Li, C.; Liu, L. Biological Activity of Lactic Acid Bacteria Exopolysaccharides and Their Applications in the Food and Pharmaceutical Industries. Foods 2024, 13, 1621. https://doi.org/10.3390/foods13111621
Liang S, Wang X, Li C, Liu L. Biological Activity of Lactic Acid Bacteria Exopolysaccharides and Their Applications in the Food and Pharmaceutical Industries. Foods. 2024; 13(11):1621. https://doi.org/10.3390/foods13111621
Chicago/Turabian StyleLiang, Shengnan, Xinyu Wang, Chun Li, and Libo Liu. 2024. "Biological Activity of Lactic Acid Bacteria Exopolysaccharides and Their Applications in the Food and Pharmaceutical Industries" Foods 13, no. 11: 1621. https://doi.org/10.3390/foods13111621




