From Extraction to Stabilization: Employing a 22 Experimental Design in Developing Nutraceutical-Grade Bixin from Bixa orellana L.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
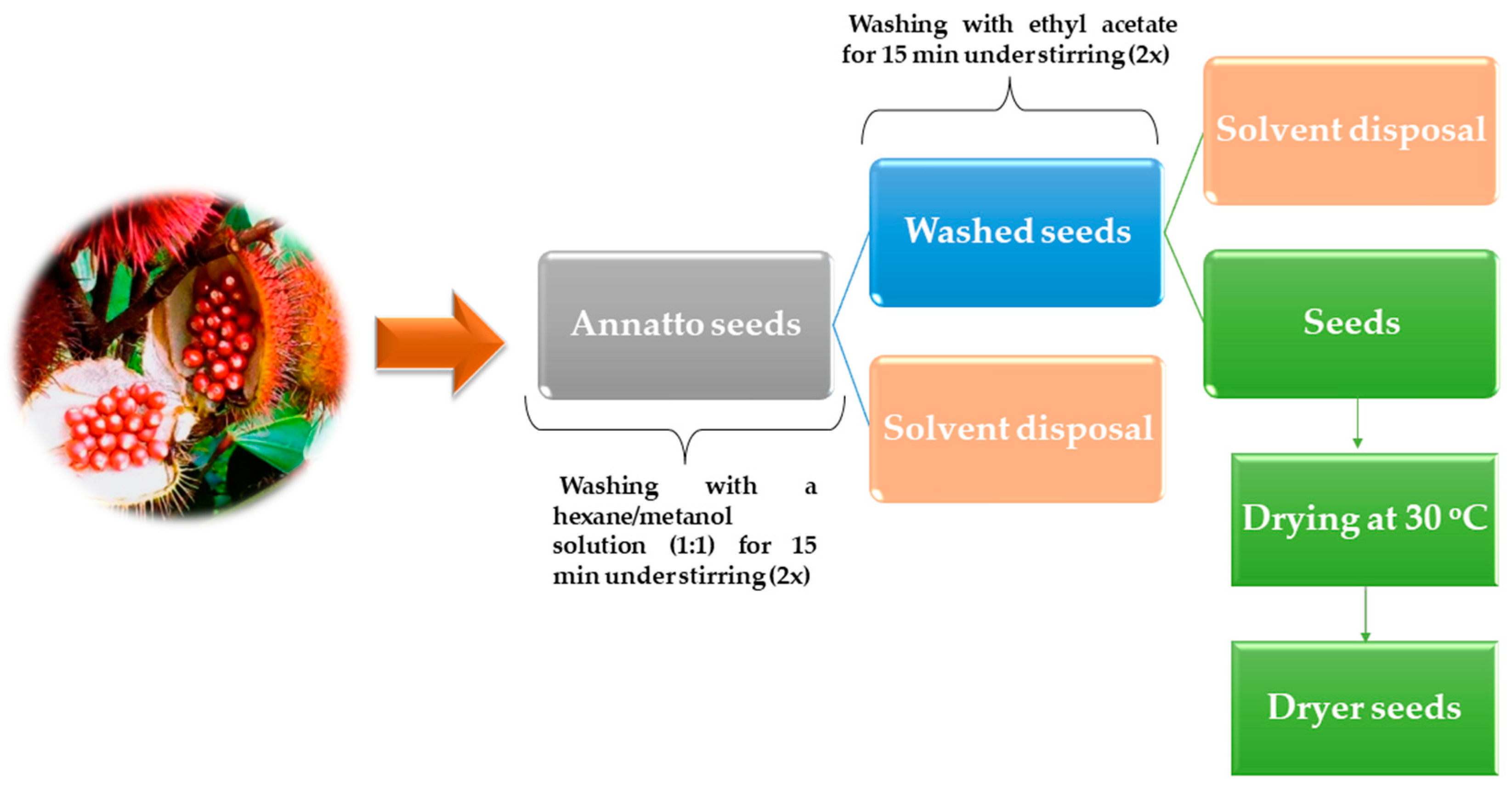
2.2. Bixin Extraction
2.3. Spectrophotometric Analysis of Bixin in Different Solvents
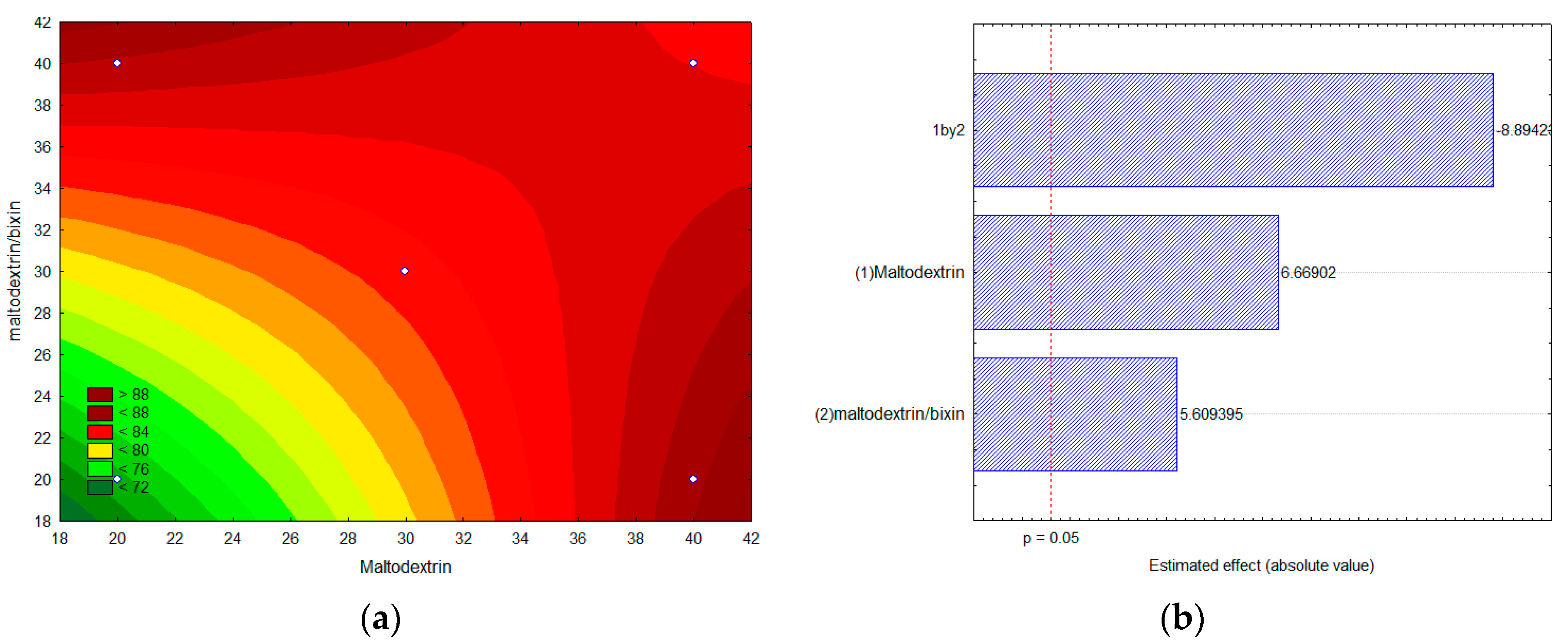
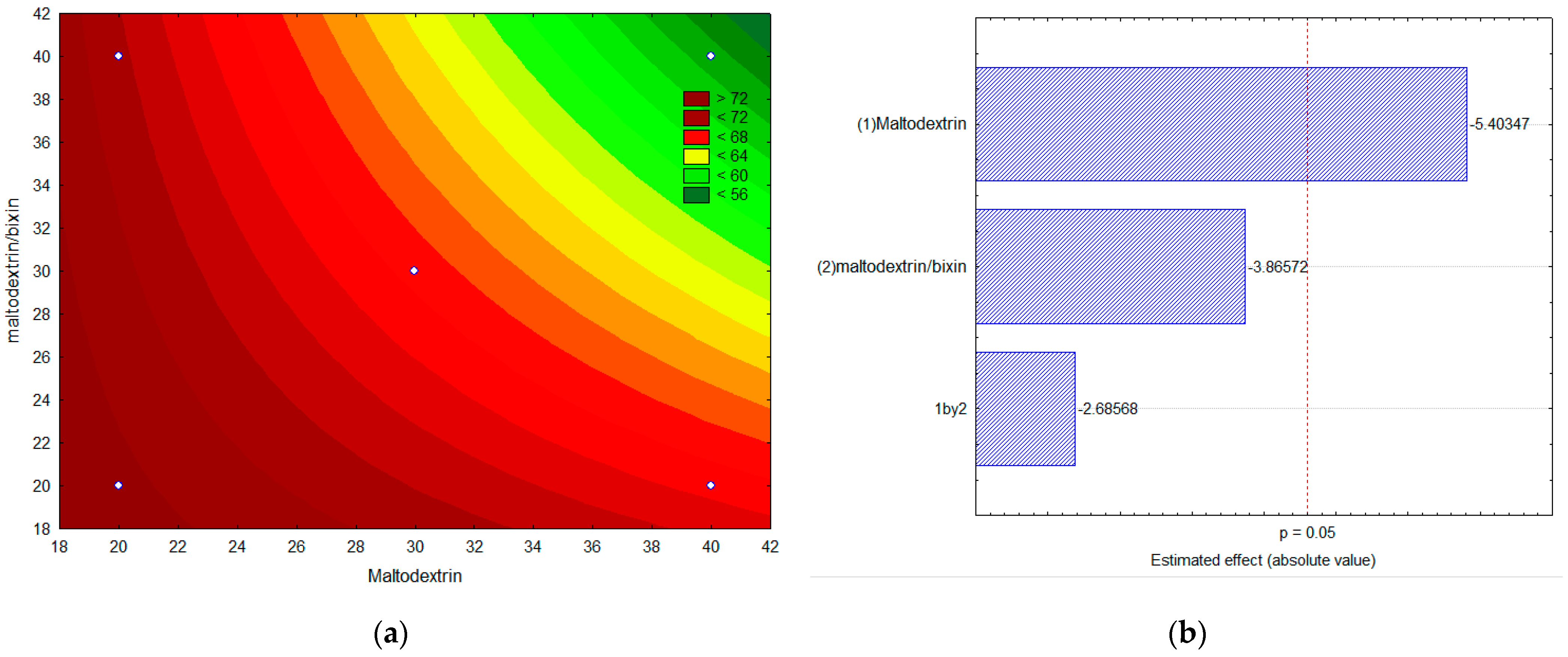
2.4. Experimental Factorial Design for the Encapsulation of Bixin by Lyophilization
2.5. Encapsulation Efficiency
2.6. Solubility in Water
2.7. Moisture
2.8. HPLC Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Molina-Romani, S.P.; Bonilla-Rivera, P.E.; de Albuquerque, R.D.D.G. A review of Bixa orellana L. (Annatto) leaves as medicinal resource: Use in the population as complementary medicine, phytotherapeutic action and quality parameters. Nat. Resour. Hum. Health 2023, 3, 277–285. [Google Scholar] [CrossRef]
- Souza, L.F.; Pagno, C.H.; Medeiros, N.d.S.; Barbosa, S.; dos Santos, P.C.; Rios, A.; Achaval, M.; Jong, E.V.d. The effect of the carotenoid bixin and annatto seeds on hematological markers and nephrotoxicity in rats subjected to chronic treatment with cisplatin. Rev. Bras. Farmacogn. 2016, 26, 446–450. [Google Scholar] [CrossRef]
- Rojas, V.; Custodio, M.Á.C.; Arnaiz, V. Use of an additive canthaxanthin based and annatto extract in diets of laying hens and its effect on the color of the yolk and the egg shelf life. Sci. Agropecu. 2015, 6, 191–199. Available online: http://www.revistas.unitru.edu.pe/index.php/scientiaagrop/article/view/957 (accessed on 25 March 2024). [CrossRef]
- Scotter, M. The chemistry and analysis of annatto food colouring: A review. Food Addit. Contam. 2009, 26, 1123–1145. [Google Scholar] [CrossRef]
- Capella, S.; Tillmann, M.; Félix, A.; Fontoura, E.; Fernandes, C.; Freitag, R.; Santos, M.; Félix, S.; Nobre, M. Therapeutic potential of Bixa orellana L. in skin wounds: A study in the rat model of open wound healing. Arq. Bras. Med. Veterinária Zootec. 2016, 68, 104–112. [Google Scholar] [CrossRef]
- Ashraf, A.; Ijaz, M.U.; Muzammil, S.; Nazir, M.M.; Zafar, S.; Zihad, S.M.N.K.; Uddin, S.J.; Hasnain, M.S.; Nayak, A.K. The role of bixin as antioxidant, anti-inflammatory, anticancer, and skin protecting natural product extracted from Bixa orellana L. Fitoterapia 2023, 169, 105612. [Google Scholar] [CrossRef] [PubMed]
- Mercadante, A.Z. Composition of Carotenoids from Annatto. In Chemistry and Physiology of Selected Food Colorants; American Chemical Society: Washington, DC, USA, 2001; Volume 775, pp. 92–101. [Google Scholar]
- Selvi, A.T.; Aravindhan, R.; Madhan, B.; Rao, J.R. Studies on the application of natural dye extract from Bixa orellana seeds for dyeing and finishing of leather. Ind. Crops Prod. 2013, 43, 84–86. [Google Scholar] [CrossRef]
- Rahmalia, W.; Fabre, J.-F.; Usman, T.; Mouloungui, Z. Aprotic solvents effect on the UV–visible absorption spectra of bixin. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2014, 131, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Humeau, C.; Rovel, B.; Girardin, M. Enzymatic esterification of bixin by L-ascorbic acid. Biotechnol. Lett. 2000, 22, 165–168. [Google Scholar] [CrossRef]
- Jahangiri, A.; Møller, A.H.; Danielsen, M.; Madsen, B.; Joernsgaard, B.; Vaerbak, S.; Adlercreutz, P.; Dalsgaard, T.K. Hydrophilization of bixin by lipase-catalyzed transesterification with sorbitol. Food Chem. 2018, 268, 203–209. [Google Scholar] [CrossRef]
- Wrolstad, R.E.; Culver, C.A. Alternatives to those artificial FD&C food colorants. Annu. Rev. Food Sci. Technol. 2012, 3, 59–77. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Concepcion, M.; Avalos, J.; Bonet, M.L.; Boronat, A.; Gomez-Gomez, L.; Hornero-Mendez, D.; Limon, M.C.; Meléndez-Martínez, A.J.; Olmedilla-Alonso, B.; Palou, A.; et al. A global perspective on carotenoids: Metabolism, biotechnology, and benefits for nutrition and health. Prog. Lipid Res. 2018, 70, 62–93. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, M.A.; Rios, A.d.O.; Mercadante, A.Z.; Nazareno, M.A.; Borsarelli, C.D. Model studies on the photosensitized isomerization of bixin. J. Agric. Food Chem. 2004, 52, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Zielinska, A.; da Ana, R.; Fonseca, J.; Szalata, M.; Wielgus, K.; Fathi, F.; Oliveira, M.; Staszewski, R.; Karczewski, J.; Souto, E.B. Phytocannabinoids: Chromatographic Screening of Cannabinoids and Loading into Lipid Nanoparticles. Molecules 2023, 28, 2875. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.B.; Zielinska, A.; Souto, S.B.; Durazzo, A.; Lucarini, M.; Santini, A.; Silva, A.M.; Atanasov, A.G.; Marques, C.; Andrade, L.N.; et al. (+)-Limonene 1,2-Epoxide-Loaded SLNs: Evaluation of Drug Release, Antioxidant Activity, and Cytotoxicity in an HaCaT Cell Line. Int. J. Mol. Sci. 2020, 21, 1449. [Google Scholar] [CrossRef]
- Souto, E.B.; Souto, S.B.; Zielinska, A.; Durazzo, A.; Lucarini, M.; Santini, A.; Horbanczuk, O.K.; Atanasov, A.G.; Marques, C.; Andrade, L.N.; et al. Perillaldehyde 1,2-epoxide Loaded SLN-Tailored mAb: Production, Physicochemical Characterization and In Vitro Cytotoxicity Profile in MCF-7 Cell Lines. Pharmaceutics 2020, 12, 161. [Google Scholar] [CrossRef] [PubMed]
- Favaro-Trindade, C.S.; de Matos Junior, F.E.; Okuro, P.K.; Dias-Ferreira, J.; Cano, A.; Severino, P.; Zielinska, A.; Souto, E.B. Encapsulation of Active Pharmaceutical Ingredients in Lipid Micro/Nanoparticles for Oral Administration by Spray-Cooling. Pharmaceutics 2021, 13, 1186. [Google Scholar] [CrossRef] [PubMed]
- Zielinska, A.; Carreiro, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef]
- Culas, M.S.; Popovich, D.G.; Rashidinejad, A. Recent advances in encapsulation techniques for cinnamon bioactive compounds: A review on stability, effectiveness, and potential applications. Food Biosci. 2024, 57, 103470. [Google Scholar] [CrossRef]
- Xiao, Z.; Xia, J.; Zhao, Q.; Niu, Y.; Zhao, D. Maltodextrin as wall material for microcapsules: A review. Carbohydr. Polym. 2022, 298, 120113. [Google Scholar] [CrossRef]
- Todorović, A.; Šturm, L.; Salević-Jelić, A.; Lević, S.; Osojnik Črnivec, I.G.; Prislan, I.; Skrt, M.; Bjeković, A.; Poklar Ulrih, N.; Nedović, V. Encapsulation of Bilberry Extract with Maltodextrin and Gum Arabic by Freeze-Drying: Formulation, Characterisation, and Storage Stability. Processes 2022, 10, 1991. [Google Scholar] [CrossRef]
- Ferreira, M.A.; de Almeida Júnior, R.F.; Onofre, T.S.; Casadei, B.R.; Farias, K.J.S.; Severino, P.; de Oliveira Franco, C.F.; Raffin, F.N.; de Lima e Moura, T.F.A.; de Melo Barbosa, R. Annatto Oil Loaded Nanostructured Lipid Carriers: A Potential New Treatment for Cutaneous Leishmaniasis. Pharmaceutics 2021, 13, 1912. [Google Scholar] [CrossRef]
- Barbosa, R.d.M.; Leite, A.M.; García-Villén, F.; Sánchez-Espejo, R.; Cerezo, P.; Viseras, C.; Faccendini, A.; Sandri, G.; Raffin, F.N. Hybrid Lipid/Clay Carrier Systems Containing Annatto Oil for Topical Formulations. Pharmaceutics 2022, 14, 1067. [Google Scholar] [CrossRef]
- Campelo, P.H.; do Carmo, E.L.; Zacarias, R.D.; Yoshida, M.I.; Ferraz, V.P.; de Barros Fernandes, R.V.; Botrel, D.A.; Borges, S.V. Effect of dextrose equivalent on physical and chemical properties of lime essential oil microparticles. Ind. Crops Prod. 2017, 102, 105–114. [Google Scholar] [CrossRef]
- Van Eck, N.J.; Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef]
- Spada, J.C.; Marczak, L.D.F.; Tessaro, I.C.; Noreña, C.P.Z. Microencapsulation of β-carotene using native pinhão starch, modified pinhão starch and gelatin by freeze-drying. Int. J. Food Sci. Technol. 2012, 47, 186–194. [Google Scholar] [CrossRef]
- Eastman, J.E.; Moore, C.O. Cold-Water-Soluble Granular Starch for Gelled Food Compositions, Staley Continental Inc (Assignee). U.S. Patent 4465702A, 14 August 1984. [Google Scholar]
- Rahmalia, W.; Fabre, J.-F.; Mouloungui, Z. Effects of Cyclohexane/Acetone Ratio on Bixin Extraction Yield by Accelerated Solvent Extraction Method. Procedia Chem. 2015, 14, 455–464. [Google Scholar] [CrossRef]
- Albuquerque, C.L.; Meireles, M.A.A. Defatting of annatto seeds using supercritical carbon dioxide as a pretreatment for the production of bixin: Experimental, modeling and economic evaluation of the process. J. Supercrit. Fluids 2012, 66, 86–95. [Google Scholar] [CrossRef]
- Alcázar-Alay, S.C.; Osorio-Tobón, J.F.; Forster-Carneiro, T.; Meireles, M.A.A. Obtaining bixin from semi-defatted annatto seeds by a mechanical method and solvent extraction: Process integration and economic evaluation. Food Res. Int. 2017, 99, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Demczuk, B., Jr.; Ribani, R.H. Updates on chemistry and use of annatto (Bixa orellana L.). Braz. J. Food Res. 2015, 6, 37–50. [Google Scholar] [CrossRef]
- Carvalho, P.R.N.; Silva, M.G.D.; Fabri, E.G.; Tavares, P.E.D.R.; Martins, A.L.M.; Spatti, L.R. Concentração de bixina e lipídios em sementes de urucum da coleção do Instituto Agronômico (IAC). Bragantia 2010, 69, 519–524. [Google Scholar] [CrossRef]
- Chisté, R.C.; Yamashita, F.; Gozzo, F.C.; Mercadante, A.Z. Simultaneous extraction and analysis by high performance liquid chromatography coupled to diode array and mass spectrometric detectors of bixin and phenolic compounds from annatto seeds. J. Chromatogr. A 2011, 1218, 57–63. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.-S. Carotenoid extraction methods: A review of recent developments. Food Chem. 2018, 240, 90–103. [Google Scholar] [CrossRef]
- Cardeñosa, V.; Lunar, M.L.; Rubio, S. Generalized and rapid supramolecular solvent-based sample treatment for the determination of annatto in food. J. Chromatogr. A 2011, 1218, 8996–9002. [Google Scholar] [CrossRef]
- Generalić Mekinić, I.; Šimat, V.; Rathod, N.B.; Hamed, I.; Čagalj, M. Algal Carotenoids: Chemistry, Sources, and Application. Foods 2023, 12, 2768. [Google Scholar] [CrossRef] [PubMed]
- Polyakov, N.E.; Leshina, T.V.; Konovalova, T.A.; Hand, E.O.; Kispert, L.D. Inclusion complexes of carotenoids with cyclodextrins: 1HNMR, EPR, and optical studies. Free Radic. Biol. Med. 2004, 36, 872–880. [Google Scholar] [CrossRef]
- Tay-Agbozo, S.; Street, S.; Kispert, L.D. The carotenoid bixin: Optical studies of aggregation in polar/water solvents. J. Photochem. Photobiol. A Chem. 2018, 362, 31–39. [Google Scholar] [CrossRef]
- Hempel, J.; Schädle, C.N.; Leptihn, S.; Carle, R.; Schweiggert, R.M. Structure related aggregation behavior of carotenoids and carotenoid esters. J. Photochem. Photobiol. A Chem. 2016, 317, 161–174. [Google Scholar] [CrossRef]
- Curi-Borda, C.K.; Linares-Pastén, J.A.; Tat, T.; Tarqui-Dueñas, R.; Chino-Flores, N.; Alvarado, J.-A.; Bergenstahl, B. Multilayer bixin microcapsules: The impact of native carbohydrates on the microencapsulation efficiency and dispersion stability. Foods 2019, 8, 108. [Google Scholar] [CrossRef] [PubMed]
- De Sousa Lobato, K.B.; Paese, K.; Forgearini, J.C.; Guterres, S.S.; Jablonski, A.; de Oliveira Rios, A. Characterisation and stability evaluation of bixin nanocapsules. Food Chem. 2013, 141, 3906–3912. [Google Scholar] [CrossRef]
- Barbosa, M.; Borsarelli, C.; Mercadante, A. Light stability of spray-dried bixin encapsulated with different edible polysaccharide preparations. Food Res. Int. 2005, 38, 989–994. [Google Scholar] [CrossRef]
- Rodríduez-Huezo, M.; Pedroza-Islas, R.; Prado-Barragán, L.; Beristain, C.; Vernon-Carter, E. Microencapsulation by spray drying of multiple emulsions containing carotenoids. J. Food Sci. 2004, 69, 351–359. [Google Scholar] [CrossRef]
- Shu, B.; Yu, W.; Zhao, Y.; Liu, X. Study on microencapsulation of lycopene by spray-drying. J. Food Eng. 2006, 76, 664–669. [Google Scholar] [CrossRef]
- Pérez-Monterroza, E.J.; Chaux-Gutiérrez, A.M.; Franco, C.M.L.; Nicoletti, V.R. Encapsulation of bixin with high amylose starch as affected by temperature and whey protein. Food Biophys. 2018, 13, 343–352. [Google Scholar] [CrossRef]
- Santos, A.B.d.; Fávaro-Trindade, C.S.; Grosso, C.R.F. Preparation and Characterization of Paprika Oleosin Microcapsules Obtained by Spray Drying. Ciência E Tecnol. Aliment. 2005, 25, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Sousdaleff, M.; Baesso, M.L.; Medina Neto, A.; Nogueira, A.C.; Marcolino, V.A.; Matioli, G. Microencapsulation by freeze-drying of potassium norbixinate and curcumin with maltodextrin: Stability, solubility, and food application. J. Agric. Food Chem. 2013, 61, 955–965. [Google Scholar] [CrossRef]
- Berton-Carabin, C.C.; Schroën, K. Pickering emulsions for food applications: Background, trends, and challenges. Annu. Rev. Food Sci. Technol. 2015, 6, 263–297. [Google Scholar] [CrossRef]
- Janiszewska, E. Microencapsulated beetroot juice as a potential source of betalain. Powder Technol. 2014, 264, 190–196. [Google Scholar] [CrossRef]
- Tupuna, D.S.; Paese, K.; Guterres, S.S.; Jablonski, A.; Flôres, S.H.; Rios, A.d.O. Encapsulation efficiency and thermal stability of norbixin microencapsulated by spray-drying using different combinations of wall materials. Ind. Crops Prod. 2018, 111, 846–855. [Google Scholar] [CrossRef]
- Shadisvaaran, S.; Chin, K.-Y.; Mohd-Said, S.; Leong, X.-F. Therapeutic potential of bixin on inflammation: A mini review. Front. Nutr. 2023, 10, 1209248. [Google Scholar] [CrossRef]
- Stoll, L.; Maillard, M.-N.; Le Roux, E.; Hickmann Flôres, S.; Nachtigall, S.M.B.; Rios, A.; Domenek, S. Bixin, a performing natural antioxidant in active food packaging for the protection of oxidation sensitive food. LWT 2023, 180, 114730. [Google Scholar] [CrossRef]





| Variable | −1 | 0 | +1 |
|---|---|---|---|
| Maltodextrin concentration (%) | 20 | 30 | 40 |
| Bixin/maltodextrin ratio (Mb/Me) * | 1:20 | 1:30 | 1:40 |
| Essay | Maltodextrin Concentration (%) | Bixin/Maltodextrin Ratio (Mb/Me) * |
|---|---|---|
| 1 | −1 | −1 |
| 2 | −1 | +1 |
| 3 | +1 | −1 |
| 4 | +1 | +1 |
| 5 | 0 | 0 |
| 6 | 0 | 0 |
| 7 | 0 | 0 |
| 8 | 0 | 0 |
| 9 | 0 | 0 |
| RT * (min) | Area | Volume (µL) | Concentration (g/L) |
|---|---|---|---|
| 6.05 | 3527.4 | 20 | 0.1350 |
| 5.67 | 4224.3 | 30 | 0.1078 |
| 5.79 | 6514.8 | 40 | 0.1247 |
| 5.68 | 7018.6 | 50 | 0.1074 |
| Average ± s.d. | 0.12 ± 0.01 |
| Bixin Mass (µg) | Area | RT * (min) |
|---|---|---|
| 0.00 | 0.00 | 0.00 |
| 0.64 | 525.60 | 5.63 |
| 1.28 | 1281.30 | 5.63 |
| 3.84 | 4952.60 | 5.67 |
| 5.12 | 6821.40 | 5.67 |
| 5.76 | 7570.80 | 5.69 |
| Component | Concentration (%) (Mean ± s.d. *) |
|---|---|
| Ash | 9.73 ± 0.45 |
| Proteins | 12.93 ± 0.15 |
| Lipids | 2.12 ± 0.20 |
| Moisture | 10.18 ± 0.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luna-Finkler, C.L.; Gomes, A.d.C.; de Aguiar Júnior, F.C.A.; Ribeiro, E.; de Melo Barbosa, R.; Severino, P.; Santini, A.; Souto, E.B. From Extraction to Stabilization: Employing a 22 Experimental Design in Developing Nutraceutical-Grade Bixin from Bixa orellana L. Foods 2024, 13, 1622. https://doi.org/10.3390/foods13111622
Luna-Finkler CL, Gomes AdC, de Aguiar Júnior FCA, Ribeiro E, de Melo Barbosa R, Severino P, Santini A, Souto EB. From Extraction to Stabilization: Employing a 22 Experimental Design in Developing Nutraceutical-Grade Bixin from Bixa orellana L. Foods. 2024; 13(11):1622. https://doi.org/10.3390/foods13111622
Chicago/Turabian StyleLuna-Finkler, Christine L., Aralí da C. Gomes, Francisco C. A. de Aguiar Júnior, Ester Ribeiro, Raquel de Melo Barbosa, Patricia Severino, Antonello Santini, and Eliana B. Souto. 2024. "From Extraction to Stabilization: Employing a 22 Experimental Design in Developing Nutraceutical-Grade Bixin from Bixa orellana L." Foods 13, no. 11: 1622. https://doi.org/10.3390/foods13111622
APA StyleLuna-Finkler, C. L., Gomes, A. d. C., de Aguiar Júnior, F. C. A., Ribeiro, E., de Melo Barbosa, R., Severino, P., Santini, A., & Souto, E. B. (2024). From Extraction to Stabilization: Employing a 22 Experimental Design in Developing Nutraceutical-Grade Bixin from Bixa orellana L. Foods, 13(11), 1622. https://doi.org/10.3390/foods13111622










