The Effect of Type 2 Resistant Starch and Indole-3-Propionic Acid on Ameliorating High-Fat-Diet-Induced Hepatic Steatosis and Gut Dysbiosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Sample Preparation
2.3. Animals and Experimental Protocols
2.4. Biochemical Analysis of the Liver Function
2.5. Assessment of Oxidative Stress and Lipid Peroxidation
2.6. Targeted Metabolomics
2.7. Histological Staining
2.8. DNA Extraction, Sequencing, and Data Analysis
2.9. Statistical Analyses
3. Results
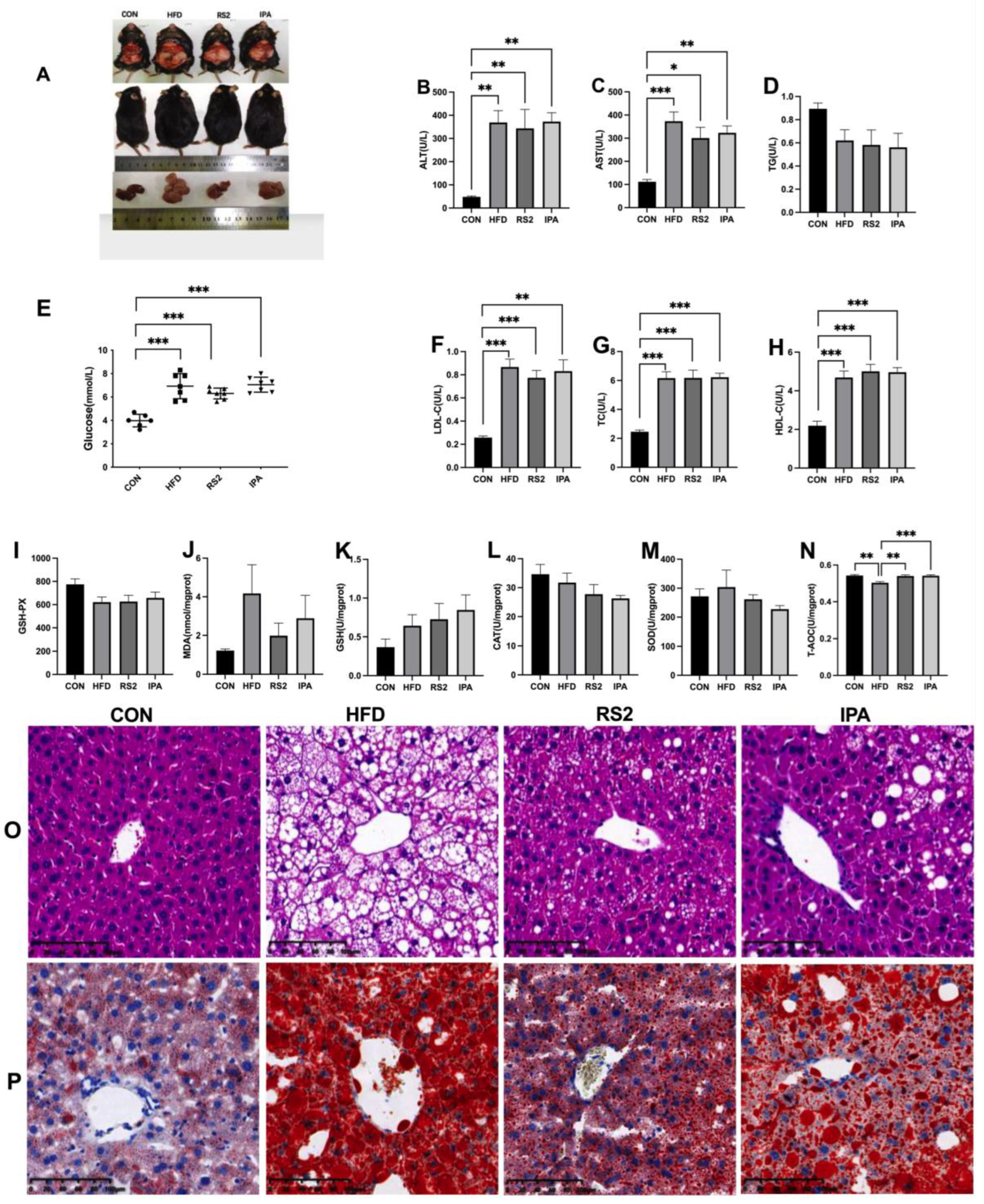
3.1. Effects of RS2 and IPA on Hepatic Steatosis in Obese Mice
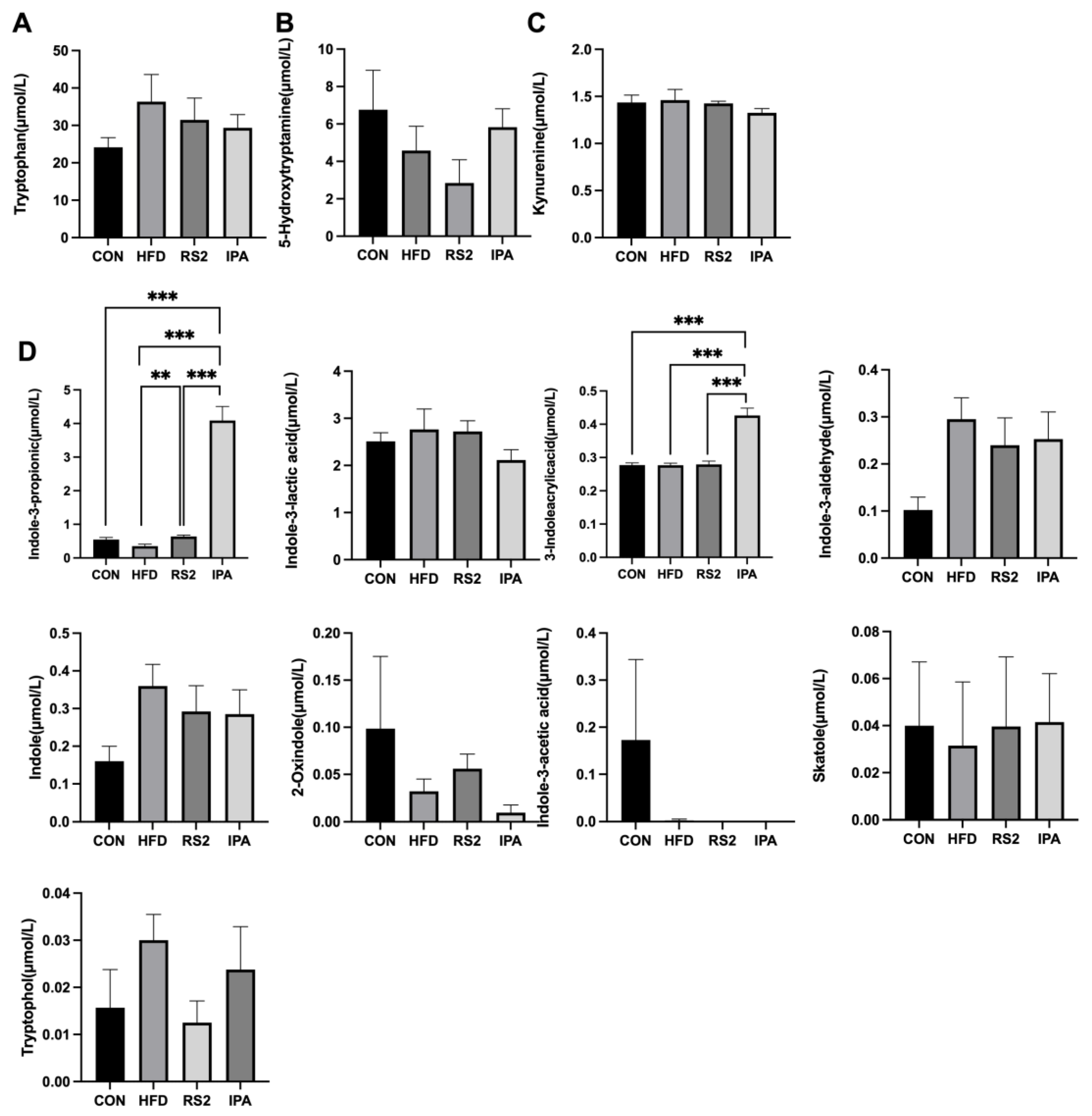
3.2. Effects of RS2 and IPA on Serum Tryptophan Metabolites in Obese Mice
3.3. Effects of RS2 and IPA on Gut Microbiota in Obese Mice
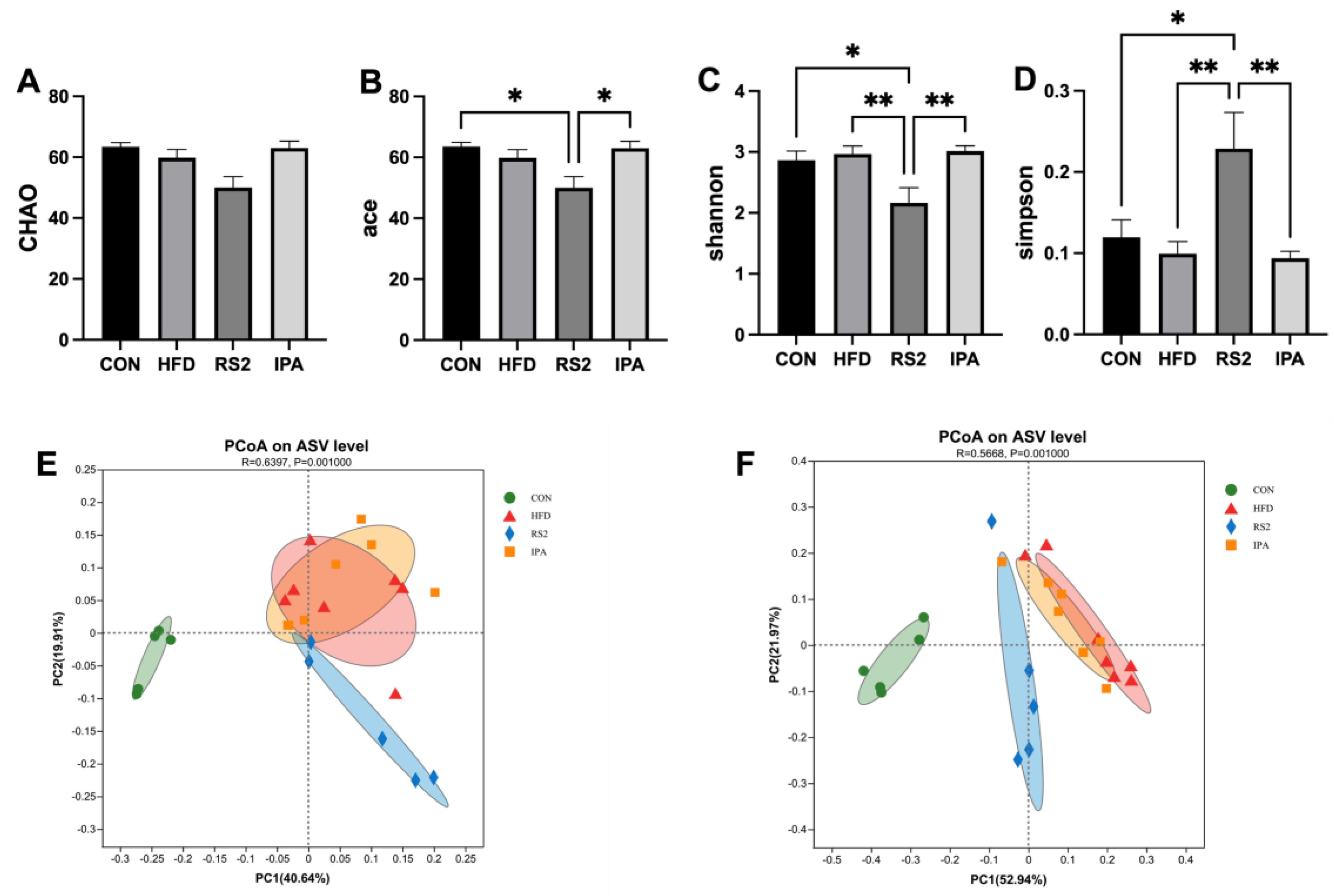
3.3.1. Effect of RS2 and IPA on Alpha and Beta Bacterial Diversity
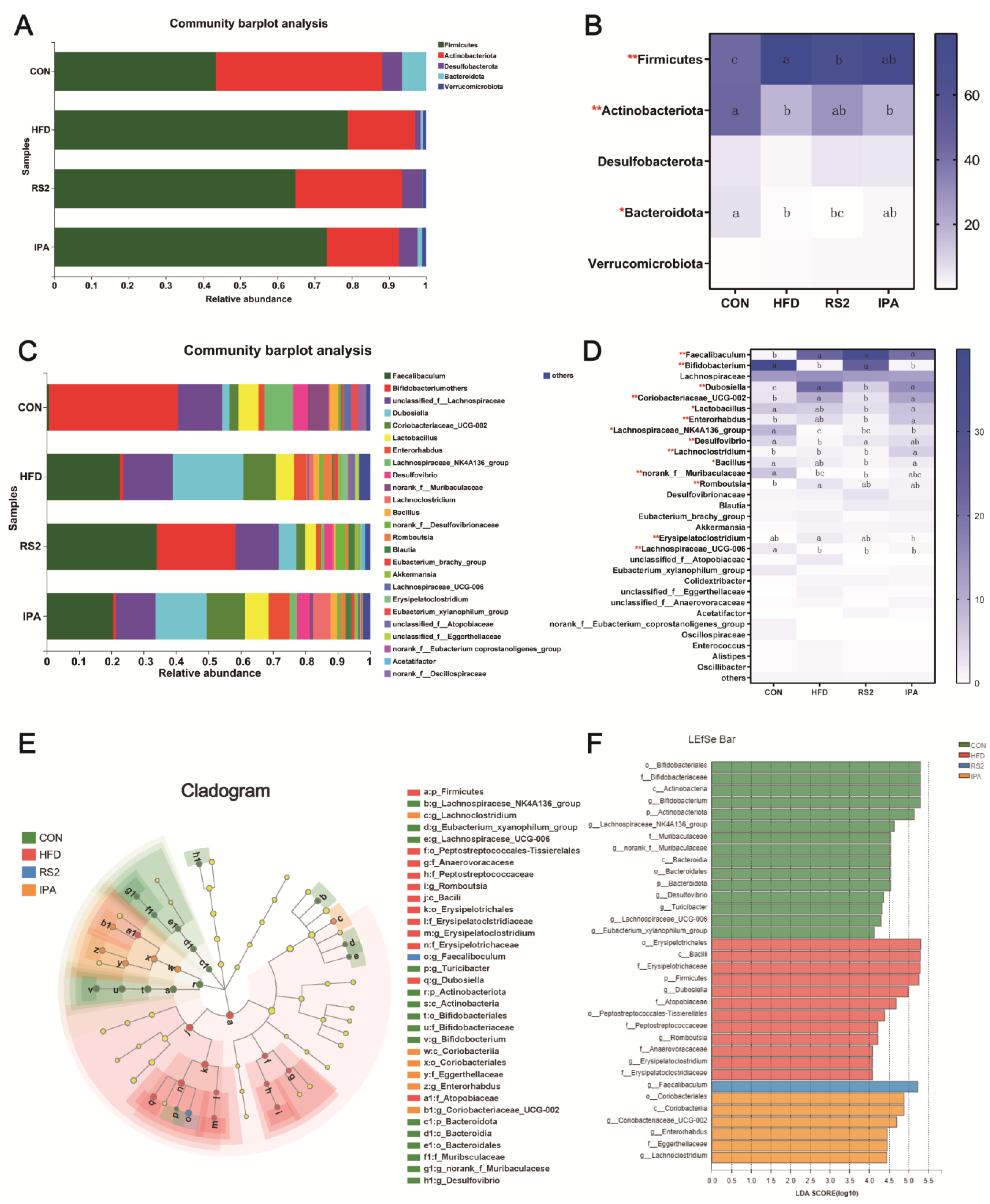
3.3.2. Effect of RS2 and IPA on Composition of the Bacterial Community Composition
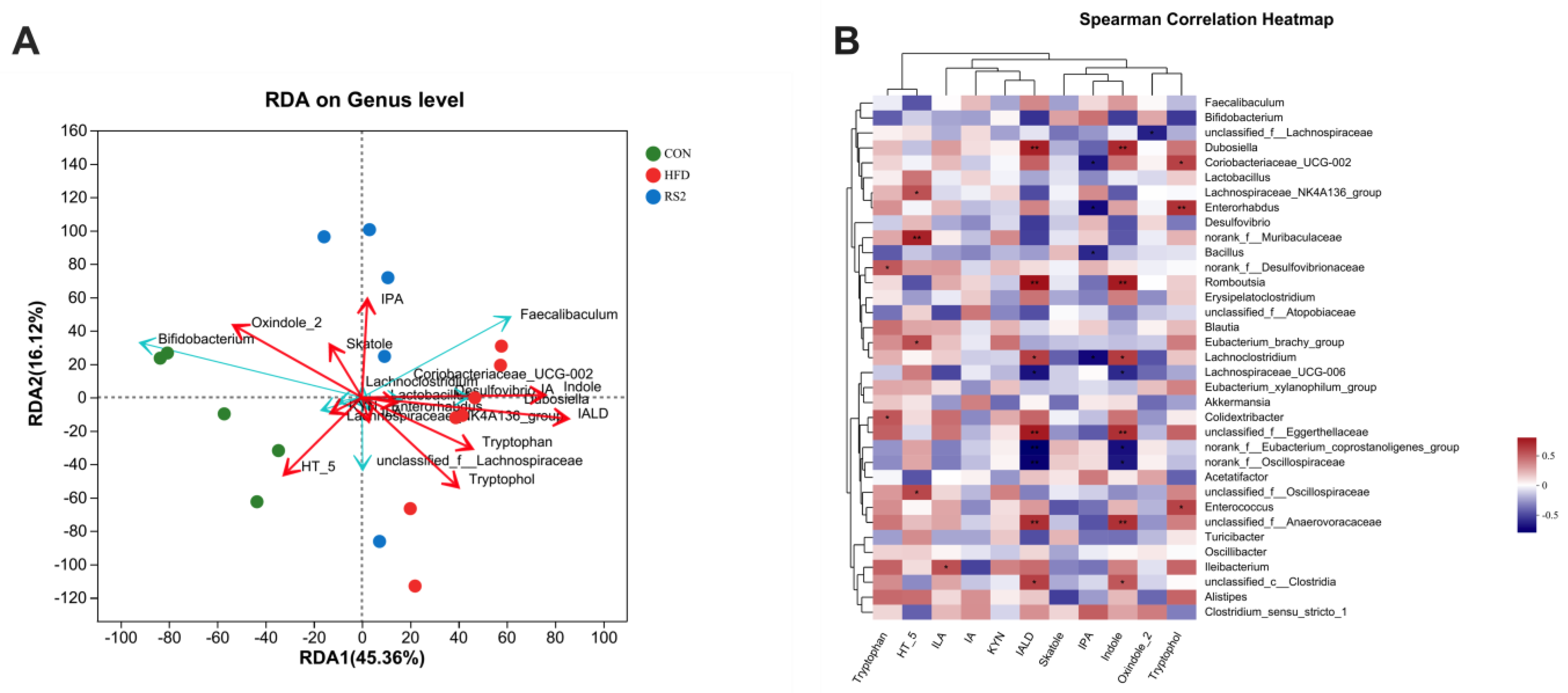
3.4. The Relationship between Bacterial Community and Tryptophan Metabolism
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cusi, K. Treatment of Patients with Type 2 Diabetes and Non-Alcoholic Fatty Liver Disease: Current Approaches and Future Directions. Diabetologia 2016, 59, 1112–1120. [Google Scholar] [CrossRef] [PubMed]
- Kopp, W. How Western Diet And Lifestyle Drive The Pandemic of Obesity and Civilization Diseases. Diabetes Metab. Syndr. Obes. 2019, 12, 2221–2236. [Google Scholar] [CrossRef] [PubMed]
- Menni, C.; Jackson, M.A.; Pallister, T.; Steves, C.J.; Spector, T.D.; Valdes, A.M. Gut Microbiome Diversity and High-Fibre Intake Are Related to Lower Long-Term Weight Gain. Int. J. Obes. 2017, 41, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.P.; Gratz, S.W.; Sheridan, P.O.; Flint, H.J.; Duncan, S.H. The Influence of Diet on the Gut Microbiota. Pharmacol. Res. 2013, 69, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Lee-Sarwar, K.A.; Lasky-Su, J.; Kelly, R.S.; Litonjua, A.A.; Weiss, S.T. Metabolome-Microbiome Crosstalk and Human Disease. Metabolites 2020, 10, 181. [Google Scholar] [CrossRef] [PubMed]
- de Mello, V.D.; Paananen, J.; Lindstrom, J.; Lankinen, M.A.; Shi, L.; Kuusisto, J.; Pihlajamaki, J.; Auriola, S.; Lehtonen, M.; Rolandsson, O.; et al. Indolepropionic Acid and Novel Lipid Metabolites Are Associated with a Lower Risk of Type 2 Diabetes in the Finnish Diabetes Prevention Study. Sci. Rep. 2017, 7, 46337. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, R.; Ilha, M.; Vaittinen, M.; Kaminska, D.; Mannisto, V.; Karja, V.; Tuomainen, M.; Hanhineva, K.; Romeo, S.; Pajukanta, P.; et al. Indole-3-Propionic Acid, a Gut-Derived Tryptophan Metabolite, Associates with Hepatic Fibrosis. Nutrients 2021, 13, 3509. [Google Scholar] [CrossRef] [PubMed]
- Menni, C.; Hernandez, M.M.; Vital, M.; Mohney, R.P.; Spector, T.D.; Valdes, A.M. Circulating Levels of the Anti-Oxidant Indoleproprionic Acid Are Associated with Higher Gut Microbiome Diversity. Gut Microbes. 2019, 10, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Dodd, D.; Spitzer, M.H.; Van Treuren, W.; Merrill, B.D.; Hryckowian, A.J.; Higginbottom, S.K.; Le, A.; Cowan, T.M.; Nolan, G.P.; Fischbach, M.A.; et al. A Gut Bacterial Pathway Metabolizes Aromatic Amino Acids into Nine Circulating Metabolites. Nature 2017, 551, 648–652. [Google Scholar] [CrossRef]
- Rothhammer, V.; Mascanfroni, I.D.; Bunse, L.; Takenaka, M.C.; Kenison, J.E.; Mayo, L.; Chao, C.C.; Patel, B.; Yan, R.; Blain, M.; et al. Type I Interferons and Microbial Metabolites of Tryptophan Modulate Astrocyte Activity and Central Nervous System Inflammation via the Aryl Hydrocarbon Receptor. Nat. Med. 2016, 22, 586–597. [Google Scholar] [CrossRef]
- Li, Y.; Xu, W.; Zhang, F.; Zhong, S.; Sun, Y.; Huo, J.; Zhu, J.; Wu, C. The Gut Microbiota-Produced Indole-3-Propionic Acid Confers the Antihyperlipidemic Effect of Mulberry-Derived 1-Deoxynojirimycin. mSystems 2020, 5, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Elsden, S.R.; Hilton, M.G.; Waller, J.M. The End Products of the Metabolism of Aromatic Amino Acids by Clostridia. Arch. Microbiol. 1976, 107, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Serger, E.; Luengo-Gutierrez, L.; Chadwick, J.S.; Kong, G.; Zhou, L.; Crawford, G.; Danzi, M.C.; Myridakis, A.; Brandis, A.; Bello, A.T.; et al. The Gut Metabolite Indole-3 Propionate Promotes Nerve Regeneration and Repair. Nature 2022, 607, 585–592. [Google Scholar] [CrossRef]
- Abildgaard, A.; Elfving, B.; Hokland, M.; Wegener, G.; Lund, S. The Microbial Metabolite Indole-3-Propionic Acid Improves Glucose Metabolism in Rats, but Does Not Affect Behaviour. Arch. Physiol. Biochem. 2018, 124, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Negatu, D.A.; Gengenbacher, M.; Dartois, V.; Dick, T. Indole Propionic Acid, an Unusual Antibiotic Produced by the Gut Microbiota, With Anti-Inflammatory and Antioxidant Properties. Front. Microbiol. 2020, 11, 575586. [Google Scholar] [CrossRef] [PubMed]
- Koay, Y.C.; Wali, J.A.; Luk, A.W.S.; Macia, L.; Cogger, V.C.; Pulpitel, T.J.; Wahl, D.; Solon-Biet, S.M.; Holmes, A.; Simpson, S.J.; et al. Ingestion of Resistant Starch by Mice Markedly Increases Microbiome-Derived Metabolites. FASEB J. 2019, 33, 8033–8042. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarczyk, M.M.; Miller, M.J.; Freund, G.G. The Health Benefits of Dietary Fiber: Beyond the Usual Suspects of Type 2 Diabetes Mellitus, Cardiovascular Disease and Colon Cancer. Metabolism 2012, 61, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Qian, L.; Siliceo, S.L.; Long, X.; Nychas, E.; Liu, Y.; Ismaiah, M.J.; Leung, H.; Zhang, L.; Gao, Q.; et al. Resistant Starch Decreases Intrahepatic Triglycerides in Patients with NAFLD via Gut Microbiome Alterations. Cell Metab. 2023, 35, 1530–1547.e8. [Google Scholar] [CrossRef] [PubMed]
- Johnston, K.L.; Thomas, E.L.; Bell, J.D.; Frost, G.S.; Robertson, M.D. Resistant Starch Improves Insulin Sensitivity in Metabolic Syndrome. Diabet. Med. 2010, 27, 391–397. [Google Scholar] [CrossRef]
- Robertson, M.D.; Bickerton, A.S.; Dennis, A.L.; Vidal, H.; Frayn, K.N. Insulin-Sensitizing Effects of Dietary Resistant Starch and Effects on Skeletal Muscle and Adipose Tissue Metabolism. Am. J. Clin. Nutr. 2005, 82, 559–567. [Google Scholar] [CrossRef]
- Keenan, M.J.; Zhou, J.; Hegsted, M.; Pelkman, C.; Durham, H.A.; Coulon, D.B.; Martin, R.J. Role of Resistant Starch in Improving Gut Health, Adiposity, and Insulin Resistance. Adv. Nutr. 2015, 6, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Vidrine, K.; Ye, J.; Martin, R.J.; McCutcheon, K.L.; Raggio, A.M.; Pelkman, C.; Durham, H.A.; Zhou, J.; Senevirathne, R.N.; Williams, C.; et al. Resistant Starch from High Amylose Maize (HAM-RS2) and Dietary Butyrate Reduce Abdominal Fat by a Different Apparent Mechanism. Obesity 2014, 22, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Rao, M.; Huang, W.; Wan, Q.; Yan, P.; Long, Y.; Guo, M.; Xu, Y.; Xu, Y. Resistant Starch Ameliorated Insulin Resistant in Patients of Type 2 Diabetes with Obesity: A Systematic Review and Meta-Analysis. Lipids Health Dis. 2019, 18, 205. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J. Fiber and Prebiotics: Mechanisms and Health Benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Molina, I.; Nieves-Hernandez, M.G.; Gutierrez-Cortez, E.; Barrón-García, O.Y.; Gaytán-Martínez, M.; Rodriguez-Garcia, M.E. Physicochemical Changes in Starch during the Conversion of Corn to Tortilla in the Traditional Nixtamalization Process Associated with RS2. Food Chem. 2024, 439, 138088. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose Translation from Animal to Human Studies Revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Sun, C.; Chen, Y.; Du, F.; Yang, Y.; Wu, G. Indole-3-Propionic Acid-Aggravated CCl4-Induced Liver Fibrosis via the TGF-Beta1/Smads Signaling Pathway. J. Clin. Transl. Hepatol. 2021, 9, 917–930. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res 2013, 41, D590-6. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, M.; Ma, Q.; Tian, B.; Nie, C.; Chen, Z.; Li, J. Health Beneficial Effects of Resistant Starch on Diabetes and Obesity via Regulation of Gut Microbiota: A Review. Food Funct. 2020, 11, 5749–5767. [Google Scholar] [CrossRef]
- Meenu, M.; Xu, B. A Critical Review on Anti-Diabetic and Anti-Obesity Effects of Dietary Resistant Starch. Crit. Rev. Food Sci. Nutr. 2019, 59, 3019–3031. [Google Scholar] [CrossRef]
- Keenan, M.J.; Martin, R.J.; Raggio, A.M.; McCutcheon, K.L.; Brown, I.L.; Birkett, A.; Newman, S.S.; Skaf, J.; Hegsted, M.; Tulley, R.T.; et al. High-Amylose Resistant Starch Increases Hormones and Improves Structure and Function of the Gastrointestinal Tract: A Microarray Study. J. Nutr. Nutr. 2012, 5, 26–44. [Google Scholar] [CrossRef] [PubMed]
- Polakof, S.; Diaz-Rubio, M.E.; Dardevet, D.; Martin, J.F.; Pujos-Guillot, E.; Scalbert, A.; Sebedio, J.L.; Mazur, A.; Comte, B. Resistant Starch Intake Partly Restores Metabolic and Inflammatory Alterations in the Liver of High-Fat-Diet-Fed Rats. J. Nutr. Biochem. 2013, 24, 1920–1930. [Google Scholar] [CrossRef] [PubMed]
- Belobrajdic, D.P.; King, R.A.; Christophersen, C.T.; Bird, A.R. Dietary Resistant Starch Dose-Dependently Reduces Adiposity in Obesity-Prone and Obesity-Resistant Male Rats. Nutr. Metab. 2012, 9, 93. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, L.; Hu, M.; Kim, J.J.; Lin, R.; Xu, J.; Fan, L.; Qi, Y.; Wang, L.; Liu, W.; et al. Dietary Type 2 Resistant Starch Improves Systemic Inflammation and Intestinal Permeability by Modulating Microbiota and Metabolites in Aged Mice on High-Fat Diet. Aging 2020, 12, 9173–9187. [Google Scholar] [CrossRef]
- Bodinham, C.L.; Smith, L.; Wright, J.; Frost, G.S.; Robertson, M.D. Dietary Fibre Improves First-Phase Insulin Secretion in Overweight Individuals. PLoS ONE 2012, 7, e40834. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Na, L.; Shan, R.; Cheng, Y.; Li, Y.; Wu, X.; Sun, C. Dietary Vitamin C Intake Reduces the Risk of Type 2 Diabetes in Chinese Adults: HOMA-IR and T-AOC as Potential Mediators. PLoS ONE 2016, 11, e0163571. [Google Scholar] [CrossRef] [PubMed]
- Nuttall, S.L.; Martin, U.; Sinclair, A.J.; Kendall, M.J. Glutathione: In Sickness and in Health. Lancet 1998, 351, 645–646. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.J. Glutathione: A Marker and Antioxidant for Aging. J. Lab. Clin. Med. 2002, 140, 380–381. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Zhen, C.; Liu, J.; Yang, P.; Hu, L.; Shang, P. Unraveling the Potential Role of Glutathione in Multiple Forms of Cell Death in Cancer Therapy. Oxid. Med. Cell Longev. 2019, 2019, 3150145. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.H.; Liu, L.K.; Chuang, C.M.; Chyau, C.C.; Huang, C.N.; Wang, C.J. Mulberry Water Extracts Possess an Anti-Obesity Effect and Ability to Inhibit Hepatic Lipogenesis and Promote Lipolysis. J. Agric. Food Chem. 2011, 59, 2663–2671. [Google Scholar] [CrossRef]
- Kim, H.Y.; Park, S.Y.; Lee, M.H.; Rho, J.H.; Oh, Y.J.; Jung, H.U.; Yoo, S.H.; Jeong, N.Y.; Lee, H.J.; Suh, S.; et al. Hepatic STAMP2 Alleviates High Fat Diet-Induced Hepatic Steatosis and Insulin Resistance. J. Hepatol. 2015, 63, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yoo, W.; Park, H.M.; Lim, S.Y.; Shin, D.H.; Kim, S.; Park, H.Y.; Jeong, T.S. Arazyme Suppresses Hepatic Steatosis and Steatohepatitis in Diet-Induced Non-Alcoholic Fatty Liver Disease-Like Mouse Model. Int. J. Mol. Sci. 2019, 20, 2325. [Google Scholar] [CrossRef]
- Rosado, C.P.; Rosa, V.H.C.; Martins, B.C.; Soares, A.C.; Santos, I.B.; Monteiro, E.B.; Moura-Nunes, N.; da Costa, C.A.; Mulder, A.; Daleprane, J.B. Resistant Starch from Green Banana (Musa Sp.) Attenuates Non-Alcoholic Fat Liver Accumulation and Increases Short-Chain Fatty Acids Production in High-Fat Diet-Induced Obesity in Mice. Int. J. Biol. Macromol. 2020, 145, 1066–1072. [Google Scholar] [CrossRef]
- Zhao, Z.H.; Xin, F.Z.; Xue, Y.; Hu, Z.; Han, Y.; Ma, F.; Zhou, D.; Liu, X.L.; Cui, A.; Liu, Z.; et al. Indole-3-Propionic Acid Inhibits Gut Dysbiosis and Endotoxin Leakage to Attenuate Steatohepatitis in Rats. Exp. Mol. Med. 2019, 51, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef]
- Jiang, H.; Chen, C.; Gao, J. Extensive Summary of the Important Roles of Indole Propionic Acid, a Gut Microbial Metabolite in Host Health and Disease. Nutrients 2022, 15, 151. [Google Scholar] [CrossRef] [PubMed]
- Konopelski, P.; Mogilnicka, I. Biological Effects of Indole-3-Propionic Acid, a Gut Microbiota-Derived Metabolite, and Its Precursor Tryptophan in Mammals’ Health and Disease. Int. J. Mol. Sci. 2022, 23, 1222. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, S.; Hemarajata, P.; Versalovic, J. The Human Gut Microbiome and Body Metabolism: Implications for Obesity and Diabetes. Clin. Chem. 2013, 59, 617–628. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An Obesity-Associated Gut Microbiome with Increased Capacity for Energy Harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef]
- Chang, D.H.; Rhee, M.S.; Ahn, S.; Bang, B.H.; Oh, J.E.; Lee, H.K.; Kim, B.C. Faecalibaculum Rodentium Gen. Nov., Sp. Nov., Isolated from the Faeces of a Laboratory Mouse. Antonie Van. Leeuwenhoek 2015, 108, 1309–1318. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.T.; Cheng, P.C.; Pan, T.M. Anti-Obesity Effects of Gut Microbiota Are Associated with Lactic Acid Bacteria. Appl. Microbiol. Biotechnol. 2014, 98, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Huang, M.; You, X.; Zhao, J.; Chen, L.; Wang, L.; Luo, Y.; Chen, Y. Gut Microbiota Mediates the Anti-Obesity Effect of Calorie Restriction in Mice. Sci. Rep. 2018, 8, 13037. [Google Scholar] [CrossRef] [PubMed]
- Roberfroid, M.; Gibson, G.R.; Hoyles, L.; McCartney, A.L.; Rastall, R.; Rowland, I.; Wolvers, D.; Watzl, B.; Szajewska, H.; Stahl, B.; et al. Prebiotic Effects: Metabolic and Health Benefits. Br. J. Nutr. 2010, 104 (Suppl. S2), S1-63. [Google Scholar] [CrossRef]
- Million, M.; Maraninchi, M.; Henry, M.; Armougom, F.; Richet, H.; Carrieri, P.; Valero, R.; Raccah, D.; Vialettes, B.; Raoult, D. Obesity-Associated Gut Microbiota Is Enriched in Lactobacillus reuteri and Depleted in Bifidobacterium animalis and Methanobrevibacter smithii. Int. J. Obes. 2012, 36, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.N.; Yu, Q.F.; Fu, N.; Liu, X.W.; Lu, F.G. Effects of Four Bifidobacteria on Obesity in High-Fat Diet Induced Rats. World J. Gastroenterol. 2010, 16, 3394–3401. [Google Scholar] [CrossRef]
- Aragozzini, F.; Ferrari, A.; Pacini, N.; Gualandris, R. Indole-3-Lactic Acid as a Tryptophan Metabolite Produced by Bifidobacterium spp. Appl. Environ. Microbiol. 1979, 38, 544–546. [Google Scholar] [CrossRef]
- Cani, P.D.; Neyrinck, A.M.; Fava, F.; Knauf, C.; Burcelin, R.G.; Tuohy, K.M.; Gibson, G.R.; Delzenne, N.M. Selective Increases of Bifidobacteria in Gut Microflora Improve High-Fat-Diet-Induced Diabetes in Mice through a Mechanism Associated with Endotoxaemia. Diabetologia 2007, 50, 2374–2383. [Google Scholar] [CrossRef]
- Tachon, S.; Zhou, J.; Keenan, M.; Martin, R.; Marco, M.L. The Intestinal Microbiota in Aged Mice Is Modulated by Dietary Resistant Starch and Correlated with Improvements in Host Responses. FEMS Microbiol. Ecol. 2013, 83, 299–309. [Google Scholar] [CrossRef]
- Hong, Y.; Sheng, L.; Zhong, J.; Tao, X.; Zhu, W.; Ma, J.; Yan, J.; Zhao, A.; Zheng, X.; Wu, G.; et al. Desulfovibrio Vulgaris, a Potent Acetic Acid-Producing Bacterium, Attenuates Nonalcoholic Fatty Liver Disease in Mice. Gut Microbes 2021, 13, 1–20. [Google Scholar] [CrossRef]
- Lin, H.; Ma, X.; Yang, X.; Chen, Q.; Wen, Z.; Yang, M.; Fu, J.; Yin, T.; Lu, G.; Qi, J.; et al. Natural Shikonin and Acetyl-Shikonin Improve Intestinal Microbial and Protein Composition to Alleviate Colitis-Associated Colorectal Cancer. Int. Immunopharmacol. 2022, 111, 109097. [Google Scholar] [CrossRef]
- Clavel, T.; Desmarchelier, C.; Haller, D.; Gerard, P.; Rohn, S.; Lepage, P.; Daniel, H. Intestinal Microbiota in Metabolic Diseases: From Bacterial Community Structure and Functions to Species of Pathophysiological Relevance. Gut Microbes 2014, 5, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Boutard, M.; Cerisy, T.; Nogue, P.Y.; Alberti, A.; Weissenbach, J.; Salanoubat, M.; Tolonen, A.C. Functional Diversity of Carbohydrate-Active Enzymes Enabling a Bacterium to Ferment Plant Biomass. PLoS Genet. 2014, 10, e1004773. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Wang, J.; Xu, Y.; Yang, H.; Wang, J.; Xue, C.; Yan, X.; Su, L. Anti-Inflammation Effects of Fucosylated Chondroitin Sulphate from Acaudina Molpadioides by Altering Gut Microbiota in Obese Mice. Food Funct. 2019, 10, 1736–1746. [Google Scholar] [CrossRef] [PubMed]
- Zelante, T.; Iannitti, R.G.; Cunha, C.; De Luca, A.; Giovannini, G.; Pieraccini, G.; Zecchi, R.; D’Angelo, C.; Massi-Benedetti, C.; Fallarino, F.; et al. Tryptophan Catabolites from Microbiota Engage Aryl Hydrocarbon Receptor and Balance Mucosal Reactivity via Interleukin-22. Immunity 2013, 39, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Natividad, J.M.; Agus, A.; Planchais, J.; Lamas, B.; Jarry, A.C.; Martin, R.; Michel, M.L.; Chong-Nguyen, C.; Roussel, R.; Straube, M.; et al. Impaired Aryl Hydrocarbon Receptor Ligand Production by the Gut Microbiota Is a Key Factor in Metabolic Syndrome. Cell Metab. 2018, 28, 737–749.e4. [Google Scholar] [CrossRef] [PubMed]
- Roager, H.M.; Licht, T.R. Microbial Tryptophan Catabolites in Health and Disease. Nat. Commun. 2018, 9, 3294. [Google Scholar] [CrossRef] [PubMed]
- Russell, W.R.; Duncan, S.H.; Scobbie, L.; Duncan, G.; Cantlay, L.; Calder, A.G.; Anderson, S.E.; Flint, H.J. Major Phenylpropanoid-Derived Metabolites in the Human Gut Can Arise from Microbial Fermentation of Protein. Mol. Nutr. Food Res. 2013, 57, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Tuomainen, M.; Lindström, J.; Lehtonen, M.; Auriola, S.; Pihlajamäki, J.; Peltonen, M.; Tuomilehto, J.; Uusitupa, M.; de Mello, V.D.; Hanhineva, K. Associations of Serum Indolepropionic Acid, a Gut Microbiota Metabolite, with Type 2 Diabetes and Low-Grade Inflammation in High-Risk Individuals. Nutr. Diabetes 2018, 8, 1–5. [Google Scholar] [CrossRef]
- Jennis, M.; Cavanaugh, C.R.; Leo, G.C.; Mabus, J.R.; Lenhard, J.; Hornby, P.J. Microbiota-Derived Tryptophan Indoles Increase after Gastric Bypass Surgery and Reduce Intestinal Permeability in Vitro and in Vivo. Neurogastroenterol. Motil. 2018, 30, e13178. [Google Scholar] [CrossRef]
- Chen, L.; Yang, Y.; Sun, S.; Xie, Y.; Pan, C.; Li, M.; Li, C.; Liu, Y.; Xu, Z.; Liu, W.; et al. Indolepropionic Acid Reduces Obesity-Induced Metabolic Dysfunction through Colonic Barrier Restoration Mediated via Tuft Cell-Derived IL-25. FEBS J. 2022, 289, 5985–6004. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, M.; Cai, W.; Li, X.; Deng, Y.; Li, J.; Wang, X.; Zhu, L.; Wang, C.; Li, X. The Effect of Type 2 Resistant Starch and Indole-3-Propionic Acid on Ameliorating High-Fat-Diet-Induced Hepatic Steatosis and Gut Dysbiosis. Foods 2024, 13, 1625. https://doi.org/10.3390/foods13111625
Yang M, Cai W, Li X, Deng Y, Li J, Wang X, Zhu L, Wang C, Li X. The Effect of Type 2 Resistant Starch and Indole-3-Propionic Acid on Ameliorating High-Fat-Diet-Induced Hepatic Steatosis and Gut Dysbiosis. Foods. 2024; 13(11):1625. https://doi.org/10.3390/foods13111625
Chicago/Turabian StyleYang, Min, Wanhao Cai, Xinxin Li, Yixuan Deng, Jinjun Li, Xin Wang, Liying Zhu, Chong Wang, and Xiaoqiong Li. 2024. "The Effect of Type 2 Resistant Starch and Indole-3-Propionic Acid on Ameliorating High-Fat-Diet-Induced Hepatic Steatosis and Gut Dysbiosis" Foods 13, no. 11: 1625. https://doi.org/10.3390/foods13111625





