Veterinary Drug Residues in the Food Chain as an Emerging Public Health Threat: Sources, Analytical Methods, Health Impacts, and Preventive Measures
Abstract
1. Introduction
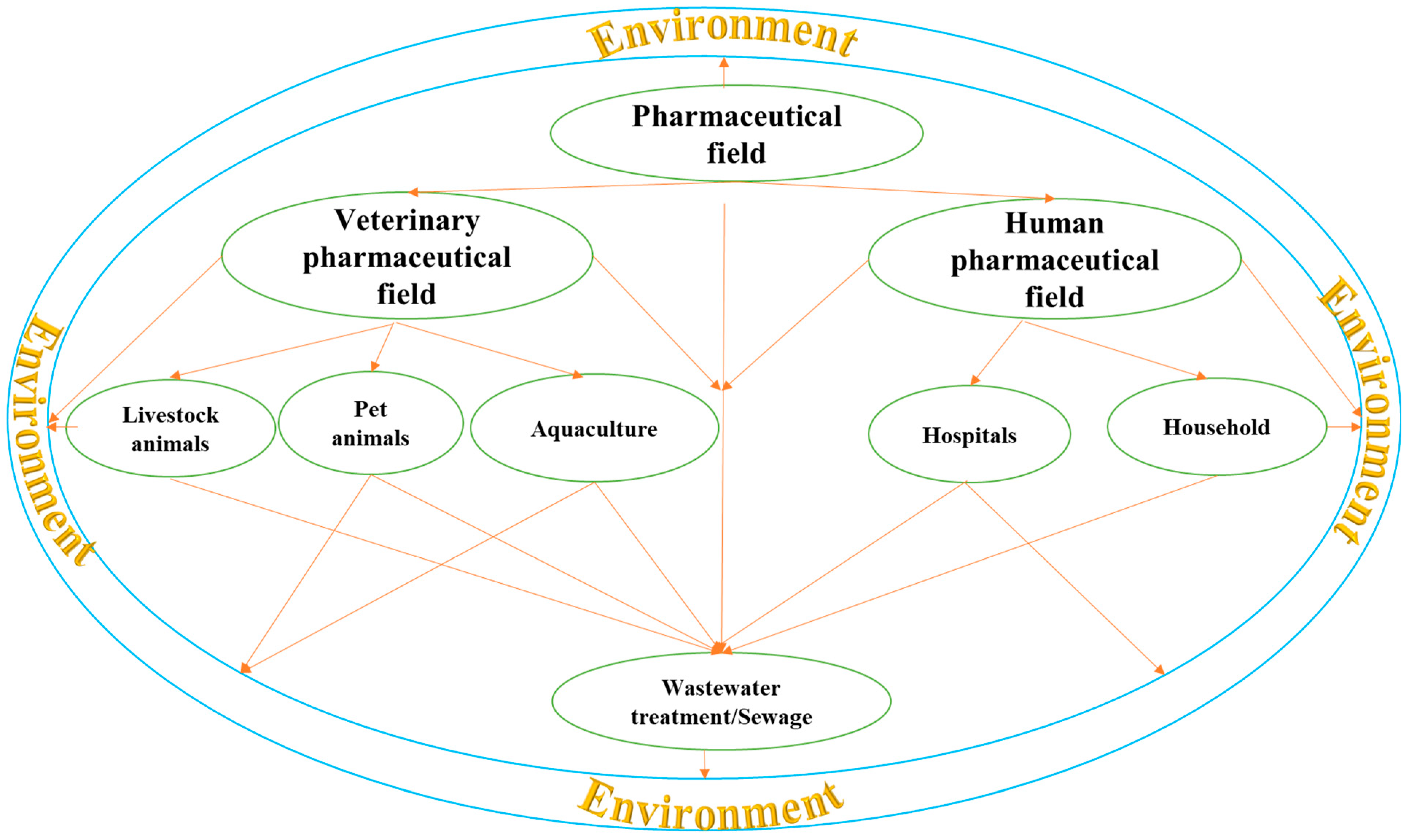
2. Sources of Drug Residues in Farm Animals
2.1. Environmental Contamination
2.2. Not following a Drug’s Withdrawal Period
2.3. Extra-Label Drug Use
3. Human Exposure to Drug Residues
3.1. Drinking Water
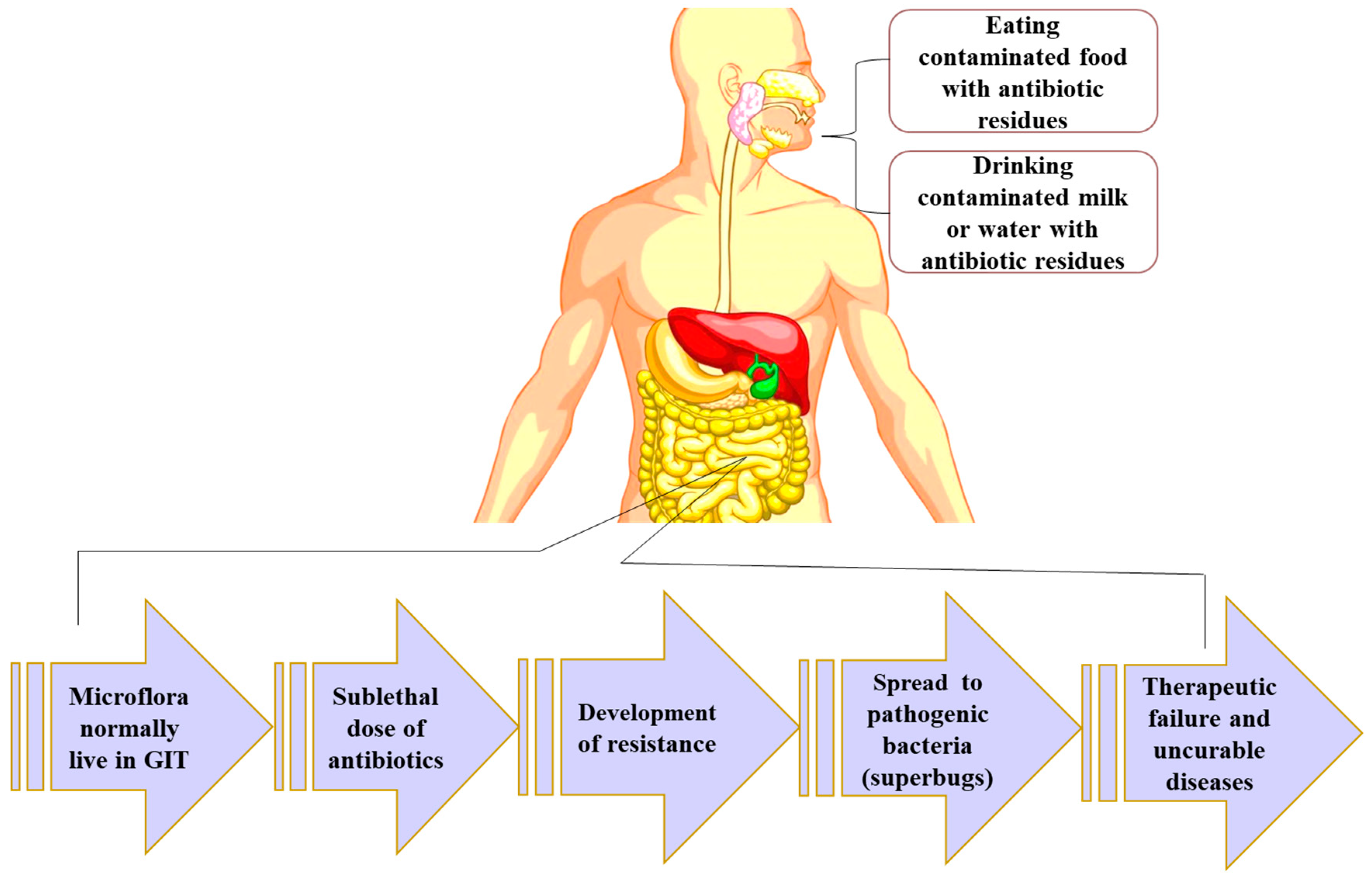
3.2. Food
3.3. Air and Dust
| Method of Exposure | Description | Example | Reference |
|---|---|---|---|
| Drinking Water | Pharmaceuticals enter water sources through excretion, disposal, industrial effluents, and agricultural runoff. Once in water sources, they can persist and accumulate. | Tap water in China was found to contain 17 drug residues, including antibiotics, NSAIDs, β-blocker, lipid regulator, psychoactive stimulant, and anticonvulsant. | [38,39,40] |
| Food | Drug residues in food arise from the treatment of food animals with antibiotics, hormones, and other drugs. These residues can persist in meat, milk, eggs, fish, fruits, vegetables, and honey. | Meat: neomycin, streptomycin, penicillin, etc. | [41,42,43,44,45,46] |
| Eggs: salinomycin, monensin, robenidine, lasalocid. Milk: NSAIDs like flunixin, meloxicam, tolfenamic acid, metamizole, diclofenac. | |||
| Air and dust | Drug residues can volatilize from animal farming and wastewater treatment, posing an occupational hazard. Inhalation of these residues can lead to respiratory diseases. | Dust from livestock barns containing fluoroquinolones, tetracyclines, tylosin, sulfamethazine, and chloramphenicol. Penicillin in airborne dust from a pharmaceutical company. | [12,18,51,52,53] |
4. Analytical Methods for Veterinary Drug Residues
4.1. Microbiological Methods
4.2. Immunological Methods
4.2.1. ELISA
4.2.2. CGIA
4.2.3. FPIA
4.2.4. TR-FIA
4.2.5. Biosensor Technology
4.2.6. QDs
4.3. Physicochemical Methods
4.3.1. LC
4.3.2. GC
4.3.3. CE
4.3.4. CE-MS
4.3.5. GC-MS
4.3.6. LC-MS
| Category | Method | Description | Examples | References |
|---|---|---|---|---|
| Microbiological methods | Microbial inhibition test | Qualitative or semi-quantitative screening based on growth inhibition of microorganisms by drug residues. | Detection of fluoroquinolone residues in animal-derived foods using E. coli strain. | [54,55] |
| Radioactive receptor assay | Competitive binding between drug residues and isotope-labeled antibiotics with a receptor on the microbial surface. | Screening tetracycline residues in food products. | [56] | |
| Immunological methods | Enzyme-linked immunosorbent assay (ELISA) | Uses specific binding between antibodies and antigens for qualitative or quantitative analysis. | Detection of tetracycline residues in milk, monoclonal antibody-based ELISA for avermectins in milk, and dual-colorimetric ELISA for fluoroquinolone and sulfonamide residues. | [57,58,59] |
| Colloidal gold immunoassay (CGIA) | Rapid screening of residues, often used alongside ELISA. | Detecting kanamycin and tobramycin residues in swine tissues, screening of streptomycin residues in milk and pig urine, and the detection of 19-nortestosterone in pork and beef. | [60,61,62] | |
| Fluorescence polarization immunoassay (FPIA) | Homogeneous assay based on competitive binding of the target analyte and fluorescein-labeled antigen with specific antibody sites. | Quantitative determination of gentamicin in milk, one-step FPIA for quinolone and fluoroquinolone in milk and chicken muscle, and multiplexed FPIA for fluoroquinolones and sulfonamides in milk. | [63,64,65] | |
| Time-resolved fluoroimmunoassay (TR-FIA) | Based on fluorescent properties of lanthanide chelates. High sensitivity and low background interference due to fluorescent properties of lanthanide chelates. | Screening narasin and salinomycin residues in poultry and eggs, the detection of chloramphenicol in shrimp and chicken muscle, and the determination of ampicillin in cow milk samples. | [66,67,68] | |
| Biosensor technology | Converts biological concentration into measurable signals. Diverse sensors employing biorecognition elements for detection. | Detection of tetracyclines in poultry muscle using luminescent bacterial biosensor, optical biosensor for amphenicol antibiotic residues in bovine, ovine, and porcine kidney, and amperometric affinity penicillin-binding protein magnetosensor for β-lactam antibiotics in milk. | [69,70,71] | |
| Quantum dots (QDs) | Semiconductor particles used for fluorescence-based detection of residues. | Indirect competitive fluorescence-linked immunosorbent assay for sulfamethazine in chicken, QD-based immunoassay for tetracyclines in bovine muscle, and QD-based lateral flow immunoassay for chloramphenicol in milk. | [72,73,74] | |
| Physicochemical methods | Liquid chromatography (LC) | High-performance (HPLC) and ultra-high performance (UHPLC) versions separate compounds based on their interaction with stationary and mobile phases. | HPLC with fluorescence detection (HPLC-FLD) for enrofloxacin in chicken muscle, UHPLC-FLD for benzimidazole residues in farm fish, HPLC with diode array detection (HPLC-DAD) for sulfonamides in milk, and HPLC with ultraviolet (HPLC-UV) for sulfonamides in pork, liver, and chicken. | [75,76,77,78] |
| Gas chromatography (GC) | Requires derivatization of analytes for volatility before detection. | GC with electron capture detector (GC-ECD) for chloramphenicol residues in animal tissues, GC with nitrogen-phosphorus detection (GC-NPD) for lincomycin and spectinomycin, and gas chromatography with an electron capture detector (GC-ECD) was utilized to detect amitraz and its metabolite residues. | [79,80,81] | |
| Capillary electrophoresis (CE) | High voltage electric field drives separation in a capillary channel. | CE with laser-induced fluorescence (CE-LIF) for sulfonamide residues detection in liver, solid phase extraction-capillary electrophoresis (SPE-CE) method was presented for the detection of sulfonamide residues in milk, and capillary zone electrophoresis (CZE) combined with post-column derivatization and laser-induced fluorescence detection for the determination of kanamycin, amikacin and tobramycin residues in milk. | [82,83,84] | |
| Capillary electrophoresis-mass spectrometry (CE-MS) | Combines CE separation with MS detection for enhanced analysis. | CE-MS for benzimidazoles in egg samples, CE-MS for screening and confirmation of sulfonamide residues in milk, CE-MS for quinolones in bovine milk, and capillary electrophoresis–quadrupole–time-of-flight mass spectrometry (CE-Q-TOF-MS) for tetracyclines and quinolones in milk. | [85,86,87,88] | |
| Gas chromatography-mass spectrometry (GC-MS) | Combines GC separation with MS detection | GC-MS for pharmaceuticals in edible animal tissues, GC-MS for hormones in milk, and ion trap GC-MS for 19-nortestosterone residues in animal tissues. | [89,90,91] | |
| Liquid chromatography-mass spectrometry (LC-MS) | Combines LC separation with MS detection for high selectivity and sensitivity. | LC-MS/MS for different veterinary drugs and pesticides in milk, and LC-MS/MS for multi-residue determination in milk powder, butter, fish tissue, and eggs. | [92,93] |
5. Public Health Impacts
5.1. Antimicrobial Resistance
5.2. Hypersensitivity
5.3. Risk of Developing Cancer
5.4. Teratogenic Effect
5.5. Disruption of Normal Intestinal Flora
5.6. Other Residue-Specific Signs
6. Discussion and Potential Solutions
6.1. Implementing Withdrawal Periods
6.2. Implement a Risk-Based Monitoring Program for Veterinary Drug Residues in Animal Food Products
6.3. Educate Farmers, Veterinarians, and Consumers about the Proper Use of Veterinary Drugs and the Risks of Drug Residues in Animal Food Products
6.4. Develop New Technologies and Methods to Detect Drug Residues in Animal Products
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. WHO Guidelines on the Use of Medically Important Antimicrobials in Food-Producing Animals. 2017. Available online: https://www.who.int/publications/i/item/9789241550130 (accessed on 23 February 2024).
- Hong, B.; Li, Q.; Li, J.; Zhou, M.; Wang, X.; He, B.; Yu, S. Spectrum of pharmaceutical residues in commercial manure-based organic fertilizers from multi-provinces of China mainland in relation to animal farming and possible environmental risks of fertilization. Sci. Total Environ. 2023, 894, 165029. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Report for 2011 on the results from the monitoring of veterinary medicinal product residues and other substances in live animals and animal products. EFSA J. 2013, 11, 3109. [Google Scholar]
- Beyene, T. Veterinary drug residues in food-animal products: Its risk factors and potential effects on public health. J. Veterinar. Sci. Technol. 2016, 7, 285. [Google Scholar] [CrossRef]
- Food and Drug Administration. CFR—Code of Federal Regulations Title 21; PART 556: Tolerances for Residues of New Animal Drugs in Food; Food and Drug Administration: Silver Spring, MD, USA, 2023.
- Commission European. Commission Regulation (EU) No 37/2010 of 22 December 2009 on Pharmacologically Active Substances and Their Classification Regarding Maximum Residue Limits in Foodstuffs of Animal Origin. Off. J. Eur. Union. 2010. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:015:0001:0072:en:PDF (accessed on 5 July 2023).
- Priyanka, P.S.; Sheoran, M.S.; Ganguly, S. Antibiotic residues in milk- a serious public health hazard. J. Environ. Life. Sci. 2017, 2, 99–102. [Google Scholar]
- Rana, M.S.; Lee, S.Y.; Kang, H.J.; Hur, S.J. Reducing veterinary drug residues in animal products: A review. Food Sci. Anim. Resour. 2019, 39, 687–703. [Google Scholar] [CrossRef] [PubMed]
- Okoye, C.O.; Okeke, E.S.; Okoye, K.C.; Echude, D.; Andong, F.A.; Chukwudozie, K.I.; Okoye, H.U.; Ezeonyejiaku, C.D. Occurrence and fate of pharmaceuticals, personal care products (PPCPs) and pesticides in African water systems: A need for timely intervention. Heliyon 2022, 8, e09143. [Google Scholar] [CrossRef]
- Ślósarczyk, K.; Jakóbczyk-Karpierz, S.; Różkowski, J.; Witkowski, A.J. Occurrence of pharmaceuticals and personal care products in the water environment of Poland: A review. Water 2021, 13, 2283. [Google Scholar] [CrossRef]
- Leichtweis, J.; Vieira, Y.; Welter, N.; Silvestri, S.; Dotto, G.L.; Carissimi, E. A review of the occurrence, disposal, determination, toxicity and remediation technologies of the tetracycline antibiotic. Process Saf. Environ. Prot. 2022, 160, 25–40. [Google Scholar] [CrossRef]
- Boxall, A.B.A.; Kolpin, D.W.; Halling-Sørensen, B.; Tolls, J. Are veterinary medicines causing environmental risks? Environ. Sci. Technol. 2003, 37, 287–294. [Google Scholar] [CrossRef]
- Mouiche, M.M.; Okah-Nnane, N.H.; Moffo, F.; Djibo, I.; Mapiefou, N.P.; Mpouam, S.E.; Mfopit, Y.M.; Mingoas, J.P.; Tebug, S.F.; Ndukum, J.A. Antibiotic residues in foods of animal origin in Cameroon: Prevalence, consumers’ risk perceptions and attitudes. J. Food Protect. 2024, 6, 100237. [Google Scholar] [CrossRef]
- Canton, L.; Canton, C.; Ceballos, L.; Domínguez, P.; Rodríguez, J.; Lanusse, C.; Alvarez, L.; Moreno, L. Oral and topical extra-label administration of fipronil to laying hens: Assessment of the egg residue patterns. J. Vet. Pharm. Ther. 2021, 44, 808–819. [Google Scholar] [CrossRef] [PubMed]
- Hamscher, G.; Abu-Quare, A.; Sczesny, S.; Höper, H.; Nau, H. Determination of tetracyclines and tylosin in soil and water samples from agricultural areas in lower Saxony. In Proceedings of the Euroresidue IV Conference, Veldhoven, The Netherlands, 8–10 May 2000; pp. 8–10. [Google Scholar]
- Wu, D.; Dai, S.; Feng, H.; Karunaratne, S.P.; Yang, M.; Zhang, Y. Persistence and potential risks of tetracyclines and their transformation products in two typical different animal manure composting treatments. Environ. Pollut. 2024, 341, 122904. [Google Scholar] [CrossRef] [PubMed]
- Marutescu, L.G.; Jaga, M.; Postolache, C.; Barbuceanu, F.; Milita, N.M.; Romascu, L.M.; Schmitt, H.; de Roda Husman, A.M.; Sefeedpari, P.; Glaeser, S.; et al. Insights into the impact of manure on the environmental antibiotic residues and resistance pool. Front. Microbiol. 2022, 13, 965132. [Google Scholar] [CrossRef] [PubMed]
- Hamscher, G.; Pawelzick, H.T.; Sczesny, S.; Nau, H.; Hartung, J. Antibiotics in dust originating from a pig-fattening farm: A new source of health hazard for farmers? Environ. Health Perspect. 2003, 111, 1590–1594. [Google Scholar] [CrossRef] [PubMed]
- Hamscher, G.; Sczesny, S.; Höper, H.; Nau, H. Determination of persistent tetracycline residues in soil fertilized with liquid manure by high-performance liquid chromatography with electrospray ionization tandem mass spectrometry. Anal. Chem. 2002, 74, 1509–1518. [Google Scholar] [CrossRef]
- Frey, L.; Tanunchai, B.; Glaser, B. Antibiotics residues in pig slurry and manure and its environmental contamination potential. A meta-analysis. Agron. Sustain. Dev. 2022, 42, 31. [Google Scholar] [CrossRef]
- EMA/CVMP/ERA/418282/2005; Guideline on Environmental Impact Assessment for Veterinary Medicinal Products. Committee for Medicinal Products for Veterinary Use, European Medicines Agency: Amsterdam, The Netherlands, 2011; pp. 1–77.
- U.S. Food and Drug Administration. Adequate Records Help Prevent Illegal Drug Residues and Ensure Food Safety. 2023. Available online: https://www.fda.gov/animal-veterinary/animal-health-literacy/adequate-records-help-prevent-illegal-drug-residues-and-ensure-food-safety (accessed on 24 February 2024).
- Vougat Ngom, B.R.; Garabed, B.R.; Rumbeiha, K.W.; Foyet, S.H.; Schrunk, E.D.; Shao, D.; Zoli, P.A. Penicillin-G and oxytetracycline residues in beef sold for human consumption in Maroua, Cameroon. Int. J. Food Contam. 2017, 4, 17. [Google Scholar] [CrossRef]
- Guetiya Wadoum, R.; Zambou, N.; Anyangwe, F.; Njimou, J.; Coman, M.; Verdenelli, M.; Cecchini, C.; Silvi, S.; Orpianesi, C.; Cresci, A.; et al. Abusive use of antibiotics in poultry farming in Cameroon and the public health implications. Br. Poult. Sci. 2016, 5, n483–n493. [Google Scholar] [CrossRef] [PubMed]
- Mouiche, M.M.M.; Njingou, B.Z.N.; Moffo, F.; Mpouam, S.E.; Feussom, J.M.K.; Awah-Ndukum, J. Veterinary pharmacovigilance in sub-Sahara Africa context: A pilot study of adverse reactions to veterinary medicine in Cameroon. BMC Vet. Res. 2019, 15, 301. [Google Scholar] [CrossRef]
- Getahun, M.; Abebe, R.B.; Sendekie, A.K.; Woldeyohanis, A.E.; Kasahun, A.E. Evaluation of antibiotics residues in milk and meat using different analytical methods. Int. J. Anal. Chem. 2023, 2023, 4380261. [Google Scholar] [CrossRef]
- Jalal, H.; Para, P.A.; Ganguly, S.; Gogai, M.; Bhat, M.M.; Praveen, P.K.; Bukhar, S.A. Chemical residues in meat and meat products: A review. World J. Pharm. Life Sci. 2015, 1, 106–122. [Google Scholar]
- Gehring, R.; Haskell, S.R.; Payne, M.A.; Craigmill, A.L.; Webb, A.I.; Riviere, J.E. Aminoglycoside residues in food of animal origin. J. Am. Vet. Med. Assoc. 2005, 227, 63–66. [Google Scholar] [CrossRef]
- Escribano, M.; San Andres, M.I.; de Lucas, J.J.; González-Canga, A. Ivermectin residue depletion in food producing species and its presence in animal foodstuffs with a view to human safety. Curr. Pharm. Biotechnol. 2012, 13, 987–998. [Google Scholar] [CrossRef]
- Jacela, J.Y.; DeRouchey, J.M.; Tokach, M.D.; Goodband, R.D.; Nelssen, J.L.; Renter, D.G.; Dritz, S.S. Feed additives for swine: Fact sheets–carcass modifiers, carbohydrate-degrading enzymes and proteases, and anthelmintics. Kans. Agric. Exp. Stn. Res. Rep. 2009, 10, 325–332. [Google Scholar]
- Hossain, D.K.; Nahar, K.; Alqahtani, A.S.; Gestier, T.; Hamid, K. Development and validation of a bioanalytical method for the determination of levamisole residue in backyard poultry egg. Res. J. Pharm. Technol. 2017, 10, 2249–2254. [Google Scholar] [CrossRef]
- Mestorino, N.; Buldain, D.; Buchamer, A.; Gortari, L.; Daniele, M.; Marchetti, M.L. Residue depletion of ivermectin in broiler poultry. Food Addit. Contam. Part A 2017, 34, 624–631. [Google Scholar] [CrossRef]
- Mund, M.D.; Khan, U.H.; Tahir, U.; Mustafa, B.E.; Fayyaz, A. Antimicrobial drug residues in poultry products and implications on public health: A review. Int. J. Food Prop. 2017, 20, 1433–1446. [Google Scholar] [CrossRef]
- Baynes, R.E.; Payne, M.; Martin-Jimenez, T.; Abdullah, A.R.; Anderson, K.L.; Webb, A.I.; Riviere, J.E. Extralabel use of ivermectin and moxidectin in food animals. J. Am. Vet. Med. Assoc. 2000, 217, 668–671. [Google Scholar] [CrossRef]
- McEwen, S.A.; Black, W.D.; Meek, A. Antibiotic residues (bacterial inhibitory substances) in the milk of cows treated under label and extra-label conditions. Can. Vet. J. 1992, 33, 545–550. [Google Scholar]
- Farger, M.V.; Eule, J.C. Availability of drugs for the treatment of cats with ocular diseases in Germany- discrepancy between theory and reality. Tierarztl. Prax. Ausg. K Kleintiere/Heimtiere 2022, 50, 82–91. [Google Scholar]
- Lyster, S. Evaluating the Clearance of Common Extra-Label Drug Combinations by Turkey Hepatocytes in Primary Culture. Ph.D. Thesis, University of Guelph, Guelph, ON, Canada, 2020. Available online: https://atrium.lib.uoguelph.ca/server/api/core/bitstreams/2b37fab4-3814-46f8-98d9-de06eb9979cc/content (accessed on 1 May 2024).
- Gracia-Lor, E.; Sancho, J.V.; Serrano, R.; Hernández, F. Occurrence and removal of pharmaceuticals in wastewater treatment plants at the Spanish Mediterranean area of Valencia. Chemosphere 2012, 87, 453–462. [Google Scholar] [CrossRef]
- Li, W.C. Occurrence, sources, and fate of pharmaceuticals in aquatic environment and soil. Environ. Pollut. 2014, 187, 193–201. [Google Scholar] [CrossRef]
- Leung, H.W.; Jin, L.; Wei, S.; Tsui, M.M.P.; Zhou, B.; Jiao, L.; Cheung, P.C.; Chun, Y.K.; Murphy, M.B.; Lam, P.K.S. Pharmaceuticals in tap water: Human health risk assessment and proposed monitoring framework in China. Environ. Health Perspect. 2013, 121, 839–846. [Google Scholar] [CrossRef]
- Constable, P.D.; Hinchcliff, K.W.; Done, S.H.; Grünberg, W. Practical antimicrobial therapeutics. Vet. Med. 2017, 1, 153–174. [Google Scholar]
- Pratiwi, R.; Ramadhanti, S.P.; Amatulloh, A.; Megantara, S.; Subra, L. Recent advances in the determination of veterinary drug residues in food. Foods 2023, 12, 3422. [Google Scholar] [CrossRef]
- Jeong, S.H.; Kang, D.; Lim, M.W.; Kang, C.S.; Sung, H.J. Risk assessment of growth hormones and antimicrobial residues in meat. Toxicol. Res. 2010, 26, 301–313. [Google Scholar] [CrossRef]
- Mortier, L.; Huet, A.C.; Charlier, C.; Daeseleire, E.; Delahaut, P.; Van Peteghem, C. Incidence of residues of nine anticoccidials in eggs. Food Addit. Contam. 2005, 22, 1120–1125. [Google Scholar] [CrossRef]
- Matus, J.L.; Boison, J.O. A multi-residue method for 17 anticoccidial drugs and ractopamine in animal tissues by liquid chromatography-tandem mass spectrometry and time-of-flight mass spectrometry. Drug Test Anal. 2016, 8, 465–476. [Google Scholar] [CrossRef]
- Jedziniak, P.; Szprengier-Juszkiewicz, T.; Pietruk, K.; Sledzinska, E.; Zmudzki, J. Determination of non-steroidal anti-inflammatory drugs and their metabolites in milk by liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2012, 403, 2955–2963. [Google Scholar] [CrossRef] [PubMed]
- Takino, M.; Yamaguchi, K. Pressure photoionization-mass spectrometry and atmospheric pressure chemical ionization-mass spectrometry. J. Agric. Food Chem. 2004, 52, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.R.; Park, S.; Kim, J.Y.; Choi, J.D.; Moon, G.I. Simultaneous determination of 31 Sulfonamide residues in various livestock matrices using liquid chromatography-tandem mass spectrometry. Appl. Biol. Chem. 2024, 67, 13. [Google Scholar] [CrossRef]
- CAC/GL 71-2009; Guidelines for the Design and Implementation of National Regulatory Food Safety Assurance Programmes Associated with the Use of Veterinary Drugs in Food Producing Animals. FAO: Rome, Italy, 2014.
- Kebede, G.; Zenebe, T.; Disassa, H.; Tolosa, T. Review on detection of antimicrobial residues in raw bulk milk in dairy farms. Afr. J. Basic Appl. Sci. 2014, 6, 87–97. [Google Scholar]
- Schulz, J.; Kemper, N.; Hartung, J.; Janusch, F.; Mohring, S.A.; Hamscher, G. Analysis of fluoroquinolones in dusts from intensive livestock farming and the co-occurrence of fluoroquinolone-resistant Escherichia coli. Sci. Rep. 2019, 9, 5117. [Google Scholar] [CrossRef] [PubMed]
- McEachran, A.D.; Blackwell, B.R.; Hanson, J.D.; Wooten, K.J.; Mayer, G.D.; Cox, S.B.; Smith, P.N. Antibiotics, bacteria, and antibiotic resistance genes: Aerial transport from cattle feed yards via particulate matter. Environ. Health Perspect. 2015, 123, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Farshad, A.A.; Enferadi, M.; Bakand, S.; Jamshidi Orak, R.; Mirkazemi, R. Penicillin dust exposure and penicillin resistance among pharmaceutical workers in Tehran, Iran. Int. J. Occup. Environ. Health 2016, 22, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Ashwin, H.; Stead, S.; Caldow, M.; Sharman, M.; Stark, J.; de Rijk, A.; Keely, B.J. A rapid microbial inhibition-based screening strategy for fluoroquinolone and quinolone residues in foods of animal origin. Anal. Chim. Acta 2009, 637, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Appicciafuoco, B.; Dragone, R.; Frazzoli, C.; Bolzoni, G.; Mantovani, A.; Ferrini, A.M. Microbial screening for quinolones residues in cow milk by bio-optical method. J. Pharm. Biomed. Anal. 2015, 106, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Al-Mazeedi, H.M.; Abbas, A.B.; Alomirah, H.F.; Al-Jouhar, W.Y.; Al-Mufty, S.A.; Ezzelregal, M.M.; Al-Owaish, R.A. Screening for tetracycline residues in food products of animal origin in the State of Kuwait using charm II radioimmunoassay and LC/MS/MS methods. Food Addit. Contam. Part A 2010, 27, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lu, S.; Liu, W.; Zhao, C.; Xi, R. Preparation of anti-tetracycline antibodies and development of an indirect heterologous competitive enzyme-linked immunosorbent assay to detect residues of tetracycline in milk. J. Agric. Food Chem. 2007, 55, 211–218. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Z.; Jiang, W.; Mi, T.; Shen, J. A Monoclonal antibody-based ELISA for multiresidue determination of avermectins in milk. Molecules 2012, 17, 7401–7414. [Google Scholar] [CrossRef]
- Jiang, W.; Wang, Z.; Beier, R.C.; Jiang, H.; Wu, Y.; Shen, J. Simultaneous determination of 13 fluoroquinolone and 22 sulfonamide residues in milk by a dual-colorimetric enzyme-linked immunosorbent assay. Anal. Chem. 2013, 85, 1995–1999. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Z.; Wang, Z.; Tang, S.; Zhu, Y.; Xiao, X. Rapid enzyme-linked immunosorbent assay and colloidal gold immunoassay for kanamycin and tobramycin in swine tissues. J. Agric. Food Chem. 2008, 56, 2944–2952. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.X.; Zhang, S.E.; Zhou, X.P. Monoclonal antibody-based ELISA and colloidal gold-based immunochromatographic assay for streptomycin residue detection in milk and swine urine. J. Zhejiang Univ. Sci. B 2010, 11, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Wang, Z.; Zhang, H.; Zhang, X.; Liu, X.; Wang, S. Monoclonal antibody-based ELISA and colloidal gold immunoassay for detecting 19-nortestosterone residue in animal tissues. J. Agric. Food Chem. 2011, 59, 9763–9769. [Google Scholar] [CrossRef] [PubMed]
- Beloglazova, N.V.; Shmelin, P.S.; Eremin, S.A. Sensitive immunochemical approaches for quantitative (FPIA) and qualitative (lateral flow tests) determination of gentamicin in milk. Talanta 2016, 149, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Mi, T.; Wang, Z.; Eremin, S.A.; Shen, J.; Zhang, S. Simultaneous determination of multiple (fluoro)quinolone antibiotics in food samples by a one-step fluorescence polarization immunoassay. J. Agric. Food Chem. 2013, 61, 9347–9355. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wen, K.; Tao, X.; Ding, S.; Xie, J.; Yu, X.; Li, J.; Xia, X.; Wang, Y.; Xie, S.; et al. A novel multiplexed fluorescence polarisation immunoassay based on a recombinant bi-specific single-chain diabody for simultaneous detection of fluoroquinolones and sulfonamides in milk. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2014, 31, 1959–1967. [Google Scholar] [CrossRef] [PubMed]
- Peippo, P.; Hagren, V.; Lovgren, T.; Tuomola, M. Rapid time-resolved fluoroimmunoassay for the screening of narasin and salinomycin residues in poultry and eggs. J. Agric. Food Chem. 2004, 52, 1824–1828. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhang, Z.; Yao, Y.; Shi, W.; Liu, Y.; Zhang, S. A Monoclonal antibody-based time-resolved fluoroimmunoassay for chloramphenicol in shrimp and chicken muscle. Anal. Chim. Acta 2006, 575, 262–266. [Google Scholar] [CrossRef]
- Bacigalupo, M.A.; Meroni, G.; Secundo, F.; Lelli, R. Time-resolved fluoroimmunoassay for quantitative determination of ampicillin in cow milk samples with different fat contents. Talanta 2008, 77, 126–130. [Google Scholar] [CrossRef]
- Pikkemaat, M.G.; Rapallini, M.L.; Karp, M.T.; Elferink, J.W. Application of a luminescent bacterial biosensor for the detection of tetracyclines in routine analysis of poultry muscle samples. Food Addit. Contam. Part A 2010, 27, 1112–1117. [Google Scholar] [CrossRef]
- Thompson, C.S.; Traynor, I.M.; Fodey, T.L.; Faulkner, D.V.; Crooks, S.R.H. Screening method for the detection of residues of amphenicol antibiotics in bovine, ovine and porcine kidney by optical biosensor. Talanta 2017, 172, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Gamella, M.; Campuzano, S.; Conzuelo, F.; Esteban-Torres, M.; de las Rivas, B.; Reviejo, A.J.; Munoz, R.; Pingarron, J.M. An amperometric affinity penicillin-binding protein magnetosensor for the detection of beta-lactam antibiotics in milk. Analyst 2013, 138, 2013–2022. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Chen, J.; Jiang, H.; He, J.; Shi, W.; Zhao, W.; Shen, J. Application of quantum dot-antibody conjugates for detection of sulfamethazine residue in chicken muscle tissue. J. Agric. Food Chem. 2006, 54, 6139–6142. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Fernandez, J.; Trapiella-Alfonso, L.; Costa-Fernandez, J.M.; Pereiro, R.; Sanz-Medel, A. A Quantum dot-based immunoassay for screening of tetracyclines in bovine muscle. J. Agric. Food Chem. 2014, 62, 1733–1740. [Google Scholar] [CrossRef] [PubMed]
- Berlina, A.N.; Taranova, N.A.; Zherdev, A.V.; Vengerov, Y.Y.; Dzantiev, B.B. Quantum dot-based lateral flow immunoassay for detection of chloramphenicol in milk. Anal. Bioanal. Chem. 2013, 405, 4997–5000. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.H.; Shen, J.Y.; Kim, M.R.; Lee, C.J.; Kim, I.S. Determination of the fluoroquinolone enrofloxacin in edible chicken muscle by supercritical fluid extraction and liquid chromatography with fluorescence detection. J. Agric. Food Chem. 2003, 51, 7528–7532. [Google Scholar] [CrossRef] [PubMed]
- Tejada-Casado, C.; Lara, F.J.; Garcia-Campana, A.M.; Del Olmo-Iruela, M. Ultra-high performance liquid chromatography with fluorescence detection following salting-out assisted liquid-liquid extraction for the analysis of benzimidazole residues in farm fish samples. J. Chromatogr. A 2018, 1543, 58–66. [Google Scholar] [CrossRef]
- Gamba, V.; Terzano, C.; Fioroni, L.; Moretti, S.; Dusi, G.; Galarini, R. Development and validation of a confirmatory method for the determination of sulphonamides in milk by liquid chromatography with diode array detection. Anal. Chim. Acta 2009, 637, 18–23. [Google Scholar] [CrossRef]
- Xu, Z.; Song, C.; Hu, Y.; Li, G. Molecularly imprinted stir bar sorptive extraction coupled with high performance liquid chromatography for trace analysis of sulfa drugs in complex samples. Talanta 2011, 85, 97–103. [Google Scholar] [CrossRef]
- Cerkvenik-Flajs, V. Performance characteristics of an analytical procedure for determining chloramphenicol residues in muscle tissue by gas chromatography-electron capture detection. Biomed. Chromatogr. 2006, 20, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Chen, D.; Yu, G.; Yu, H.; Pan, Y.; Wang, Y.; Huang, L.; Yuan, Z. Simultaneous determination of lincomycin and spectinomycin residues in animal tissues by gas chromatography-nitrogen phosphorus detection and gas chromatography-mass spectrometry with accelerated solvent extraction. Food Addit. Contam. Part A 2011, 28, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Tao, Y.; Le, T.; Chen, D.; Ishsan, A.; Liu, Y.; Wang, Y.; Yuan, Z. Simultaneous determination of amitraz and its metabolite residue in food animal tissues by gas chromatography-electron capture detector and gas chromatography–mass spectrometry with accelerated solvent extraction. J. Chromatogr. B 2010, 878, 1746–1752. [Google Scholar] [CrossRef]
- Hoff, R.B.; Barreto, F.; Kist, T.B. Use of capillary electrophoresis with laser-induced fluorescence detection to screen and liquid chromatography-tandem mass spectrometry to confirm sulfonamide residues: Validation According to European Union 2002/657/EC. J. Chromatogr. A 2009, 1216, 8254–8261. [Google Scholar] [CrossRef]
- Polo-Luque, M.L.; Simonet, B.M.; Valcarcel, M. Solid phase extraction-capillary electrophoresis determination of sulphonamide residues in milk samples by use of C18- carbon nanotubes as hybrid sorbent materials. Analyst 2013, 138, 3786–3791. [Google Scholar] [CrossRef]
- Yu, C.Z.; He, Y.Z.; Fu, G.N.; Xie, H.Y.; Gan, W.E. Determination of kanamycin A, amikacin and tobramycin residues in milk by capillary zone electrophoresis with post-column derivatization and laser-induced fluorescence detection. J. Chromatogr. B 2009, 877, 333–338. [Google Scholar] [CrossRef]
- Dominguez-Alvarez, J.; Mateos-Vivas, M.; Garcia-Gomez, D.; Rodriguez-Gonzalo, E.; Carabias-Martinez, R. Capillary electrophoresis coupled to mass spectrometry for the determination of anthelmintic benzimidazoles in eggs using a QuEChERS with preconcentration as sample treatment. J. Chromatogr. A 2013, 1278, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Santos, B.; Lista, A.; Simonet, B.M.; Rios, A.; Valcarcel, M. Screening and analytical confirmation of sulfonamide residues in milk by capillary electrophoresis-mass spectrometry. Electrophoresis 2005, 26, 1567–1575. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Gonzalez, D.; Lara, F.J.; Gamiz-Gracia, L.; GarciaCampana, A.M. Molecularly imprinted polymer as in-line concentrator in capillary electrophoresis coupled with mass spectrometry for the determination of quinolones in bovine milk samples. J. Chromatogr. A 2014, 1360, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Gonzalez, D.; Hamed, A.M.; Gilbert-Lopez, B.; Gamiz-Gracia, L.; Garcia-Campana, A.M. Evaluation of a multiresidue capillary electrophoresis-quadrupole-time-of flight mass spectrometry method for the determination of antibiotics in milk samples. J. Chromatogr. A 2017, 1510, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Azzouz, A.; Souhail, B.; Ballesteros, E. Determination of residual pharmaceuticals in edible animal tissues by continuous solid-phase extraction and gas chromatography-mass spectrometry. Talanta 2011, 84, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liang, F.; Shi, J.; Zhao, X.; Liu, Z.; Wu, L.; Song, Y.; Zhang, H.; Wang, Z. Determination of hormones in milk by hollow fiber-based stirring extraction bar liquid-liquid microextraction gas chromatography-mass spectrometry. Anal. Chim. Acta 2013, 790, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.Q.; Zhang, L.; Li, G.L.; Zhang, H.T.; Yang, X.F.; Liu, J.W.; Li, R.F.; Wang, Z.L.; Wang, J.H. Analysis of 19-Nortestosterone residue in animal tissues by ion-trap gas chromatography-tandem mass spectrometry. J. Zhejiang Univ. Sci. B 2011, 12, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Imamoglu, H.; Oktem Olgun, E. Analysis of veterinary drug and pesticide residues using the ethyl acetate multiclass/multiresidue method in milk by liquid chromatography-tandem mass spectrometry. J. Anal. Meth. Chem. 2016, 2016, 2170165. [Google Scholar] [CrossRef] [PubMed]
- Dasenaki, M.E.; Thomaidis, N.S. Multi-residue determination of 115 veterinary drugs and pharmaceutical residues in milk powder, butter, fish tissue and eggs using liquid chromatography-tandem mass spectrometry. Anal. Chim. Acta 2015, 880, 103–121. [Google Scholar] [CrossRef] [PubMed]
- Arsène, M.M.J.; Davares, A.K.L.; Viktorovna, P.I.; Andreevna, S.L.; Sarra, S.; Khelifi, I.; Sergueïevna, D.M. The public health issue of antibiotic residues in food and feed: Causes, consequences, and potential solutions. Vet. World 2022, 15, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-L.; Deng, J.-F.; Chen, Y.; Chu, W.-L.; Hung, D.-Z.; Yang, C.-C. Late diagnosis of an outbreak of leanness-enhancing agent–related food poisoning. Am. J. Emerg. Med. 2013, 31, 1501–1503. [Google Scholar] [CrossRef]
- Khalifa, H.O.; Okanda, T.; Abd El-Hafeez, A.A.; Abd El Latif, A.; Habib, A.G.; Yano, H.; Kato, Y.; Matsumoto, T. Comparative evaluation of five assays for detection of carbapenemases with a proposed scheme for their precise application. J. Mol. Diagn. 2020, 22, 1129–1138. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, H.O.; Oreiby, A.F.; Abd El-Hafeez, A.A.; Okanda, T.; Haque, A.; Anwar, K.S.; Tanaka, M.; Miyako, K.; Tsuji, S.; Kato, Y.; et al. First report of multidrug-resistant carbapenemase-producing bacteria coharboring mcr-9 associated with respiratory disease complex in pets: Potential of animal-human transmission. Antimicrob. Agents Chemoth. 2020, 65, e01890-20. [Google Scholar] [CrossRef]
- Khalifa, H.O.; Oreiby, A.; Abd El-Hafeez, A.A.; Abd El Latif, A.; Okanda, T.; Kato, Y.; Matsumoto, T. High β-lactam and quinolone resistance of Enterobacteriaceae from the respiratory tract of sheep and goat with respiratory disease. Animals 2021, 11, 2258. [Google Scholar] [CrossRef]
- Khalifa, H.O.; Oreiby, A.F.; Okanda, T.; Kato, Y.; Matsumoto, T. High β-lactam resistance in Gram-negative bacteria associated with kennel cough and cat flu in Egypt. Sci. Rep. 2021, 11, 3347. [Google Scholar] [CrossRef] [PubMed]
- Habib, I.; Elbediwi, M.; Mohamed, M.Y.I.; Ghazawi, A.; Abdalla, A.; Khalifa, H.O.; Khan, M. Enumeration, antimicrobial resistance and genomic characterization of extended-spectrum β-lactamases producing Escherichia coli from supermarket chicken meat in the United Arab Emirates. Int. J. Food Microbiol. 2023, 398, 110224. [Google Scholar] [CrossRef]
- Oreiby, A.; Khalifa, H.; Eid, A.; Ahmed, A.; Shimamoto, T. Staphylococcus aureus and bovine mastitis: Molecular typing of methicillin resistance and clinical description of infected quarters. J. Hellenic Vet. Med. Soc. 2019, 70, 1511–1516. [Google Scholar] [CrossRef]
- Lu, Q.; Okanda, T.; Yang, Y.; Khalifa, H.O.; Haque, A.; Takemura, H.; Matsumoto, T. High-speed quenching probe-polymerase chain reaction assay for the rapid detection of carbapenemase-producing gene using GENECUBE: A fully automatic gene analyzer. Mol. Diagn. Ther. 2021, 25, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Menkem, Z.E.; Ngangom, B.L.; Tamunjoh, S.S.A.; Boyom, F.F. Antibiotic residues in food animals: Public health concern. Acta Ecol. Sin. 2019, 39, 411–415. [Google Scholar] [CrossRef]
- Landers, T.F.; Cohen, B.; Wittum, T.E.; Larson, E.L. A review of antibiotic use in food animals: Perspective, policy, and potential. Public Health Rep. 2012, 127, 4–22. [Google Scholar] [CrossRef]
- Elafify, M.; Khalifa, H.O.; Al-Ashmawy, M.; Elsherbini, M.; El Latif, A.A.; Okanda, T.; Matsumoto, T.; Koseki, S.; Abdelkhalek, A. Prevalence and antimicrobial resistance of Shiga toxin-producing Escherichia coli in milk and dairy products in Egypt. J. Environ. Sci. Health B 2020, 55, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Habib, I.; Elbediwi, M.; Mohteshamuddin, K.; Mohamed, M.Y.I.; Lakshmi, G.B.; Abdalla, A.; Anis, F.; Ghazawi, A.; Khan, M.; Khalifa, H.O. Genomic profiling of extended-spectrum β-lactamase-producing Escherichia coli from pets in the United Arab Emirates: Unveiling colistin resistance mediated by mcr-1.1 and its probable transmission from chicken meat-A One Health perspective. J. Infect. Public Health 2023, 1, 163–171. [Google Scholar] [CrossRef]
- National Research Council (NRC). The Use of Drugs in Food Animal Benefits and Risks; The National academies Press: Washington, DC, USA, 1999; Volume 253, p. 284.
- Schlomann, B.H.; Wiles, T.J.; Wall, E.S.; Guillemin, K.; Parthasarathy, R. Sublethal antibiotics collapse gut bacterial populations by enhancing aggregation and expulsion. Proc. Natl. Acad. Sci. USA 2019, 116, 21392–21400. [Google Scholar] [CrossRef]
- Ghimpețeanu, O.M.; Pogurschi, E.N.; Popa, D.C.; Dragomir, N.; Drăgotoiu, T.; Mihai, O.D.; Petcu, C.D. Antibiotic use in livestock and residues in food—A public health threat: A review. Foods 2022, 11, 1430. [Google Scholar] [CrossRef] [PubMed]
- Allen, H.K. Antibiotic resistance gene discovery in food-producing animals. Curr. Opin. Microbiol. 2014, 19, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Kyuchukova, R. Antibiotic residues and human health hazard—Review. Bulg. J. Agric. Sci. 2020, 26, 664–668. [Google Scholar]
- Bacanlı, M.; Başaran, N. Importance of antibiotic residues in animal food. Food Chem. Toxicol. 2019, 125, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Minaldi, E.; Phillips, E.J.; Norton, A. Immediate and delayed hypersensitivity reactions to beta-lactam antibiotics. Clin. Rev. Allergy Immunol. 2021, 62, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Szegedi, A.; Remenyik, E.; Gellén, E. Drug hypersensitivity reactions. In European Handbook of Dermatological Treatments; Springer International Publishing: Cham, Switzerland, 2023; pp. 229–245. [Google Scholar]
- Ulitzka, M.; Carrara, S.; Grzeschik, J.; Kornmann, H.; Hock, B.; Kolmar, H. Engineering therapeutic antibodies for patient safety: Tackling the immunogenicity problem. Protein Eng. Des. Sel. 2020, 33, gzaa025. [Google Scholar] [CrossRef]
- Kanny, G.; Puygrenier, J.; Beaudoin, E.; Moneret-Vautrin, D.A. Choc anaphylactique alimentaire: Implication des résidus de pénicilline. Allerg. Immunol. 1994, 26, 181–183. [Google Scholar]
- Raison-Peyron, N.; Messaad, D.; Bousquet, J.; Demoly, P. Anaphylaxis to beef in penicillin-allergic patient. Allergy 2001, 56, 796–797. [Google Scholar]
- Tinkelman, D.G.; Bock, S.A. Anaphylaxis presumed to be caused by beef containing streptomycin. Ann. Allergy 1984, 53, 243–244. [Google Scholar]
- Schwartz, M.H.; Sher, T.H. Anaphylaxis to penicillin in a frozen dinner. Ann. Allergy 1984, 52, 342–343. [Google Scholar]
- Falowo, A.; Akinmoladun, O. Veterinary drug residues in meat and meat products: Occurrence, detection and implications. Vet. Med. Pharm. 2019, 3, 194. [Google Scholar]
- Okocha, R.C.; Olatoye, I.O.; Adedeji, O.B. Food safety impacts of antimicrobial use and their residues in aquaculture. Public Health Rev. 2018, 39, 21. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, J.; Kang, R.; Klionsky, D.J.; Tang, D. Ferroptosis: Machinery and regulation. Autophagy 2021, 17, 2054–2081. [Google Scholar] [CrossRef] [PubMed]
- Elisabeth, Z.M. Antibiotic residues in food. In Antibiotics-Therapeutic Spectrum and Limitations; Elsevier: Amsterdam, The Netherlands, 2023; pp. 645–675. [Google Scholar]
- Sundlof, S.F. Human health risks associated with drug residues in animal-derived foods. J. Agromed. 1994, 1, 5–20. [Google Scholar] [CrossRef]
- Treiber, F.M.; Beranek-Knauer, H. Antimicrobial residues in food from animal origin—A review of the literature focusing on products collected in stores and markets worldwide. Antibiotics 2021, 10, 534. [Google Scholar] [CrossRef]
- Uchiyama, K.; Naito, Y.; Takagi, T. Intestinal microbiome as a novel therapeutic target for local and systemic inflammation. Pharm. Ther. 2019, 199, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Guida, S.; Venema, K. Gut microbiota and obesity: Involvement of the adipose tissue. J. Funct. Foods 2015, 14, 407–423. [Google Scholar] [CrossRef]
- Zhu, Y.; Luo, T.M.; Jobin, C.; Young, H.A. Gut microbiota and probiotics in colon tumorigenesis. Cancer Lett. 2011, 309, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Wang, Y.; Huang, L.; Shen, M.; Yu, Y.; Yu, Q.; Chen, Y.; Xie, J. Review of the relationships among polysaccharides, gut microbiota, and human health. Food Res. Int. 2021, 140, 109858. [Google Scholar] [CrossRef]
- Cabello, F.C. Heavy use of prophylactic antibiotics in aquaculture: A growing problem for human and animal health and for the environment. Environ. Microbiol. 2006, 8, 1137–1144. [Google Scholar] [CrossRef]
- Linge, B.; Gonzalo, C.; Carriedo, J.A.; Asensio, J.A.; Blanco, M.A.; De La Fuente, L.F.; San Primitivo, F. Performance of blue-yellow screening test for antimicrobial detection in ovine milk. J. Dairy Sci. 2007, 90, 5374–5379. [Google Scholar] [CrossRef]
- Herago, T.; Agonafir, A. Drug residues in foods of animal origin and their impact on human health: Review. Food Sci. Qual. Manag. 2021, 108, 12–21. [Google Scholar]
- Ben, Y.; Hu, M.; Zhang, X.; Wu, S.; Wong, M.H.; Wang, M.; Andrews, C.B.; Zheng, C. Efficient detection and assessment of human exposure to trace antibiotic residues in drinking water. Water Res. 2020, 175, 115699. [Google Scholar] [CrossRef]
- Baynes, R.E.; Dedonder, K.; Kissell, L.; Mzyk, D.; Marmulak, T.; Smith, G.; Tell, L.; Gehring, R.; Davis, J.; Riviere, J.E. Health concerns and management of select veterinary drug residues. Food Chem. Toxicol. 2016, 88, 112–122. [Google Scholar] [CrossRef]
- Kemp, S.A.; Pinchbeck, G.L.; Fèvre, E.M.; Williams, N.J. A cross-sectional survey of the knowledge, attitudes, and practices of antimicrobial users and providers in an area of high-density livestock-human population in Western Kenya. Front. Vet. Sci. 2021, 8, 727365. [Google Scholar] [CrossRef]
- Al Sattar, A.; Chisty, N.N.; Irin, N.; Uddin, M.H.; Hasib, F.Y.; Hoque, M.A. Knowledge and practice of antimicrobial usage and resistance among poultry farmers: A systematic review, meta-analysis, and meta-regression. Vet. Res. Commun. 2023, 47, 1047–1066. [Google Scholar] [CrossRef]
- Caudell, M.A.; Quinlan, M.B.; Subbiah, M.; Call, D.R.; Roulette, C.J.; Roulette, J.W.; Roth, A.; Matthews, L.; Quinlan, R.J. Antimicrobial use and veterinary care among agro-pastoralists in Northern Tanzania. PLoS ONE 2017, 12, e0170328. [Google Scholar] [CrossRef]
- Kigozi, M.; Higenyi, J. Evaluation of farmers knowledge and application of guidelines on use of veterinary antibiotics in layer poultry production in Mukono district, central Uganda. Livest. Res. Rural Dev. 2017, 29, 176. [Google Scholar]
- Kimera, Z.I.; Frumence, G.; Mboera, L.E.G.; Rweyemamu, M.; Mshana, S.E.; Matee, M.I. Assessment of drivers of antimicrobial use and resistance in poultry and domestic pig farming in the Msimbazi river basin in Tanzania. Antibiotics 2020, 9, 838. [Google Scholar] [CrossRef]
- European Union. Regulation 2017/625 of the European Parliament and of the Council of 15 March 2017 on official controls and other official activities performed to ensure the application of food and feed law, rules on animal health and welfare, plant health and plant protection products. Off. J. Eur. Union 2017, L95, 1–142. [Google Scholar]
- van Asselt, E.D.; Jager, J.; Jansen, L.J.M.; Hoek-van den Hil, E.F.; Barbu, I.; Rutgers, P.; Pikkemaat, M.G. Prioritizing veterinary drug residues in animal products for risk-based monitoring. Food Control 2023, 123, 109782. [Google Scholar] [CrossRef]
- Focker, M.; van Asselt, E.D.; Van Der Fels-Klerx, H.J. Designing a risk-based monitoring plan for pathogens in food: A review. Food Control 2023, 143, 109319. [Google Scholar] [CrossRef]
- van Asselt, E.D.; Noordam, M.Y.; Pikkemaat, M.G.; Dorgelo, F.O. Risk-based monitoring of chemical substances in food: Prioritization by decision trees. Food Control 2018, 93, 112–120. [Google Scholar] [CrossRef]
- van der Fels-Klerx, H.J.; van Asselt, E.D.; Raley, M.; Poulsen, M.; Korsgaard, H.; Bredsdorff, L.; Nauta, M.; D’agostino, M.; Coles, D.; Marvin, H.J.; et al. Critical review of methods for risk ranking of food related hazards, based on risks for human health. Crit. Rev. Food Sci. Nutr. 2018, 58, 178–193. [Google Scholar] [CrossRef]
- van der Fels-Klerx, H.J.; Van Asselt, E.D.; Raley, M.; Poulsen, M.; Korsgaard, H.; Bredsdorff, L.; Nauta, M.; Flari, V.; d’Agostino, M.; Coles, D.; et al. Critical review of methodology and application of risk ranking for prioritisation of food and feed related issues, on the basis of the size of anticipated health impact. EFSA Support. Publ. 2015, EN-710, 106. [Google Scholar] [CrossRef]
- Kang, H.S.; Han, S.; Cho, B.H.; Lee, H. Risk-based approach to develop a national residue program: Prioritizing the residue control of veterinary drugs in fishery products. Fish Aquat. Sci. 2019, 22, 29. [Google Scholar] [CrossRef]
- Chah, J.M.; Nwankwo, S.C.; Uddin, I.O.; Chah, K.F. Knowledge and practices regarding antibiotic use among small-scale poultry farmers in Enugu State, Nigeria. Heliyon 2022, 8, e09342. [Google Scholar] [CrossRef]
- Mudenda, S.; Malama, S.; Munyeme, M.; Hang’ombe, B.M.; Mainda, G.; Kapona, O.; Mukosha, M.; Yamba, K.; Bumbangi, F.N.; Mfune, R.L.; et al. Awareness of antimicrobial resistance and associated factors among layer poultry farmers in Zambia: Implications for surveillance and antimicrobial stewardship programs. Antibiotics 2022, 11, 383. [Google Scholar] [CrossRef]
- Food and Drug Administration. FDA’s Strategy on Antimicrobial Resistance. 2013. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/fdas-strategy-antimicrobial-resistance-questions-and-answers (accessed on 17 January 2024).
- Food and Agriculture Organization. The FAO Action Plan on Antimicrobial Resistance 2016–2020. Supporting the Food and Agriculture Sectors in Implementing the Global Action Plan on Antimicrobial Resistance to Minimize the Impact of Antimicrobial Resistance. 2020. Available online: https://www.fao.org/poultry-production-products/production/en/ (accessed on 17 January 2024).
- World Health Organization. Annual Report on Antimicrobial Agents Intended for the Use in Animals: Better Understanding of the Global Situation (Fourth Report). Paris, France. 2020. Available online: https://www.woah.org/fleadmin/Home/eng/Our_scientifc_expertise/docs/pdf/A_Fourth_Annual_Report_AMU.pdf (accessed on 17 January 2024).
- Nonga, H.E.; Mariki, M.; Karimuribo, E.D.; Mdegela, R.H. Assessment of antimicrobial usage and antimicrobial residues in broiler chickens in Morogoro Municipality, Tanzania. Pak. J. Nutr. 2009, 8, 203–207. [Google Scholar] [CrossRef]
- Kiambi, S.; Mwanza, R.; Sirma, A.; Czerniak, C.; Kimani, T.; Kabali, E.; Dorado-Garcia, A.; Eckford, S.; Price, C.; Gikonyo, S.; et al. Understanding antimicrobial use contexts in the poultry sector: Challenges for smallscale layer farms in Kenya. Antibiotics 2021, 10, 106. [Google Scholar] [CrossRef]
- Tufa, T.B.; Gurmu, F.; Beyi, A.F.; Hogeveen, H.; Beyene, T.J.; Ayana, D.; Woldemariyam, F.T.; Hailemariam, E.; Gutema, F.D.; Stegeman, J.A. Veterinary medicinal product usage among food animal producers and its health implications in Central Ethiopia. BMC Vet. Res. 2018, 14, 409. [Google Scholar] [CrossRef]
- Majdinasab, M.; Yaqub, M.; Rahim, A.; Catanante, G.; Hayat, A.; Marty, J.L. An overview on recent progress in electrochemical biosensors for antimicrobial drug residues in animal-derived food. Sensors 2017, 17, 1947. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Liu, Q.; Hao, N.; Kun, W. Recent developments of photoelectrochemical biosensors for food analysis. J. Mater. Chem. B 2019, 7, 7283–7300. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, A.S.; Ansari, N.; Ramezani, M.; Abnous, K.; Mohsenzadeh, M.; Taghdisi, S.M.; Alibolandi, M. Optical and electrochemical aptasensors for the detection of amphenicols. Biosens. Bioelectron. 2018, 118, 137–152. [Google Scholar] [CrossRef]
- Majdinasab, M.; Mitsubayashi, K.; Marty, J.L. Optical and electrochemical sensors and biosensors for the detection of quinolones. Trends Biotechnol. 2019, 37, 898–915. [Google Scholar] [CrossRef] [PubMed]
- Mehlhorn, A.; Rahimi, P.; Joseph, Y. Aptamer-based biosensors for antibiotic detection: A review. Biosensors 2018, 8, 54. [Google Scholar] [CrossRef]
- Kharewal, T.; Verma, N.; Gahlaut, A.; Hooda, V. Biosensors for penicillin quantification: A comprehensive review. Biotechnol. Lett. 2020, 42, 1829–1846. [Google Scholar] [CrossRef]
- Cervera-Chiner, L.; Jiménez, Y.; Montoya, Á.; Juan-Borrás, M.; Pascual, N.; Arnau, A.; Escriche, I. High fundamental frequency quartz crystal microbalance (HFF-QCMD) immunosensor for detection of sulfathiazole in honey. Food Control 2020, 115, 107296. [Google Scholar] [CrossRef]
- Mirecki, S.; Nikolić, N. Influence of preservative concentration, pH Value and fat content in raw milk at detection limit of microbial inhibitor tests (Delvotest® Accelerator) for amoxicillin and oxytetracycline. Food Anal. Meth. 2016, 9, 2864–2871. [Google Scholar] [CrossRef]
- Bion, C.; Beck Henzelin, A.; Qu, Y.; Pizzocri, G.; Bolzoni, G.; Buffoli, E. Analysis of 27 antibiotic residues in raw cow’s milk and milk-based products–validation of Delvotest® T. Food Addit. Contam. Part A 2016, 33, 54–59. [Google Scholar] [CrossRef]


| Animal Species | Drug | Withdrawal Period/Days | Reference |
|---|---|---|---|
| Cattle | Ampicillin | 15 days (oral) and 6 days (injection) | [27] |
| Chlortetracycline | 10 days (oral and injection) | [27] | |
| Dihydrostreptomycine | 10 days (oral) and 30 days (injection) | [27] | |
| Erythromycin | 14 days (injection) | [27] | |
| Procaine penicillin | 10 days (injection) | [27] | |
| Oxytetracycline | 7 days (oral) and 22 days (injection) | [27] | |
| Sulphamezathine | 7 days (oral) | [27] | |
| Dihydrostreptomycine | 4 days (intramammary) | [28] | |
| Streptomycin | 2 days (oral) | [28] | |
| Neomycin | 1 days (oral) * | [28] | |
| Ivermectin | 49–66 days (subcutaneous) ** | [29] | |
| Sheep and goat | Dihydrostreptomycine | 30 days (injection) | [27] |
| Erythromycin | 3 days (injection) | [27] | |
| Procaine penicillin G | 9 days (injection) | [27] | |
| Chlortetracycline | 2 days (oral) | [27] | |
| Sulphamezathine | 10 days (oral and injection) | [27] | |
| Sulphaquinoxaline | 10 days (oral) | [27] | |
| Neomycin | 2 days (oral) | [28] | |
| Swine | Streptomycin | 0 days (oral) | [28] |
| Gentamicin | 3–14 days (oral) *** and 40 days (intramuscular) | [28] | |
| Neomycin | 3 days (oral) | [28] | |
| Apramycin | 28 days (oral) | [28] | |
| Ivermectin | 5 days (oral) | [30] | |
| Levamisole | 3 days (oral) | [30] | |
| Piperazine | 21 days (oral) | [30] | |
| Pyrantel tartrate | 1 day (oral) | [30] | |
| Dichlorvos | 0 day (oral) | [30] | |
| Fenbendazole | 0 day (oral) | [30] | |
| Chickens | Streptomycin | 4 days (oral) | [28] |
| Gentamicin | 35 days (subcutaneous) | [28] | |
| Chlortetracycline | 1 day | [27] | |
| Erythromycin | 2 days | [27] | |
| Monensin | 5 days | [27] | |
| Tylosine | 5 days | [27] | |
| Levamisole | 0–7 days **** | [31] | |
| Ivermectin | 0–12 days ***** (oral) | [32] | |
| Nicarbazin narasin combination | 5 days ****** | [33] | |
| Lasalocid, salinomycin narasin, maduramicin, and semduramicin | 5 days ******* | [33] | |
| Ciprofloxacin | 15–19 days ******** | [33] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalifa, H.O.; Shikoray, L.; Mohamed, M.-Y.I.; Habib, I.; Matsumoto, T. Veterinary Drug Residues in the Food Chain as an Emerging Public Health Threat: Sources, Analytical Methods, Health Impacts, and Preventive Measures. Foods 2024, 13, 1629. https://doi.org/10.3390/foods13111629
Khalifa HO, Shikoray L, Mohamed M-YI, Habib I, Matsumoto T. Veterinary Drug Residues in the Food Chain as an Emerging Public Health Threat: Sources, Analytical Methods, Health Impacts, and Preventive Measures. Foods. 2024; 13(11):1629. https://doi.org/10.3390/foods13111629
Chicago/Turabian StyleKhalifa, Hazim O., Lamek Shikoray, Mohamed-Yousif Ibrahim Mohamed, Ihab Habib, and Tetsuya Matsumoto. 2024. "Veterinary Drug Residues in the Food Chain as an Emerging Public Health Threat: Sources, Analytical Methods, Health Impacts, and Preventive Measures" Foods 13, no. 11: 1629. https://doi.org/10.3390/foods13111629
APA StyleKhalifa, H. O., Shikoray, L., Mohamed, M.-Y. I., Habib, I., & Matsumoto, T. (2024). Veterinary Drug Residues in the Food Chain as an Emerging Public Health Threat: Sources, Analytical Methods, Health Impacts, and Preventive Measures. Foods, 13(11), 1629. https://doi.org/10.3390/foods13111629








