Thawing of Frozen Hairtail (Trichiurus lepturus) with Graphene Nanoparticles Combined with Radio Frequency: Variations in Protein Aggregation, Structural Characteristics, and Stability
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Experimental Setup
2.2.1. AT
2.2.2. RT
2.2.3. GO-RT
2.2.4. GM-RT
2.3. Texture Analysis
2.4. Centrifugal Loss
2.5. Scanning Electron Microscope
2.6. Extraction of MP
2.7. Measurement of Particle Size
2.8. Measurement of Zeta Potential
2.9. X-ray Powder Diffraction
2.10. Fluorescence Spectroscopy
2.11. Differential Scanning Calorimetry
2.12. Statistical Analysis
3. Results and Discussion
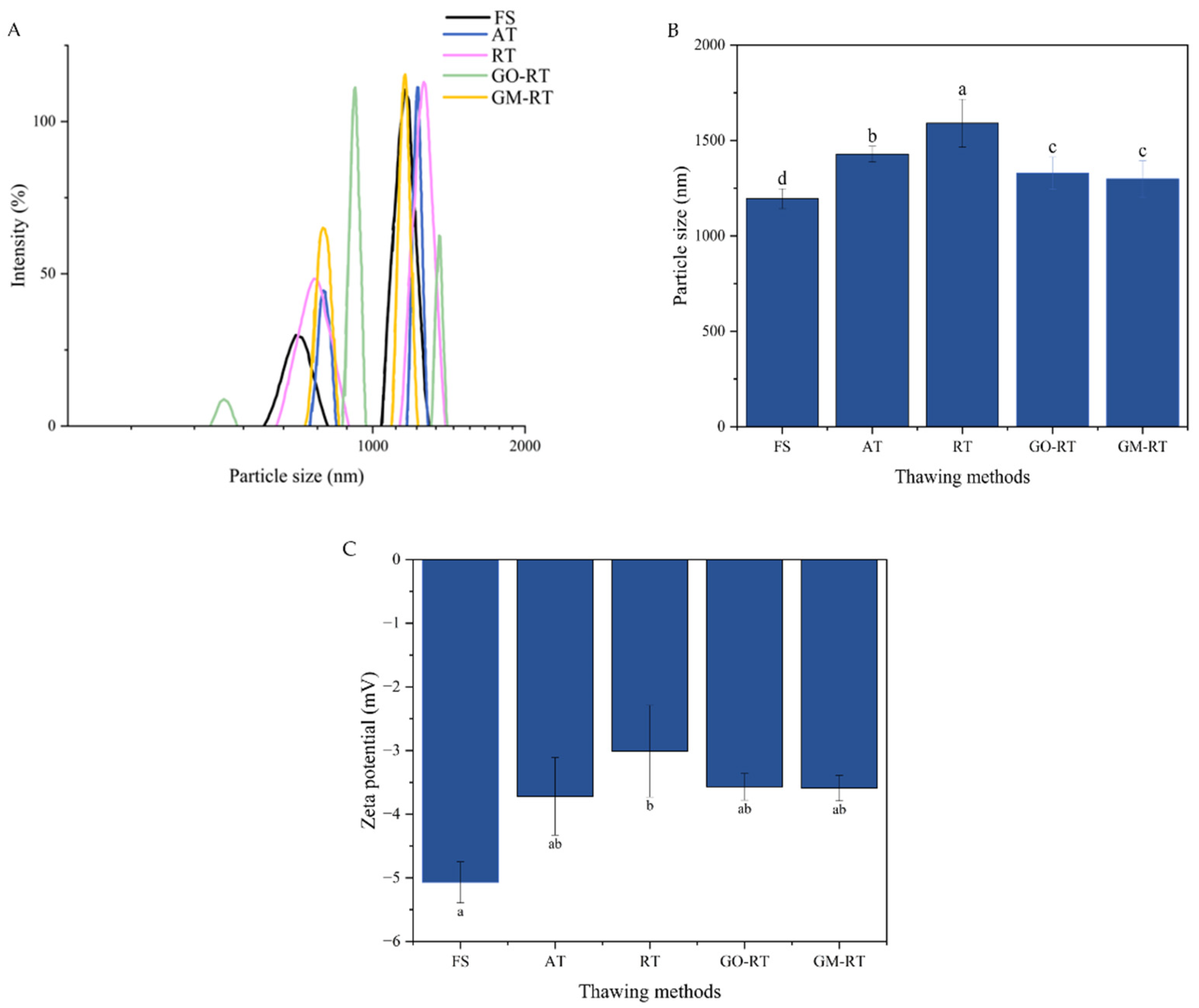
3.1. Effects of Different Thawing Methods on the Particle Size and Zeta Potential of Hairtail MP
3.2. Effects of Different Thawing on MP Secondary and Tertiary Structures in Hairtail
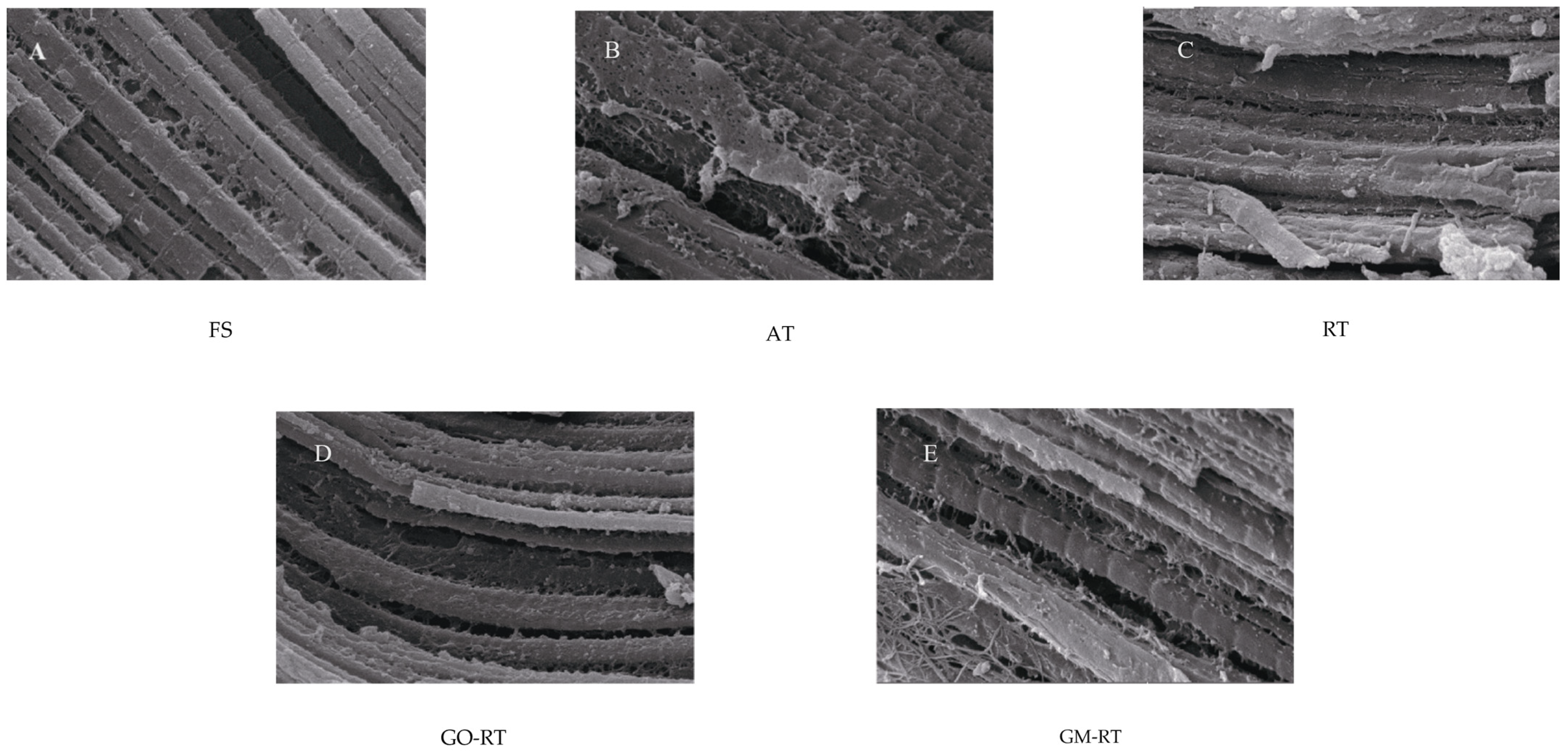
3.3. Effects of Different Thawing on Microstructure in Hairtail
3.4. Effects of Different Thawing Methods on Texture and Centrifugal Loss of Hairtail Muscle
3.5. Differential Scanning Calorimetry
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yan, B.; Bai, W.; Tao, Y.; Ye, W.; Zhang, W.; Zhang, N.; Huang, J.; Chen, W.; Fan, D. Physicochemical changes and comparative proteomics analysis of hairtail (Trichiurus lepturus) fish muscles during frozen storage. Food Biosci. 2023, 55, 103021. [Google Scholar] [CrossRef]
- Liao, B.; Karim, E.; Zhang, K. Comparative performance of catch-based and surplus production models on evaluating largehead hairtail (Trichiurus lepturus) fishery in the East China Sea. Reg. Stud. Mar. Sci. 2021, 48, 102026. [Google Scholar] [CrossRef]
- Mousakhani, G.A.; Hamdami, N.; Soltanizadeh, N. Impact of high voltage electric field thawing on the quality of frozen tuna fish (Thunnus albacares). J. Food Eng. 2015, 156, 39–44. [Google Scholar] [CrossRef]
- Peng, Z.; Zhu, M.; Zhang, J.; Zhao, S.; He, H.; Kang, Z.; Ma, H.; Xu, B. Physicochemical and structural changes in myofibrillar proteins from porcine longissimus dorsi subjected to microwave combined with air convection thawing treatment. Food Chem. 2021, 343, 128412. [Google Scholar] [CrossRef]
- Zhou, P.C.; Xie, J. Effect of different thawing methods on the quality of mackerel (Pneumatophorus japonicus). Food Sci. Biotechnol. 2021, 30, 1213–1223. [Google Scholar] [CrossRef]
- Cai, L.; Wan, J.; Li, X.; Li, J. Effects of different thawing methods on conformation and oxidation of myofibrillar protein from largemouth bass (Micropterus salmoides). J. Food Sci. 2022, 85, 2470–2480. [Google Scholar] [CrossRef]
- Li, F.; Wang, B.; Kong, B.; Xia, X.; Bao, Y. Impact of Ultrasound-assisted Saline Thawing on the Technological Properties of mirror carp (Cyprinuscarpio L.). Ultrason. Sonochemistry 2022, 86, 106014. [Google Scholar] [CrossRef]
- Zhang, W.; Guan, W.; Cai, L.; Wang, X.; Zhang, Z.; Ni, Z. Effects of magnetic nanometer combined with radio frequency or microwave thawing on physicochemical properties of myofibrillary protein in sea bass. LWT Food Sci. Technol. 2022, 154, 112585. [Google Scholar] [CrossRef]
- Hassoun, A.; Shumilina, E.; Di Donato, F.; Foschi, M.; Simal-Gandara, J.; Biancolillo, A. Emerging techniques for differentiation of fresh and frozen–thawed seafoods: Highlighting the potential of spectroscopic techniques. Molecules 2020, 25, 4472. [Google Scholar] [CrossRef]
- Cao, M.; Cao, A.; Wang, J.; Cai, L.; Regenstein, J.; Ruan, Y.; Li, X. Effect of magnetic nanoparticles plus microwave or far-infrared thawing on protein conformation changes and moisture migration of red seabream (Pagrus major) fillets. Food Chem. 2018, 266, 498–507. [Google Scholar] [CrossRef]
- Zhu, W.; Guo, H.; Han, M.; Shan, C.; Bu, Y.; Li, J.; Li, X. Evaluating the effects of nanoparticles combined ultrasonic-microwave thawing on water holding capacity, oxidation, and protein conformation in jumbo squid (Dosidicus gigas) mantles. Food Chem. 2023, 402, 134250. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, Y.; Guo, L.; Ouyang, N.; Wang, B. Application of multi-frequency ultrasonic thawing on pork: Thawing rate, quality properties and microstructure. Food Phys. 2023, 1, 100002. [Google Scholar] [CrossRef]
- Ramaswamy, H.; Tang, J. Microwave and radio frequency heating. Food Sci. Technol. Int. 2008, 14, 423–427. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Zhou, F.; Wu, Y.; Ma, H.; Zhao, R.; Jian, H.; Gu, Z. Effect of ultrasonic thawing temperature on the quality of quick-frozen small yellow croaker (Larimichthys polyactis) and its possible mechanisms. LWT Food Sci. Technol. 2023, 179, 114620. [Google Scholar] [CrossRef]
- Chen, J.; Xu, Y.; Pius, B.A.; Wang, P.; Xu, X. Changes of myofibrillar protein structure improved the stability and distribution of baicalein in emulsion. LWT Food Sci. Technol. 2021, 137, 110404. [Google Scholar] [CrossRef]
- Fathi, M.; Amir, B.; Hosein, R. Development and characterization of locust bean gum-Viola anthocyanin-graphene oxide ternary nanocomposite as an efficient pH indicator for food packaging application. Food Packag. Shelf Life 2022, 34, 100934. [Google Scholar] [CrossRef]
- He, Y.; Yi, C.; Zhang, X.; Zhao, W.; Yu, D. Magnetic graphene oxide: Synthesis approaches, physicochemical characteristics, and biomedical applications. TrAC Trends Anal. Chem. 2021, 136, 116191. [Google Scholar] [CrossRef]
- Kim, M.D.; Rutka, M.D.; Chan, C.W. Nanomedicine. N. Engl. J. Med. 2010, 363, 2434–2443. [Google Scholar] [CrossRef]
- Koddy, J.K.; Miao, W.; Hatab, S.; Tang, L.; Xu, H.; Nyaisaba, B.M.; Chen, M.; Deng, S. Understanding the role of atmospheric cold plasma (ACP) in maintaining the quality of hairtail (Trichiurus Lepturus). Food Chem. 2021, 343, 128418. [Google Scholar] [CrossRef]
- Tian, F.; Gu, X.; Li, Y.; Cai, L. Evaluating the effects of graphene nanoparticles combined radio-frequency thawing on the physicochemical quality and protein conformation in hairtail (Trichiurus lepturus) dorsal muscle. J. Sci. Food Agric. 2024, 104, 2809–2819. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, Y.; Ding, Y.; Xiang, X.; Yang, Q.; Wei, Z.; Song, H.; Liu, S.; Zhou, X. Improved texture properties and toughening mechanisms of surimi gels by double network strategies. Food Hydrocoll. 2024, 152, 109900. [Google Scholar] [CrossRef]
- Lefevre, F.; Fauconneau, B.; Thompson, J.W.; Gill, T.A. Thermal denaturation and aggregation properties of Atlantic salmon myofibrils and myosin from white and red muscles. J. Agric. Food Chem. 2007, 55, 4761–4770. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, M.; Rezaei, M.; Jafarpour, A.; Undeland, I. Dynamic rheological, microstructural and physicochemical properties of blend fish protein recovered from kilka (Clupeonella cultriventris) and silver carp (Hypophthalmichthys molitrix) by the pH-shift process or washing-based technology. Food Chem. 2017, 229, 695–709. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Kong, B.; Liu, J.; Diao, X.; Liu, Q. Influence of different thawing methods on physicochemical changes and protein oxidation of porcine longissimus muscle. LWT Food Sci. Technol. 2012, 46, 280–286. [Google Scholar] [CrossRef]
- Negi, J.S.; Chattopadhyay, P.; Sharma, A.K. Development of solid lipid nanoparticles (SLNs) of lopinavir using hot self nano-emulsification (SNE) technique. Eur. J. Pharm. Sci. 2013, 48, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Bejraphaab, P.; Mina, S.G.; Surassmoab, S.; Choi, M. Physicothermal properties of freeze-dried fish oil nanocapsules frozen under different conditions. Dry. Technol. 2010, 28, 481–489. [Google Scholar] [CrossRef]
- Promeyrat, A.; Gatellier, P.; Lebret, B.; Kajak-Siemaszko, K.; Aubry, L.; Santé-lhoutellier, V. Evaluation of protein aggregation in cooked meat. Food Chem. 2010, 121, 412–417. [Google Scholar] [CrossRef]
- Sun, Q.; Dong, X.; Zheng, O.; Liu, S.; Kong, B. Protein oxidation/aggregation during ultrasound thawing at different powers impair the gel properties of common carp (Cyprinus carpio) myofibrillar protein. LWT Food Sci. Technol. 2024, 191, 115592. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, R. The effect of pulsed electric fields on the inactivation and structure of lysozyme. Food Chem. 2008, 110, 334–343. [Google Scholar] [CrossRef]
- Li, Z.; Zhong, X.; Luan, C.; Wen, N.; Shi, C.; Lin, X.; Zhao, C.; Zhang, Y.; Luo, L.; Zhang, L.; et al. Fabrication of high-preformance emulsifier from conjugating maltodextrin onto myofibrillar protein peptide with microwave-ultrasound synergy. Ultrason. Sonochemistry 2024, 20, 106818. [Google Scholar] [CrossRef]
- Han, M.; Zhang, Y.; Fei, Y.; Xu, X.; Zhou, G. Effect of microbial transglutaminase on NMR relaxometery and microstructure of pork myofibrillar protein gel. Eur. Food Res. Technol. 2009, 228, 665–670. [Google Scholar] [CrossRef]
- Purohit, S.R.; Jayachandran, L.E.; Raj, A.S.; Nayak, D.; Rao, P.S. X-ray diffraction for food quality evaluation. In Evaluation Technologies for Food Quality; Woodhead Publishing: Sawston, UK, 2019; pp. 579–594. [Google Scholar]
- Farahnak, R.; Nourani, M.; Riahi, E. Ultrasound thawing of mushroom (Agaricus bisporus): Effects on thawing rate, protein denaturation and some physical properties. LWT Food Sci. Technol. 2021, 151, 112150. [Google Scholar] [CrossRef]
- Gan, S.; Zhang, A.; Mujumdar, A.S.; Jiang, Q. Effects of different thawing methods on quality of unfrozen meats. Int. J. Refrig. 2022, 134, 168–175. [Google Scholar] [CrossRef]
- Xie, F.; Zheng, W.; Fu, T.; Zhu, K.; Zhang, H.; Song, Z.; Ai, L. Cryoprotective effect of tamarind seed polysaccharide on grass carp surimi: Characteristics, interactions, and mechanisms. Food Hydrocoll. 2024, 153, 110022. [Google Scholar] [CrossRef]
- Cao, Y.; Xiong, Y. Chlorogenic acid-mediated gel formation of oxidatively stressed myofibrillar protein. Food Chem. 2015, 180, 235–243. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, X.; Yang, W.; Mei, J.; Xie, J. Effect of magnetic nano-particles combined with multi-frequency ultrasound-assisted thawing on the quality and myofibrillar protein-related properties of salmon (Salmo salar). Food Chem. 2024, 445, 138701. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Mei, J.; Xie, J. Effects of multi-frequency ultrasound on the freezing rates, quality properties and structural characteristics of cultured large yellow croaker (Larimichthys crocea). Ultrason. Sonochemistry 2021, 76, 105657. [Google Scholar] [CrossRef]
- Zhu, J.; Li, S.; Yang, L.; Zhao, Z.; Xia, J.; Zhu, Y.; Li, C. Effect of multiple freeze–thaw cycles on water migration, protein conformation and quality attributes of beef longissimus dorsi muscle by real-time low field nuclear magnetic resonance and Raman spectroscopy. Food Res. Int. 2023, 166, 112644. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Kong, B.; Zheng, O.; Liu, S.; Dong, X. Effect of protein structure changes during different power ultrasound thawing on emulsification properties of common carp (Cyprinus carpio) myofibrillar protein. Ultrason. Sonochemistry 2023, 101, 106719. [Google Scholar] [CrossRef]
- Vaskoska, R.; Ha, M.; Ong, L.; Chen, G.; White, J.; Gras, S.; Warner, R. Myosin sensitivity to thermal denaturation explains differences in water loss and shrinkage during cooking in muscles of distinct fibre types. Meat Sci. 2021, 179, 108521. [Google Scholar] [CrossRef]
- Jantakoson, T.; Kongkarn, K. Effect of high pressure and heat treatments on black tiger shrimp (Penaeus monodon Fabricius) muscle protein. Int. Aquat. Res. 2012, 4, 19. [Google Scholar] [CrossRef]



| λmax | FI | |
|---|---|---|
| FS | 297.67 ± 4.02 a | 789.23 ± 44.30 ab |
| AT | 301.01 ± 6.61 a | 933.12 ± 74.83 a |
| RT | 302.14 ± 6.23 a | 943.11 ± 98.33 a |
| GO-RT | 300.04 ± 3.12 a | 779.15 ± 53.63 ab |
| GM-RT | 299.87 ± 2.11 a | 780.09 ± 43.18 ab |
| Hardness (g) | Springiness (mm) | Chewiness (mJ) | |
|---|---|---|---|
| FS | 1475.5 ± 175.1 a | 2.37 ± 0.26 a | 6.22 ± 1.51 ab |
| AT | 1065.2 ± 83.5 ab | 1.53 ± 0.43 b | 4.59 ± 1.91 b |
| RT | 931.5 ± 76.5 b | 1.17 ± 0.55 b | 3.64 ± 2.38 b |
| GO-RT | 1179.9 ± 66.5 ab | 2.01 ± 0.35 a | 5.71 ± 2.63 a |
| GM-RT | 1185.25 ± 131.8 ab | 2.25 ± 1.33 a | 5.75 ± 2.26 ab |
| Peak1 | Peak2 | Peak3 | ||||
|---|---|---|---|---|---|---|
| Tmax1 | ΔH1 | Tmax2 | ΔH2 | Tmax3 | ΔH3 | |
| FS | 44.23 ± 0.02 ab | 0.92 ± 0.09 a | 59.60 ± 0.04 a | 0.03 ± 0.01 a | 70.22 ± 0.61 a | 0.13 ± 0.04 ab |
| AT | 43.78 ± 0.66 b | 0.33 ± 0.87 a | 59.98 ± 0.13 a | 0.03 ± 0.19 a | 70.98 ± 0.98 a | 0.13 ± 0.97 ab |
| RT | 43.98 ± 0.23 b | 0.34 ± 0.09 a | 59.23 ± 0.10 a | 0.02 ± 0.01 a | 71.59 ± 0.78 a | 0.19 ± 0.02 a |
| GO-RT | 44.71 ± 0.22 a | 0.78 ± 0.53 a | 59.19 ± 0.09 a | 0.11 ± 0.21 a | 71.23 ± 0.43 a | 0.14 ± 0.02 ab |
| GM-RT | 44.62 ± 0.21 a | 0.83 ± 0.18 a | 59.21 ± 0.11 a | 0.11 ± 0.13 a | 71.65 ± 0.33 a | 0.13 ± 0.03 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, F.; Chen, W.; Gu, X.; Guan, W.; Cai, L. Thawing of Frozen Hairtail (Trichiurus lepturus) with Graphene Nanoparticles Combined with Radio Frequency: Variations in Protein Aggregation, Structural Characteristics, and Stability. Foods 2024, 13, 1632. https://doi.org/10.3390/foods13111632
Tian F, Chen W, Gu X, Guan W, Cai L. Thawing of Frozen Hairtail (Trichiurus lepturus) with Graphene Nanoparticles Combined with Radio Frequency: Variations in Protein Aggregation, Structural Characteristics, and Stability. Foods. 2024; 13(11):1632. https://doi.org/10.3390/foods13111632
Chicago/Turabian StyleTian, Fang, Wenyuchu Chen, Xiaohan Gu, Weiliang Guan, and Luyun Cai. 2024. "Thawing of Frozen Hairtail (Trichiurus lepturus) with Graphene Nanoparticles Combined with Radio Frequency: Variations in Protein Aggregation, Structural Characteristics, and Stability" Foods 13, no. 11: 1632. https://doi.org/10.3390/foods13111632
APA StyleTian, F., Chen, W., Gu, X., Guan, W., & Cai, L. (2024). Thawing of Frozen Hairtail (Trichiurus lepturus) with Graphene Nanoparticles Combined with Radio Frequency: Variations in Protein Aggregation, Structural Characteristics, and Stability. Foods, 13(11), 1632. https://doi.org/10.3390/foods13111632









