A Dual and Rapid RPA-CRISPR/Cas12a Method for Simultaneous Detection of Cattle and Soybean-Derived Adulteration in Goat Milk Powder
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
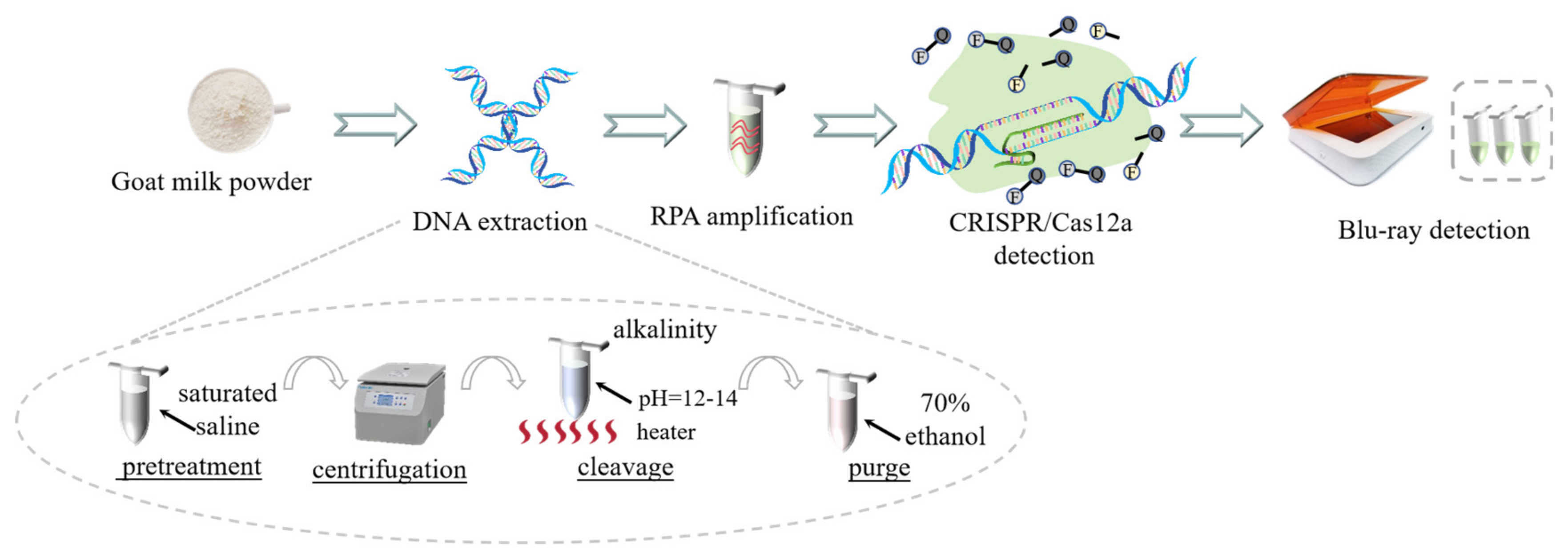
2.2. DNA Extraction
2.3. Primer Screening
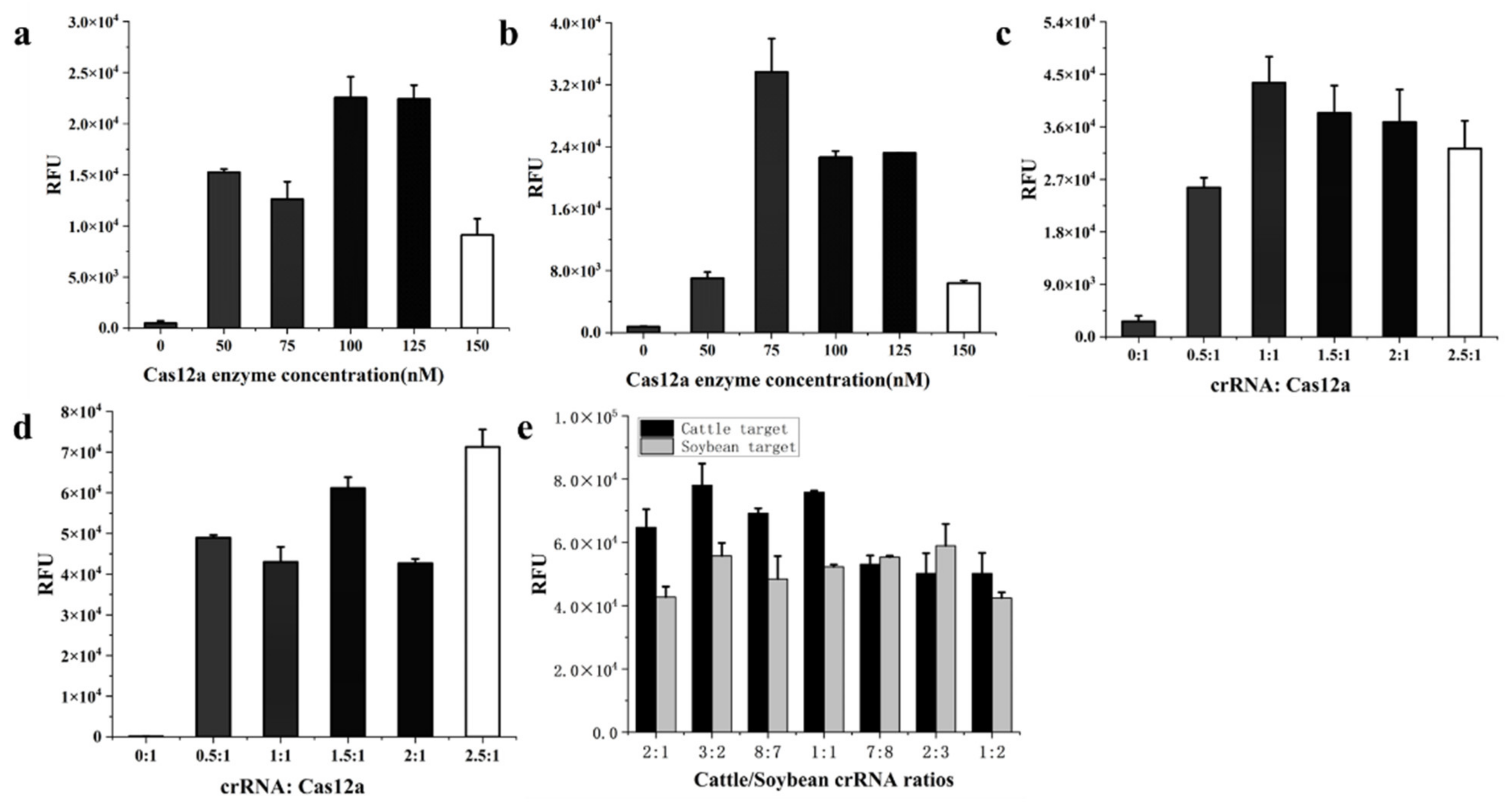
2.4. RPA Optimization
2.5. Establishment of Dual RPA-CRISPR/Cas12a Assay
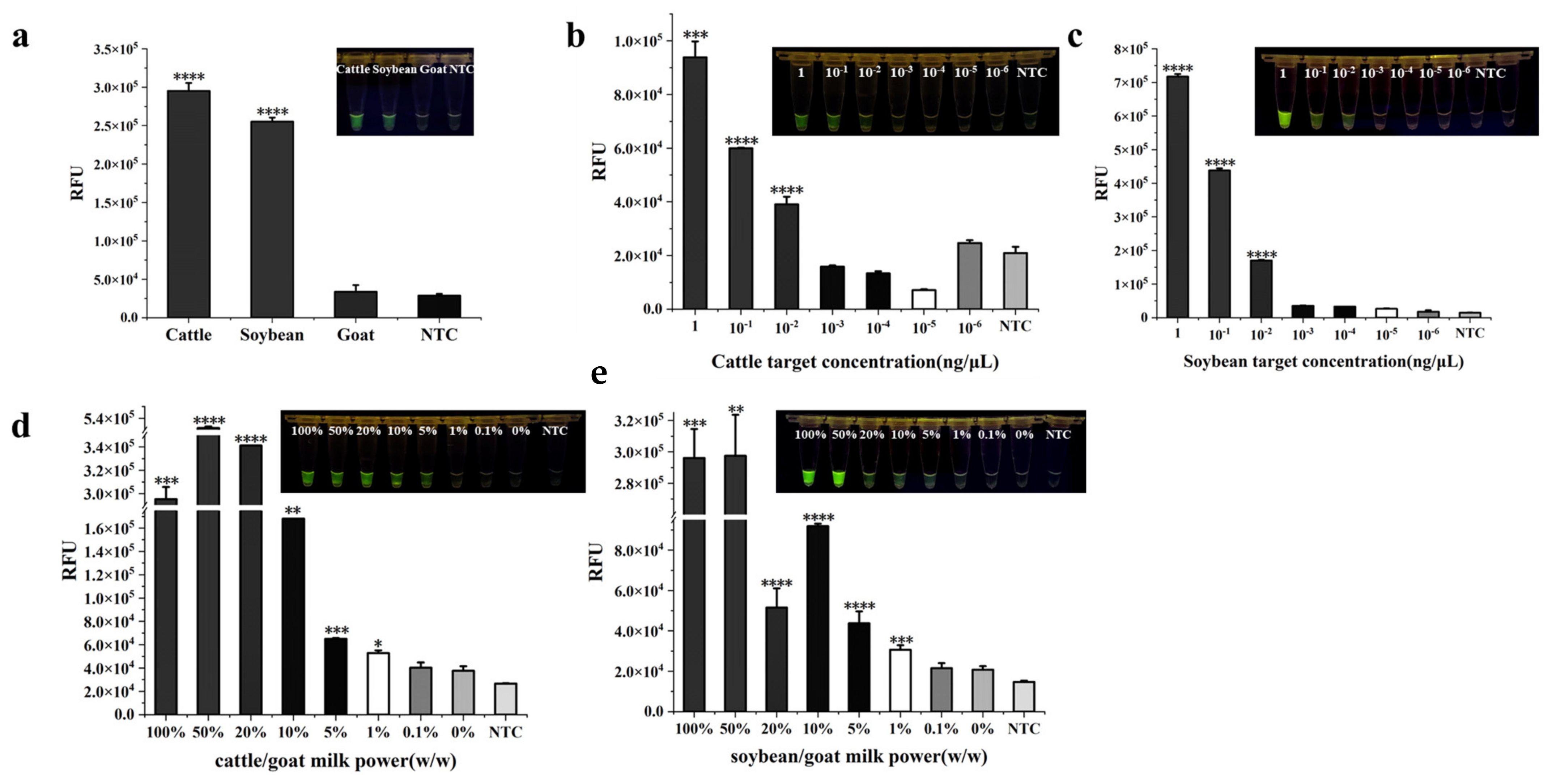
2.6. Specificity and Detectability of Dual RPA-CRISPR/Cas12a
2.7. Real Sample Testing and Statistical Analysis
2.8. Method Validation
3. Results and Discussion
3.1. DNA Extraction
3.2. Primer Screening and RPA Optimization
3.3. Establishment of Dual RPA-CRISPR/Cas12a Method
3.4. The Specificity and Sensitivity of Dual RPA-CRISPR/Cas12a Method
3.5. Real Sample Testing
3.6. Method Validation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Parkash, S.; Jenness, R. The composition and characteristics of goats’ milk: A review. Dairy Sci. Abstr. 1968, 30, 67–87. [Google Scholar]
- Shu, G.; Chen, H.; Lv, J.; Zhang, L. Comparison of Physicochemical Properties of Sheep Milk and Goat Milk. Food Ind. Sci. Technol. 2008, 11, 280–284. (In Chinese) [Google Scholar]
- Ballabio, C.; Chessa, S.; Rignanese, D.; Gigliotti, C.; Pagnacco, G.; Terracciano, L.; Fiocchi, A.; Restani, P.; Caroli, A.M. Goat milk allergenicity as a function of αS1-casein genetic polymorphism. J. Dairy Sci. 2011, 94, 998–1004. [Google Scholar] [CrossRef] [PubMed]
- Di Pinto, A.; Terio, V.; Marchetti, P.; Bottaro, M.; Mottola, A.; Bozzo, G.; Bonerba, E.; Ceci, E.; Tantillo, G. DNA-based approach for species identification of goat-milk products. Food Chem. 2017, 229, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Kemal Seçkin, A.; Yilmaz, B.; Tosun, H. Real-time PCR is a potential tool to determine the origin of milk used in cheese production. LWT 2017, 77, 332–336. [Google Scholar] [CrossRef]
- Zhang, H.; Yan, L.; Dang, G.; Liu, Y. Research Progress on Adulteration Detection Technology of Sheep Milk Products. China Dairy Ind. 2019, 8, 132–136. [Google Scholar]
- Gimonkar, S.; Van Fleet, E.E.; Boys, K.A. Chapter 13—Dairy product fraud. In Food Fraud; Hellberg, R.S., Everstine, K., Sklare, S.A., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 249–279. [Google Scholar] [CrossRef]
- Jamnik, P.; Volk, H.; Ogrinc, N.; Jeršek, B. Potential of bovine kappa-casein as biomarker for detection of adulteration of goat’s milk with cow’s milk. Mljekarstv 2019, 69, 78–84. [Google Scholar] [CrossRef]
- Müller-Maatsch, J.; Alewijn, M.; Wijtten, M.; Weesepoel, Y. Detecting fraudulent additions in skimmed milk powder using a portable, hyphenated, optical multi-sensor approach in combination with one-class classification. Food Control 2021, 121, 107744. [Google Scholar] [CrossRef]
- Piras, C.; Hale, O.J.; Reynolds, C.K.; Jones, A.K.; Taylor, N.; Morris, M.; Cramer, R. Speciation and milk adulteration analysis by rapid ambient liquid MALDI mass spectrometry profiling using machine learning. Sci. Rep. 2021, 11, 3305. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zeng, W.; Zhao, Y.; Zhu, X.; Wan, H.; Zhang, M.; Li, Z. Rapid detection of adulteration of goat milk and goat infant formulas using near-infrared spectroscopy fingerprints. Int. Dairy J. 2023, 137, 105536. [Google Scholar] [CrossRef]
- Baptista, M.; Cunha, J.T.; Domingues, L. DNA-based approaches for dairy products authentication: A review and perspectives. Trends Food Sci. Technol. 2021, 109, 386–397. [Google Scholar] [CrossRef]
- Chen, A. Research Progress on Qualitative Discrimination Technology of Food Authenticity Based on Amplification Detection of Spe-cies-Specific Single DNA Marker. Agric. Prod. Qual. Saf. 2022, 1, 10–18. (In Chinese) [Google Scholar]
- Mustafa, J.Y. Using Species-Specific Polymerase Chain Reaction Technique for Detection of Cows, Buffallos, Seeps and Goats Milk and Cheeses in Basra City. Indian J. Public Health Res. Dev. 2019, 10, 1082–1087. [Google Scholar] [CrossRef]
- Kounelli, M.L.; Kalogianni, D.P. A sensitive DNA-based fluorometric method for milk authenticity of dairy products based on spectrally distinct microspheres. Eur. Food Res. Technol. 2017, 243, 1773–1781. [Google Scholar] [CrossRef]
- He, X.; Ma, X.; Xu, Q. PCR High-Resolution Melting Detection Method for Differentiating Cattle and Sheep Milk. Food Ferment. Ind. 2021, 47, 225–230. [Google Scholar]
- Deng, L.; Li, A.; Gao, Y.; Shen, T.; Yue, H.; Miao, J.; Li, R.; Yang, J. Detection of the Bovine Milk Adulterated in Camel, Horse, and Goat Milk Using Duplex PCR. Food Anal. Methods 2020, 13, 560–567. [Google Scholar] [CrossRef]
- Klančnik, A.; Toplak, N.; Kovač, M.; Ogrinc, N.; Jeršek, B. Robust PCR-based method for quantification of bovine milk in cheeses made from caprine and ovine milk. Int. J. Dairy Technol. 2016, 69, 540–549. [Google Scholar] [CrossRef]
- Kim, M.-J.; Kim, H.-Y. Direct duplex real-time loop mediated isothermal amplification assay for the simultaneous detection of cow and goat species origin of milk and yogurt products for field use. Food Chem. 2018, 246, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Chen, Y.; Wang, Z.; Qiao, L.; Xie, R.; Zhang, J.; Bian, S.; Li, H.; Zhang, Y.; Chen, A. Multiple authentications of high-value milk by centrifugal microfluidic chip-based real-time fluorescent LAMP. Food Chem. 2021, 351, 129348. [Google Scholar] [CrossRef]
- Li, K.; Luo, Y.; Xu, W. Research Progress on CRISPR-Cas Biosensors. Biotechnol. Adv. 2019, 9, 579–591. [Google Scholar]
- Li, Y.; Li, J. Crispr bioanalytical chemistry technology. Prog. Chem. 2020, 32, 5–13. [Google Scholar]
- Liu, H.; Wang, J.; Zeng, H.; Liu, X.; Jiang, W.; Wang, Y.; Ouyang, W.; Tang, X. RPA-Cas12a-FS: A frontline nucleic acid rapid detection system for food safety based on CRISPR-Cas12a combined with recombinase polymerase amplification. Food Chem. 2021, 334, 127608. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Wang, J.; Yao, C.; Xie, P.; Li, X.; Xu, Z.; Xian, Y.; Lei, H.; Shen, X. Alkaline lysis-recombinase polymerase amplification combined with CRISPR/Cas12a assay for the ultrafast visual identification of pork in meat products. Food Chem. 2022, 383, 132318. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhao, G.; Li, X.; Xu, Z.; Lei, H.; Shen, X. Development of rapid and easy detection of Salmonella in food matrics using RPA-CRISPR/Cas12a method. LWT 2022, 162, 113443. [Google Scholar] [CrossRef]
- Wang, Y.; Ke, Y.; Liu, W.; Sun, Y.; Ding, X. A One-Pot Toolbox Based on Cas12a/crRNA Enables Rapid Foodborne Pathogen Detection at Attomolar Level. ACS Sens. 2020, 5, 1427–1435. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Liu, Y.; Zhang, X.; Gai, Z.; Lei, H.; Shen, X. A Rapid RPA-CRISPR/Cas12a Detection Method for Adulteration of Goat Milk Powder. Foods 2023, 12, 1569. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, T.; Yu, W.; Chen, A. A Study on a Rapid Genomic DNA Extraction Method Suitable for Dairy Products. J. Food Saf. Qual. Insp. 2020, 11, 134–139. (In Chinese) [Google Scholar]
- Ma, X. Study on PCR High-Resolution Melting Detection Method for Cattle and Ovine-Derived Components in Milk and Dairy Products. Master’s Thesis, Shaanxi University of Science and Technology, Xi’an, China, 2019. [Google Scholar]
- Tan, T. Study on On-Site LAMP Detection Method for Cattle and Ovine-Derived Components in Milk and Dairy Products. Master’s Thesis, Shaanxi University of Science and Technology, Xi’an, China, 2020. [Google Scholar]
- Fan, Y.; Zhang, L.; Liu, Y.; Bian, R. Establishment of a Multiple Real-Time Fluorescent Quantitative PCR Method for Detecting Cattle Milk and Soybean Com-ponents in Goat Milk. Shandong Agric. Sci. 2016, 48, 118–123. (In Chinese) [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, Y.; Huang, S.; Lei, H.; Li, X.; Shen, X. A Dual and Rapid RPA-CRISPR/Cas12a Method for Simultaneous Detection of Cattle and Soybean-Derived Adulteration in Goat Milk Powder. Foods 2024, 13, 1637. https://doi.org/10.3390/foods13111637
Wen Y, Huang S, Lei H, Li X, Shen X. A Dual and Rapid RPA-CRISPR/Cas12a Method for Simultaneous Detection of Cattle and Soybean-Derived Adulteration in Goat Milk Powder. Foods. 2024; 13(11):1637. https://doi.org/10.3390/foods13111637
Chicago/Turabian StyleWen, Yuanjun, Shuqin Huang, Hongtao Lei, Xiangmei Li, and Xing Shen. 2024. "A Dual and Rapid RPA-CRISPR/Cas12a Method for Simultaneous Detection of Cattle and Soybean-Derived Adulteration in Goat Milk Powder" Foods 13, no. 11: 1637. https://doi.org/10.3390/foods13111637
APA StyleWen, Y., Huang, S., Lei, H., Li, X., & Shen, X. (2024). A Dual and Rapid RPA-CRISPR/Cas12a Method for Simultaneous Detection of Cattle and Soybean-Derived Adulteration in Goat Milk Powder. Foods, 13(11), 1637. https://doi.org/10.3390/foods13111637






