Prevalence and Antimicrobial Resistance of Listeria monocytogenes in Different Raw Food from Reynosa, Tamaulipas, Mexico
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Isolation and Identification of Listeria monocytogenes
2.3. Classification of L. monocytogenes Serogroups
2.4. Virulence Factors
2.5. Antimicrobial Susceptibility Testing
2.6. Statistical Analysis
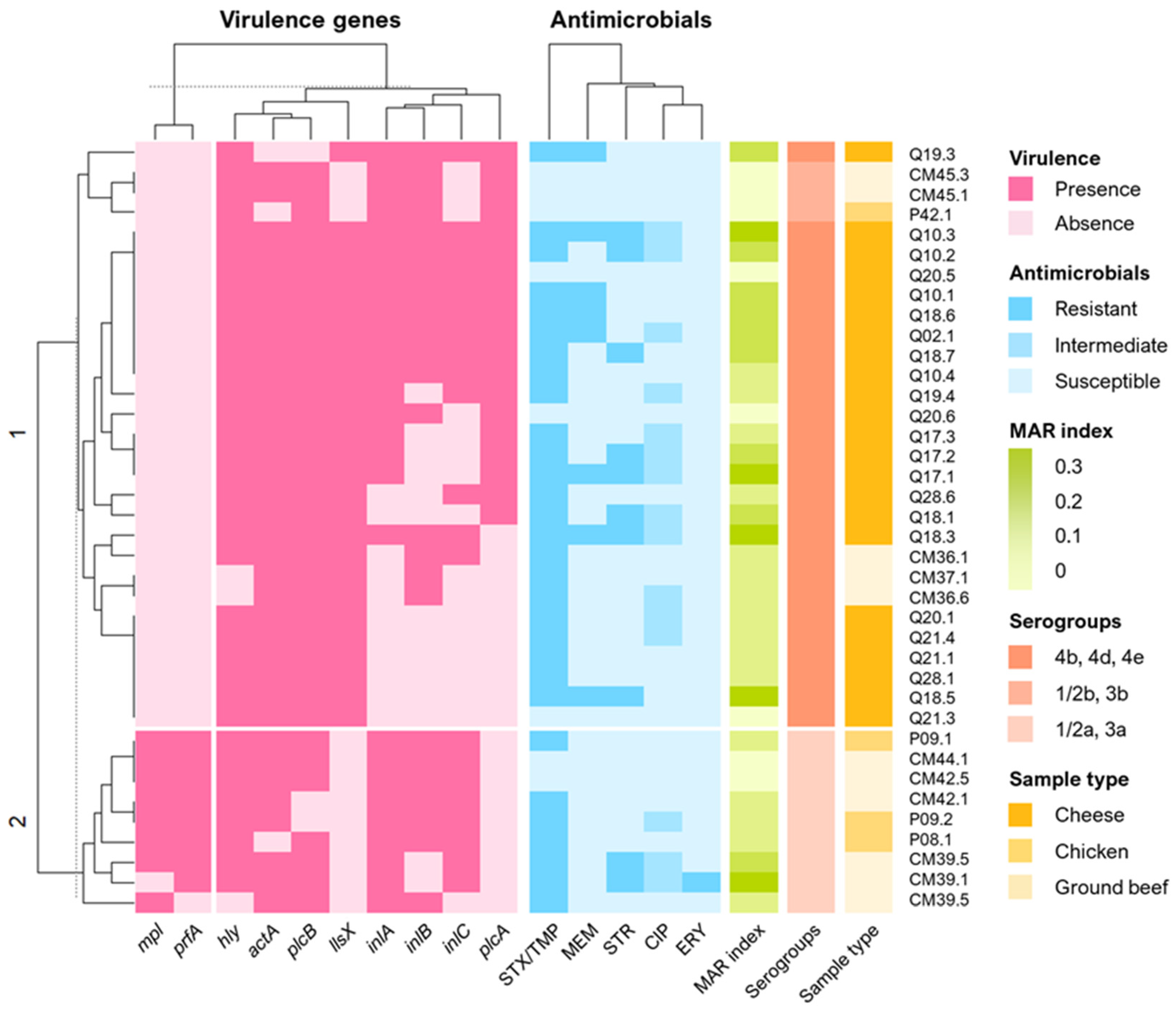
3. Results
3.1. Sample Collection
3.2. Isolation and Identification of Listeria monocytogenes
3.3. Classification of Serogroups
3.4. Virulence Factors
3.5. Antimicrobial Susceptibility Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wiktorczyk-Kapischke, N.; Skowron, K.; Grudlewska-Buda, K.; Wałecka-Zacharska, E.; Korkus, J.; Gospodarek-Komkowska, E. Adaptive Response of Listeria monocytogenes to the Stress Factors in the Food Processing Environment. Front. Microbiol. 2021, 12, 710085. [Google Scholar] [CrossRef]
- Osek, J.; Lachtara, B.; Wieczorek, K. Listeria monocytogenes—How This Pathogen Survives in Food-Production Environments? Front. Microbiol. 2022, 13, 866462. [Google Scholar] [CrossRef]
- Kayode, A.J.; Semerjian, L.; Osaili, T.; Olapade, O.; Okoh, A.I. Occurrence of Multidrug-Resistant Listeria monocytogenes in Environmental Waters: A Menace of Environmental and Public Health Concern. Front. Environ. Sci. 2021, 9, 737435. [Google Scholar] [CrossRef]
- Quereda, J.J.; Morón-García, A.; Palacios-Gorba, C.; Dessaux, C.; García-del Portillo, F.; Pucciarelli, M.G.; Ortega, A.D. Pathogenicity and Virulence of Listeria monocytogenes: A Trip from Environmental to Medical Microbiology. Virulence 2021, 12, 2509–2545. [Google Scholar] [CrossRef] [PubMed]
- Sibanda, T.; Buys, E.M. Listeria monocytogenes Pathogenesis: The Role of Stress Adaptation. Microorganisms 2022, 10, 1522. [Google Scholar] [CrossRef]
- Avila-Novoa, M.G.; Navarrete-Sahagún, V.; González-Gómez, J.P.; Novoa-Valdovinos, C.; Guerrero-Medina, P.J.; García-Frutos, R.; Martínez-Chávez, L.; Martínez-Gonzáles, N.E.; Gutiérrez-Lomelí, M. Conditions of in Vitro Biofilm Formation by Serogroups of Listeria monocytogenes Isolated from Hass Avocados Sold at Markets in Mexico. Foods 2021, 10, 2097. [Google Scholar] [CrossRef] [PubMed]
- Pirone-Davies, C.; Chen, Y.; Pightling, A.; Ryan, G.; Wang, Y.; Yao, K.; Hoffmann, M.; Allard, M.W. Genes Significantly Associated with Lineage II Food Isolates of Listeria monocytogenes. BMC Genom. 2018, 19, 708. [Google Scholar] [CrossRef]
- Ravindhiran, R.; Sivarajan, K.; Sekar, J.N.; Murugesan, R.; Dhandapani, K. Listeria monocytogenes an Emerging Pathogen: A Comprehensive Overview on Listeriosis, Virulence Determinants, Detection, and Anti-Listerial Interventions. Microb. Ecol. 2023, 86, 2231–2251. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, M.; Wang, J.; Wu, Q.; Cheng, J.; Zhang, J.; Sun, Q.; Xue, L.; Zeng, H.; Lei, T.; et al. Heterogeneity, Characteristics, and Public Health Implications of Listeria monocytogenes in Ready-to-Eat Foods and Pasteurized Milk in China. Front. Microbiol. 2020, 11, 642. [Google Scholar] [CrossRef]
- Anwar, T.M.; Pan, H.; Chai, W.; Ed-Dra, A.; Fang, W.; Li, Y.; Yue, M. Genetic Diversity, Virulence Factors, and Antimicrobial Resistance of Listeria monocytogenes from Food, Livestock, and Clinical Samples between 2002 and 2019 in China. Int. J. Food Microbiol. 2022, 366, 109572. [Google Scholar] [CrossRef]
- Grosboillot, V.; Keller, I.; Ernst, C.; Loessner, M.J.; Schuppler, M. Ampicillin Treatment of Intracellular Listeria monocytogenes Triggers Formation of Persistent, Drug-Resistant L-Form Cells. Front. Cell. Infect. Microbiol. 2022, 12, 869339. [Google Scholar] [CrossRef] [PubMed]
- Thønnings, S.; Knudsen, J.D.; Schønheyder, H.C.; Søgaard, M.; Arpi, M.; Gradel, K.O.; Østergaard, C.; Danish Collaborative Bacteraemia Network (DACOBAN). Antibiotic Treatment and Mortality in Patients with Listeria monocytogenes Meningitis or Bacteraemia. Clin. Microbiol. Infect. 2016, 22, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Amaya-Villar, R.; García-Cabrera, E.; Sulleiro-Igual, E.; Fernández-Viladrich, P.; Fontanals-Aymerich, D.; Catalán-Alonso, P.; Rodrigo-Gonzalo de Liria, C.; Coloma-Conde, A.; Grill-Díaz, F.; Guerrero-Espejo, A.; et al. Three-Year Multicenter Surveillance of Community-Acquired Listeria monocytogenes Meningitis in Adults. BMC Infect. Dis. 2010, 10, 324. [Google Scholar] [CrossRef]
- Torres, K.; Sierra, S.; Potou, R.; Carrascal, A.; Mercado, M. Patogénesis de Listeria monocytogenes, Microorganismo Zoonótico Emergente. Rev. MVZ Córdoba 2005, 10, 511–543. [Google Scholar] [CrossRef]
- Baquero, F.; Lanza, V.F.; Duval, M.; Coque, T.M. Ecogenetics of Antibiotic Resistance in Listeria monocytogenes. Mol. Microbiol. 2020, 113, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Akrami-Mohajeri, F.; Derakhshan, Z.; Ferrante, M.; Hamidiyan, N.; Soleymani, M.; Conti, G.O.; Tafti, R.D. The Prevalence and Antimicrobial Resistance of Listeria Spp in Raw Milk and Traditional Dairy Products Delivered in Yazd, Central Iran (2016). Food Chem. Toxicol. 2018, 114, 141–144. [Google Scholar] [CrossRef]
- Bouymajane, A.; Rhazi Filali, F.; Oulghazi, S.; Lafkih, N.; Ed-Dra, A.; Aboulkacem, A.; El Allaoui, A.; Ouhmidou, B.; Moumni, M. Occurrence, Antimicrobial Resistance, Serotyping and Virulence Genes of Listeria monocytogenes Isolated from Foods. Heliyon 2021, 7, e06169. [Google Scholar] [CrossRef]
- Maćkiw, E.; Korsak, D.; Kowalska, J.; Felix, B.; Stasiak, M.; Kucharek, K.; Antoszewska, A.; Postupolski, J. Genetic Diversity of Listeria monocytogenes Isolated from Ready-to-Eat Food Products in Retail in Poland. Int. J. Food Microbiol. 2021, 358, 109397. [Google Scholar] [CrossRef]
- Cruz-Pulido, W.L.; Téllez-Luis, S.J.; Rivera-Sánchez, G.; Ávila-Aguilar, S.; Cantú-Ramírez, R.; Garza-González, E.; Sierra-Juárez, G.; Bocanegra-García, V. Listeria sp. y Listeria monocytogenes En Pollo Congelado: Detección Por NOM-143-SSA1-1995 y PCR de Expendios Comerciales de Matamoros y Reynosa, Tamaulipas, México. CienciaUAT 2012, 6, 41. [Google Scholar] [CrossRef]
- Castañeda-Ruelas, G.; Eslava-Campos, C.; Castro-del-Campo, N.; León-Félix, J.; Chaidez-Quiroz, C. Listeriosis En México: Importancia Clínica y Epidemiológica. Salud Publica Mex. 2014, 56, 654–659. [Google Scholar]
- Salud Rubio Lozano, M.; Fernando Martínez Bruno, J.; Hernández Castro, R.; Bonilla Contreras, C.; Danilo Méndez Medina, R.; Fernando Núñez Espinosa, J.; Echeverry, A.; Brashears, M.M. Detection of Listeria monocytogenes, Salmonella and Yersinia Enterocolitica in Beef at Points of Sale in Mexico. RES Rev Mex Cienc Pecu 2013, 4, 107–115. [Google Scholar]
- Rosas-Barbosa, B.T.; Morales, A.L.-J.; Alaniz, R.; Ramírez-Álvarez, A.; Soltero-Ramos, J.P.; de la Mora-Quiroz, R.; Martin, P.; Jacquet, C. Presencia y Persistencia de Listeria En Cuatro Queserías Artesanales de Jalisco, México. e-CUCBA 2014, 2, 3–37. [Google Scholar]
- Chávez-Martínez, A.; Paredes-Montoya, P.; Rentería-Monterrubio, A.L.; Corral-Luna, A.; Lechuga-Valles, R.; Dominguez-Viveros, J.; Sánchez-Vega, R.; Santellano-Estrada, E. Microbial Quality and Prevalence of Foodborne Pathogens of Cheeses Commercialized at Different Retail Points in Mexico. Food Sci. Technol. 2019, 39, 703–710. [Google Scholar] [CrossRef]
- Güssow, D.; Clackson, T. Direct Clone Characterization from Plaques and Colonies by the Polymerase Chain Reaction. Nucleic Acids Res. 1989, 17, 4000. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Knabel, S.J. Multiplex PCR for Simultaneous Detection of Bacteria of the Genus Listeria, Listeria monocytogenes, and Major Serotypes and Epidemic Clones of L. monocytogenes. Appl. Environ. Microbiol. 2007, 73, 6299–6304. [Google Scholar] [CrossRef] [PubMed]
- Doumith, M.; Buchrieser, C.; Glaser, P.; Jacquet, C.; Martin, P. Differentiation of the Major Listeria monocytogenes Serovars by Multiplex PCR. J. Clin. Microbiol. 2004, 42, 3819–3822. [Google Scholar] [CrossRef] [PubMed]
- Suárez, M.; González-Zorn, B.; Vega, Y.; Chico-Calero, I.; Vázquez-Boland, J.A. A Role for ActA in Epithelial Cell Invasion by Listeria monocytogenes. Cell. Microbiol. 2001, 3, 853–864. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.K.; Wu, Q.P.; Zhang, J.M.; Deng, M.Q.; Zhou, Y.H. Studies on Specific Detection of Listeria monocytogenes in Foods by Duplex PCR. Chin. J. Heal. Lab. Technol. 2009, 19, 1199–1201. [Google Scholar]
- Chen, M.; Wu, Q.; Zhang, J.; Wang, J. Prevalence and Characterization of Listeria monocytogenes Isolated from Retail-Level Ready-to-Eat Foods in South China. Food Control 2014, 38, 1–7. [Google Scholar] [CrossRef]
- Liu, D.; Lawrence, M.L.; Austin, F.W.; Ainsworth, A.J. A Multiplex PCR for Species-and Virulence-Specific Determination of Listeria monocytogenes. J. Microbiol. Methods 2007, 71, 133–140. [Google Scholar] [CrossRef]
- Clayton, E.M.; Hill, C.; Cotter, P.D.; Ross, R.P. Real-Time PCR Assay to Differentiate Listeriolysin S-Positive and-Negative Strains of Listeria monocytogenes. Appl. Environ. Microbiol. 2011, 77, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Notermans, S.H.; Dufrenne, J.; Leimeister-Wächter, M.; Domann, E.; Chakraborty, T. Phosphatidylinositol-Specific Phospholipase C Activity as a Marker to Distinguish between Pathogenic and Nonpathogenic Listeria Species. Appl. Environ. Microbiol. 1991, 57, 2666–2670. [Google Scholar] [CrossRef]
- Zhang, W.; Jayarao, B.M.; Knabel, S.J. Multi-Virulence-Locus Sequence Typing of Listeria monocytogenes. Appl. Environ. Microbiol. 2004, 70, 913–920. [Google Scholar] [CrossRef] [PubMed]
- EUCAST The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters 2020. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Disk_criteria/Validation_2024/L._monocytogenes_v_2.2_January_2020.pdf (accessed on 20 January 2020).
- Wayne, P.A. CLSI Clinical and Laboratory Standards Institute. M100 Performance Standards for Antimicrobial Susceptibility Testing 2020. Inform Suppl. 2011, 31, 100–121. [Google Scholar]
- Elbar, S.; Elkenany, R.; Elhadidy, M.; Younis, G. Prevalence, Virulence and Antibiotic Susceptibility of Listeria monocytogenes Isolated from Sheep. Mansoura Vet. Med. J. 2020, 21, 48–52. [Google Scholar] [CrossRef]
- Elsayed, M.M.; Elkenany, R.M.; Zakaria, A.I.; Badawy, B.M. Epidemiological Study on Listeria monocytogenes in Egyptian Dairy Cattle Farms’ Insights into Genetic Diversity of Multi-Antibiotic-Resistant Strains by ERIC-PCR. Environ. Sci. Pollut. Res. 2022, 29, 54359–54377. [Google Scholar] [CrossRef] [PubMed]
- Allaire, J. RStudio: Integrated Development Environment for R. Boston MA 2012, 770, 165–171. [Google Scholar]
- Gu, Z.; Eils, R.; Schlesner, M. Complex Heatmaps Reveal Patterns and Correlations in Multidimensional Genomic Data. Bioinformatics 2016, 32, 2847–2849. [Google Scholar] [CrossRef]
- Olivoto, T.; Lúcio, A.D. Metan: An R Package for Multi-environment Trial Analysis. Methods Ecol. Evol. 2020, 11, 783–789. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, S.; Chen, H.; Chen, J.; Zhang, J.; Zhang, Z.; Yang, Y.; Xu, Z.; Zhan, L.; Mei, L. Prevalence, Genotypic Characteristics and Antibiotic Resistance of Listeria monocytogenes from Retail Foods in Bulk in Zhejiang Province, China. Front. Microbiol. 2019, 10, 1710. [Google Scholar] [CrossRef]
- Mashak, Z.; Banisharif, F.; Banisharif, G.; Reza Pourian, M.; Eskandari, S.; Seif, A.; Safarpoor Dehkordi, F.; Alavi, I. Prevalence of Listeria Species and Serotyping of Listeria monocytogenes Bacteria Isolated from Seafood Samples. Egypt. J. Vet. Sci. 2021, 52, 1–9. [Google Scholar] [CrossRef]
- Menon, K.V.; Sunil, B.; Latha, C. Prevalence and Antibiotic Resistance Profile of Listeria Spp. Associated with Seafoods from Fish Catchment Areas in Kerala, India. Vet. World 2021, 14, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Abdeen, E.E.; Mousa, W.S.; Harb, O.H.; Fath-Elbab, G.A.; Nooruzzaman, M.; Gaber, A.; Alsanie, W.F.; Abdeen, A. Prevalence, Antibiogram and Genetic Characterization of Listeria monocytogenes from Food Products in Egypt. Foods 2021, 10, 1381. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewski, A.J.; Gajewska, J.; Chajęcka-Wierzchowska, W.; Załuski, D.; Zadernowska, A. Prevalence of Listeria monocytogenes and Other Listeria Species in Fish, Fish Products and Fish Processing Environment: A Systematic Review and Meta-Analysis. Sci. Total Environ. 2023, 907, 167912. [Google Scholar] [CrossRef] [PubMed]
- Kayode, A.J.; Okoh, A.I. Assessment of Multidrug-Resistant Listeria monocytogenes in Milk and Milk Product and One Health Perspective. PLoS ONE 2022, 17, e0270993. [Google Scholar] [CrossRef] [PubMed]
- Oluwafemi, Y.D.; Igere, B.E.; Ekundayo, T.C.; Ijabadeniyi, O.A. Prevalence of Listeria monocytogenes in Milk in Africa: A Generalized Logistic Mixed-Effects and Meta-Regression Modelling. Sci. Rep. 2023, 13, 12646. [Google Scholar] [CrossRef] [PubMed]
- Seyoum, E.T.; Woldetsadik, D.A.; Mekonen, T.K.; Gezahegn, H.A.; Gebreyes, W.A. Prevalence of Listeria monocytogenes in Raw Bovine Milk and Milk Products from Central Highlands of Ethiopia. J. Infect. Dev. Ctries. 2015, 9, 1204–1209. [Google Scholar] [CrossRef] [PubMed]
- Rios-Muñiz, D.; Cerna-Cortes, J.F.; Lopez-Saucedo, C.; Angeles-Morales, E.; Bobadilla-Del Valle, M.; Ponce-De Leon, A.; Estrada-Garcia, T. Longitudinal Analysis of the Microbiological Quality of Raw Cow’s Milk Samples Collected from Three Small Family Dairy Farms in Mexico over a 2-Year Period. J. Food Prot. 2019, 82, 2194–2200. [Google Scholar] [CrossRef] [PubMed]
- Silva-Paz, L.E.; Medina-Basulto, G.E.; López-Valencia, G.; Montaño-Gómez, M.F.; Villa-Angulo, R.; Herrera Ramírez, J.C.; González-Silva, A.L.; Monge-Navarro, F.; Cueto-González, S.A.; Felipe-García, G. Characterization of the Milk and Artisanal Cheese of the Region of Ojos Negros, Baja California, México. Rev. Mex. ciencias Pecu. 2020, 11, 553–564. [Google Scholar] [CrossRef]
- Lee, J.; Seo, Y.; Ha, J.; Kim, S.; Choi, Y.; Oh, H.; Lee, Y.; Kim, Y.; Kang, J.; Park, E.; et al. Influence of Milk Microbiota on Listeria monocytogenes Survival during Cheese Ripening. Food Sci. Nutr. 2020, 8, 5071–5076. [Google Scholar] [CrossRef]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control). The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSA J. 2018, 16, 5500. [Google Scholar] [CrossRef] [PubMed]
- Pyz-łukasik, R.; Gondek, M.; Winiarczyk, D.; Michalak, K.; Paszkiewicz, W.; Piróg-Komorowska, A.; Policht, A.; Ziomek, M. Occurrence of Listeria monocytogenes in Artisanal Cheeses from Poland and Its Identification by Maldi-Tof Ms. Pathogens 2021, 10, 632. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, N.; Ceniti, C.; Santoro, A.; Clausi, M.T.; Casalinuovo, F. Foodborne Pathogen Assessment in Raw Milk Cheeses. Int. J. Food Sci. 2020, 2020, 3616713. [Google Scholar] [CrossRef] [PubMed]
- Merchán, N.; Zurymar, T.S.; Niño, L.; Urbano, E. Determinación de la Inocuidad Microbiológica de Quesos Artesanales Según Las Normas Técnicas Colombianas. Rev. Chil. Nutr. 2019, 46, 288–294. [Google Scholar] [CrossRef]
- Cufaoglu, G.; Ambarcioglu, P.; Ayaz, N.D. Meta-Analysis of the Prevalence of Listeria Spp. and Antibiotic Resistant, L. monocytogenes Isolates from Foods in Turkey. LWT 2021, 144, 111210. [Google Scholar] [CrossRef]
- Shourav, A.H.; Salma, K.P.; Ahmed, S.; Khan, R. Listeria monocytogenes in Ready-to-Eat Chicken Products, Their Antibiotic Resistance and Virulence Genes. Biores. Commun. 2023, 9, 1160–1169. [Google Scholar] [CrossRef]
- Benavides-Sánchez, D.A.; Pena-Serna, C. Approaching the Sensory Profile of Paipa Cheese, the Colombian Ripened Cheese with Protected Designation of Origin. Braz. J. Food Technol. 2022, 25, e2022121. [Google Scholar] [CrossRef]
- Martinez-Rios, V.; Dalgaard, P. Prevalence of Listeria monocytogenes in European Cheeses: A Systematic Review and Meta-Analysis. Food Control 2018, 84, 205–214. [Google Scholar] [CrossRef]
- Campagnollo, F.B.; Gonzales-Barron, U.; Pilão Cadavez, V.A.; Sant’Ana, A.S.; Schaffner, D.W. Quantitative Risk Assessment of Listeria monocytogenes in Traditional Minas Cheeses: The Cases of Artisanal Semi-Hard and Fresh Soft Cheeses. Food Control 2018, 92, 370–379. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Chang, X.; Qin, S.; Song, Y.; Tian, J.; Ma, A. Analysis of 90 Listeria monocytogenes Contaminated in Poultry and Livestock Meat through Whole-Genome Sequencing. Food Res. Int. 2022, 159, 111641. [Google Scholar] [CrossRef]
- Oliveira, T.S.; Varjão, L.M.; da Silva, L.N.N.; de Castro Lisboa Pereira, R.; Hofer, E.; Vallim, D.C.; de Castro Almeida, R.C. Listeria monocytogenes at Chicken Slaughterhouse: Occurrence, Genetic Relationship among Isolates and Evaluation of Antimicrobial Susceptibility. Food Control 2018, 88, 131–138. [Google Scholar] [CrossRef]
- Aziz, S.A.A.A.; Mohamed, M.B.E.D. Prevalence, Virulence Genes, and Antimicrobial Resistance Profile of Listeria monocytogenes Isolated from Retail Poultry Shops in Beni-Suef City, Egypt. J. Adv. Vet. Anim. Res. 2020, 7, 710–717. [Google Scholar] [CrossRef]
- Shakuntala, I.; Das, S.; Ghatak, S.; Milton, A.A.P.; Sanjukta, R.; Puro, K.-U.U.; Pegu, R.K.; Duarah, A.; Barbuddhe, S.B.; Sen, A. Prevalence, Characterization, and Genetic Diversity of Listeria monocytogenes Isolated from Foods of Animal Origin in North East India. Food Biotechnol. 2019, 33, 237–250. [Google Scholar] [CrossRef]
- Aksono, E.B.; Riwu, K.H.P.; Estoepangestie, A.T.S.; Pertiwi, H. Phylogenetic Analysis and Antibiotics Resistance of Listeria monocytogenes Contaminating Chicken Meat in Surabaya, Indonesia. Vet. Med. Int. 2020, 2020, 9761812. [Google Scholar] [CrossRef]
- Arslan, S.; Baytur, S. Prevalence and Antimicrobial Resistance of Listeria Species and Subtyping and Virulence Factors of Listeria monocytogenes from Retail Meat. J. Food Saf. 2019, 39, e12578. [Google Scholar] [CrossRef]
- Coban, A.; Pennone, V.; Sudagidan, M.; Molva, C.; Jordan, K.; Aydin, A. Prevalence, Virulence Characterization, and Genetic Relatedness of Listeria monocytogenes Isolated from Chicken Retail Points and Poultry Slaughterhouses in Turkey. Braz. J. Microbiol. 2019, 50, 1063–1073. [Google Scholar] [CrossRef]
- Mamber, S.W.; Mohr, T.B.; Leathers, C.; Mbandi, E.; Bronstein, P.A.; Barlow, K.; Silverman, M.; Aston, C.; Izsak, Y.; Saini, N.S.; et al. Occurrence of Listeria Monocytogenes in Ready-to-Eat Meat and Poultry Product Verification Testing Samples from U.S. Department of Agriculture-Regulated Producing Establishments, 2005 through 2017. J. Food Prot. 2020, 83, 1598–1606. [Google Scholar] [CrossRef]
- Moabelo, K.C.; Gcebe, N.; Gana, J.; Ngoshe, Y.B.; Adesiyun, A.A. Contamination of Beef and Beef Products by Listeria Spp. and Molecular Characterization of L. Monocytogenes in Mpumalanga, South Africa. J. Food Saf. 2023, 43, e13055. [Google Scholar] [CrossRef]
- Zhang, H.; Luo, X.; Aspridou, Z.; Misiou, O.; Dong, P.; Zhang, Y. The Prevalence and Antibiotic-Resistant of Listeria Monocytogenes in Livestock and Poultry Meat in China and the EU from 2001 to 2022: A Systematic Review and Meta-Analysis. Foods 2023, 12, 769. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, W.; Sun, T.; Gorris, L.G.M.; Wang, X.; Liu, B.; Dong, Q. The Prevalence of Listeria Monocytogenes in Meat Products in China: A Systematic Literature Review and Novel Meta-Analysis Approach. Int. J. Food Microbiol. 2020, 312, 108358. [Google Scholar] [CrossRef]
- Cavalcanti, A.A.C.; Limeira, C.H.; de Siqueira, I.N.; de Lima, A.C.; Medeiros, F.J.P.d.; de Souza, J.G.; de Araújo Medeiros, N.G.; de Oliveira Filho, A.A.; de Melo, M.A. The Prevalence of Listeria Monocytogenes in Meat Products in Brazil: A Systematic Literature Review and Meta-Analysis. Res. Vet. Sci. 2022, 145, 169–176. [Google Scholar] [CrossRef]
- Datta, A.R.; Burall, L.S. Serotype to Genotype: The Changing Landscape of Listeriosis Outbreak Investigations. Food Microbiol. 2018, 75, 18–27. [Google Scholar] [CrossRef]
- Capita, R.; Felices-Mercado, A.; García-Fernández, C.; Alonso-Calleja, C. Characterization of Listeria Monocytogenes Originating from the Spanish Meat-Processing Chain. Foods 2019, 8, 542. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, A.; Lewis, M.A.; Rothrock, M.J. The Distribution of Listeria in Pasture-Raised Broiler Farm Soils Is Potentially Related to University of Vermont Medium Enrichment Bias toward Listeria Innocua over Listeria Monocytogenes. Front. Vet. Sci. 2017, 4, 227. [Google Scholar] [CrossRef] [PubMed]
- Orsi, R.H.; den Bakker, H.C.; Wiedmann, M. Listeria Monocytogenes Lineages: Genomics, Evolution, Ecology, and Phenotypic Characteristics. Int. J. Med. Microbiol. 2011, 301, 79–96. [Google Scholar] [CrossRef]
- Andritsos, N.D.; Paramithiotis, S.; Mataragas, M.; Drosinos, E.H. Listeria Monocytogenes Serogroup 1/2 Strains Have a Competitive Growth Advantage over Serotype 4b during Refrigerated Storage of an Artificially Contaminated Ready-to-Eat Pork Meat Product. Appl. Sci. 2021, 11, 6096. [Google Scholar] [CrossRef]
- Ricci, A.; Allende, A.; Bolton, D.; Chemaly, M.; Davies, R.; Fernández Escámez, P.S.; Girones, R.; Herman, L.; Koutsoumanis, K.; Nørrung, B.; et al. Listeria Monocytogenes Contamination of Ready-to-Eat Foods and the Risk for Human Health in the EU. EFSA J. 2018, 16, e5134. [Google Scholar] [CrossRef]
- Shamloo, E.; Hosseini, H.; Moghadam, A.Z.; Larsen, H.M.; Haslberger, A.; Alebouyeh, M. Importance of Listeria Monocytogenes in Food Safety: A Review of Its Prevalence, Detection, and Antibiotic Resistance. Iran. J. Vet. Res. 2019, 20, 241–254. [Google Scholar] [PubMed]
- Radoshevich, L.; Cossart, P. Listeria Monocytogenes: Towards a Complete Picture of Its Physiology and Pathogenesis. Nat. Rev. Microbiol. 2018, 16, 32–46. [Google Scholar] [CrossRef]
- Disson, O.; Moura, A.; Lecuit, M. Making Sense of the Biodiversity and Virulence of Listeria Monocytogenes. Trends Microbiol. 2021, 29, 811–822. [Google Scholar] [CrossRef]
- Coelho, C.; Brown, L.; Maryam, M.; Vij, R.; Smith, D.F.Q.; Burnet, M.C.; Kyle, J.E.; Heyman, H.M.; Ramirez, J.; Prados-Rosales, R.; et al. Listeria Monocytogenes Virulence Factors, Including Listeriolysin O, Are Secreted in Biologically Active Extracellular Vesicles. J. Biol. Chem. 2019, 294, 1202–1217. [Google Scholar] [CrossRef] [PubMed]
- Lan, Z.-W.; Xiao, M.-J.; Guan, Y.; Zhan, Y.-J.; Tang, X.-Q. Detection of Listeria Monocytogenes in a Patient with Meningoencephalitis Using Next-Generation Sequencing: A Case Report. BMC Infect. Dis. 2020, 20, 721. [Google Scholar] [CrossRef] [PubMed]
- Adjei, P.C. Listeria Monocytogenes Meningoencephalitis and Cerebral Abscess in a Heart Transplant Recipient. Case Rep. Infect. Dis. 2020, 2020, 8496216. [Google Scholar] [CrossRef] [PubMed]

| Primer’s Name | Primers Sequences (5’→3’) | Product Size (pb) |
|---|---|---|
| lmo0737 | AGGGCTTCAAGGACTTACCC ACGATTTCTGCTTGCCATTC | 691 |
| lmo1118 | AGGGGTCTTAAATCCTGGAA CGGCTTGTTCGGCATACTTA | 906 |
| ORF2819 | AGCAAAATGCCAAAACTCGT CATCACTAAAGCCTCCCATTG | 471 |
| ORF2110 | AGTGGACAATTGATTGGTGAA CATCCATCCCTTACTTTGGAC | 597 |
| prs | GCTGAAGAGATTGCGAAAGAAG CAAAGAAACCTTGGATTTGCGG | 370 |
| Gene | bp | Serogroups | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1/2a | 3a | 1/2c | 3c | 4b | 4d | 4e | 1/2b | 3b | ||
| lmo1118 | 906 | ✓ | ✓ | |||||||
| lmo0737 | 691 | ✓ | ✓ | ✓ | ✓ | |||||
| ORF2110 | 597 | ✓ | ✓ | ✓ | ||||||
| ORF2819 | 471 | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| prs | 370 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Prime’s Name | Primers Sequences (5’→3’) | T/A (°C) |
|---|---|---|
| actA | CGCCGCGGAAATTAAAAAAAGA ACGAAGGAACCGGGCTGCTAG | 60 |
| hly | GTTAATGAACCTACAAGACCTTCC ACCGTTCTCCACCATTCCCA | 60 |
| llsX | TTATTGCATCAATTGTTCTA CCCCTATAAACATCATGCTAGTG | 52 |
| mpl | GCTTTGCCGGATTCCTGCG CTTCTTATTCGCCCATCTCGCG | 55 |
| plcA | CTGCTTGAGCGTTCATGTCTCATCCCCC CATGGGTTTCACTCTCCTTCTAC | 60 |
| plcB | ATGTGCTTGACCGCAAGTGT CTTCTCGGTAATCAGCCACC | 60 |
| prfA | AACGGGATAAAACCAAAACCA TGCGATGCCACTTGAATATC | 60 |
| inlA | CGGATGCAGGAGAAAATCC CTTTCACACTATCCTCTCC | 60 |
| inlB | GATATTGTGCCACTTTCAGGT CCTCTTTCAGTGGTTGGGT | 60 |
| inlC | AATTCCCACAGGACACAACC CGGGAATGCAATTTTTCACTA | 55 |
| Type of Sample | % (n) | ||
|---|---|---|---|
| 4b, 4d, 4e | 1/2b, 3b | 1/2a, 3a | |
| Cheese (n = 23) | 100% (23/23) | 0.0% (0/23) | 0.0% (0/23) |
| Chicken (n = 4) | 0.0% (0/4) | 25.0% (1/4) | 75.0% (3/4) |
| Ground beef (n = 11) | 27.3% (3/11) | 18.2% (2/11) | 54.5% (6/11) |
| Pathogenicity Islands | Virulence Factors | Serogroups | ||
|---|---|---|---|---|
| 1/2a, 3a | 1/2b, 3b | 4b, 4d, 4e | ||
| LIPI-1 | actA | 88.8% (8/9) | 66.6% (2/3) | 96.1% (25/26) |
| hly | 88.8% (8/9) | 100.0% (3/3) | 92.3% (24/26) | |
| mpl | 88.8% (8/9) | 0.0% (0/3) | 0.0% (0/26) | |
| plcA | 0.0% (0/9) | 100.0% (3/3) | 61.5% (16/26) | |
| plcB | 77.7% (7/9) | 100.0% (3/3) | 96.1% (25/26) | |
| prfA | 88.8% (8/9) | 0.0% (0/3) | 0.0% (0/26) | |
| LIPI-2 | inlA | 100.0 (9/9) | 100.0% (3/3) | 57.6% (15/26) |
| inlB | 77.7% (7/9) | 100.0% (3/3) | 53.8% (14/26) | |
| inlC | 88.8% (8/9) | 0.0% (0/3) | 50.0% (13/26) | |
| LIPI-3 | llsX | 0.0% (0/9) | 0.0% (0/3) | 100.0% (26/26) |
| Antimicrobials | Lineage I | Lineage II | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 4b, 4d, 4e | 1/2b, 3b | 1/2a, 3a | |||||||
| S | I | R | S | I | R | S | I | R | |
| Ampicillin | 100% (26/26) | 0.0% (0/26) | 0.0% (0/26) | 100% (3/3) | 0.0% (0/3) | 0.0% (0/3) | 100% (9/9) | 0.0% (0/9) | 0.0% (0/9) |
| Chloramphenicol | 100% (26/26) | 0.0% (0/26) | 0.0% (0/26) | 100% (3/3) | 0.0% (0/3) | 0.0% (0/3) | 100% (9/9) | 0.0% (0/9) | 0.0% (0/9) |
| Ciprofloxacin | 53.8% (14/26) | 46.1% (12/26) | 0.0% (0/26) | 100% (3/3) | 0.0% (0/3) | 0.0% (0/3) | 66.6% (6/9) | 33.3% (3/9) | 0.0% (0/9) |
| Erythromycin | 100% (26/26) | 0.0% (0/26) | 0.0% (0/26) | 100% (3/3) | 0.0% (0/3) | 0.0% (0/3) | 88.8% (8/9) | 0.0 (0/9) | 11.1% (8/9) |
| Gentamicin | 100% (26/26) | 0.0% (0/26) | 0.0% (0/26) | 100% (3/3) | 0.0% (0/3) | 0.0% (0/3) | 100% (9/9) | 0.0% (0/9) | 0.0% (0/9) |
| Levofloxacin | 100% (26/26) | 0.0% (0/26) | 0.0% (0/26) | 100% (3/3) | 0.0% (0/3) | 0.0% (0/3) | 100% (9/9) | 0.0% (0/9) | 0.0% (0/9) |
| Meropenem | 69.7% (20/26) | 0.0% (0/26) | 30.7% (8/26) | 100% (3/3) | 0.0% (0/3) | 0.0% (0/3) | 100% (9/9) | 0.0% (0/9) | 0.0% (0/9) |
| Penicillin | 100% (26/26) | 0.0% (0/26) | 0.0% (0/26) | 100% (3/3) | 0.0% (0/3) | 0.0% (0/3) | 100% (9/9) | 0.0% (0/9) | 0.0% (0/9) |
| Streptomycin | 69.7% (20/26) | 0.0% (0/26) | 30.7% (8/26) | 100% (3/3) | 0.0% (0/3) | 0.0% (0/3) | 77.7% (7/9) | 0.0% (0/9) | 22.2% (2/9) |
| Sulfamethoxazole/trimethoprim | 11.5% (3/26) | 0.0% (0/26) | 88.4% (3/26) | 100% (3/3) | 0.0% (0/3) | 0.0% (0/3) | 22.2% (2/9) | 0.0% (0/9) | 77.7% (7/9) |
| Tetracycline | 100% (26/26) | 0.0% (0/26) | 0.0% (0/26) | 100% (3/3) | 0.0% (0/3) | 0.0% (0/3) | 100% (9/9) | 0.0% (0/9) | 0.0% (0/9) |
| Vancomycin | 100% (26/26) | 0.0% (0/26) | 0.0% (0/26) | 100% (3/3) | 0.0% (0/3) | 0.0% (0/3) | 100% (9/9) | 0.0% (0/9) | 0.0% (0/9) |
| MARI | Pattern | n | Total | % |
|---|---|---|---|---|
| 0.083 | STX-TMP | 16 | 16/38 | 42.1 |
| 0.167 | STX-TMP + STR STX-TMP + MEM | 5 4 | 9/38 | 23.6 |
| 0.250 | STX-TMP + STR + MEM STX-TMP + STR + ERY | 4 1 | 5/38 | 13.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guel-García, P.; García De León, F.J.; Aguilera-Arreola, G.; Mandujano, A.; Mireles-Martínez, M.; Oliva-Hernández, A.; Cruz-Hernández, M.A.; Vasquez-Villanueva, J.; Rivera, G.; Bocanegra-García, V.; et al. Prevalence and Antimicrobial Resistance of Listeria monocytogenes in Different Raw Food from Reynosa, Tamaulipas, Mexico. Foods 2024, 13, 1656. https://doi.org/10.3390/foods13111656
Guel-García P, García De León FJ, Aguilera-Arreola G, Mandujano A, Mireles-Martínez M, Oliva-Hernández A, Cruz-Hernández MA, Vasquez-Villanueva J, Rivera G, Bocanegra-García V, et al. Prevalence and Antimicrobial Resistance of Listeria monocytogenes in Different Raw Food from Reynosa, Tamaulipas, Mexico. Foods. 2024; 13(11):1656. https://doi.org/10.3390/foods13111656
Chicago/Turabian StyleGuel-García, Paulina, Francisco Javier García De León, Guadalupe Aguilera-Arreola, Antonio Mandujano, Maribel Mireles-Martínez, Amanda Oliva-Hernández, María Antonia Cruz-Hernández, Jose Vasquez-Villanueva, Gildardo Rivera, Virgilio Bocanegra-García, and et al. 2024. "Prevalence and Antimicrobial Resistance of Listeria monocytogenes in Different Raw Food from Reynosa, Tamaulipas, Mexico" Foods 13, no. 11: 1656. https://doi.org/10.3390/foods13111656
APA StyleGuel-García, P., García De León, F. J., Aguilera-Arreola, G., Mandujano, A., Mireles-Martínez, M., Oliva-Hernández, A., Cruz-Hernández, M. A., Vasquez-Villanueva, J., Rivera, G., Bocanegra-García, V., & Martínez-Vázquez, A. V. (2024). Prevalence and Antimicrobial Resistance of Listeria monocytogenes in Different Raw Food from Reynosa, Tamaulipas, Mexico. Foods, 13(11), 1656. https://doi.org/10.3390/foods13111656







