Antioxidant Activity, Physicochemical and Sensory Properties of Stingless Bee Honey from Australia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Solutions
2.2. Honey Samples
2.3. Physicochemical Analysis
2.3.1. Soluble Solids (°Brix) and Moisture
2.3.2. Electrical Conductivity
2.3.3. pH
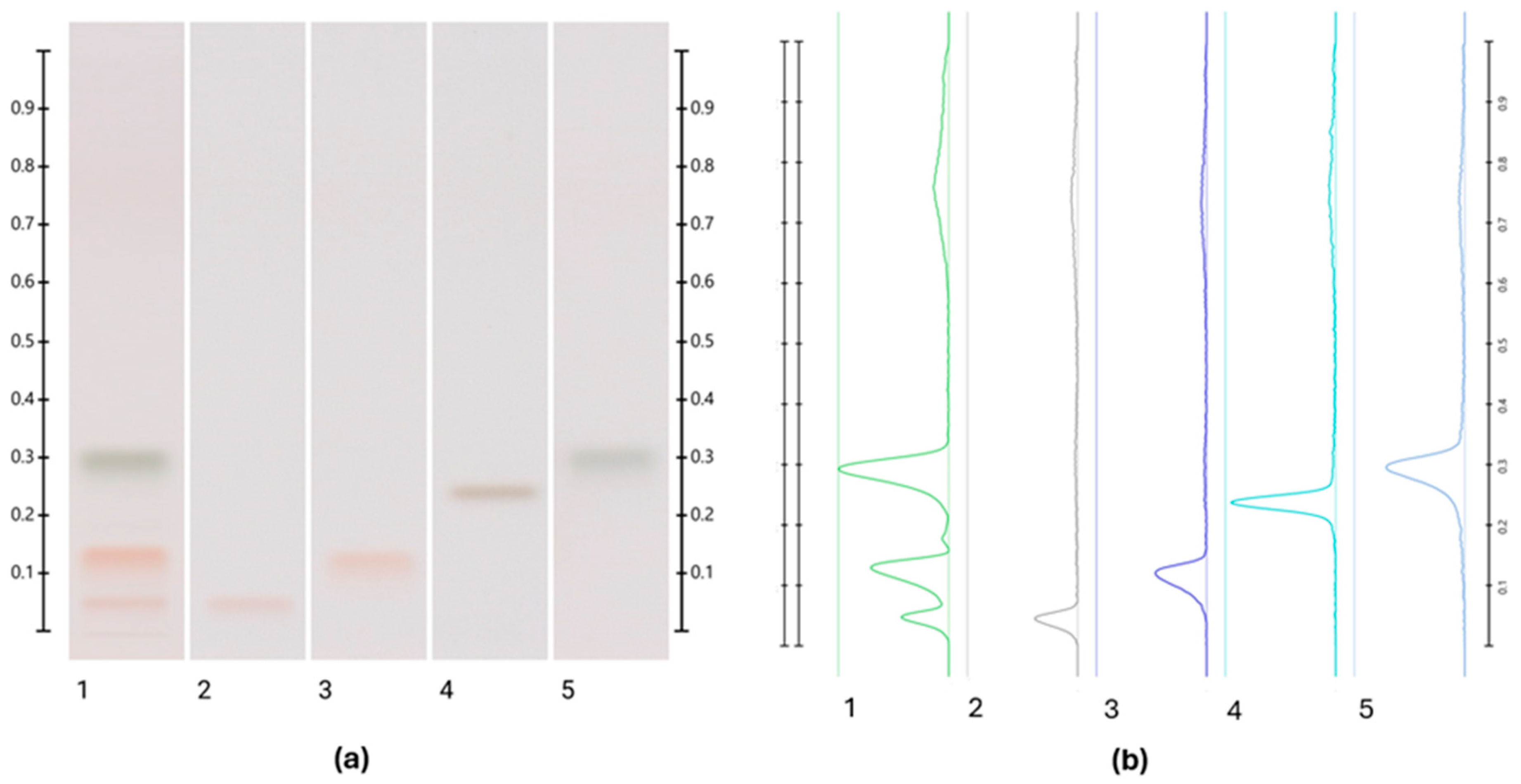
2.4. Sugar Analysis
2.5. Consumer Evaluation
2.6. Determination of Total Phenolic Content (TPC)
2.7. Determination of Antioxidant Activity Using the Ferric Reducing Antioxidant Power (FRAP) Assay
2.8. Determination of Antioxidant Activity Using the 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Radical Scavenging Assay
2.9. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Parameters
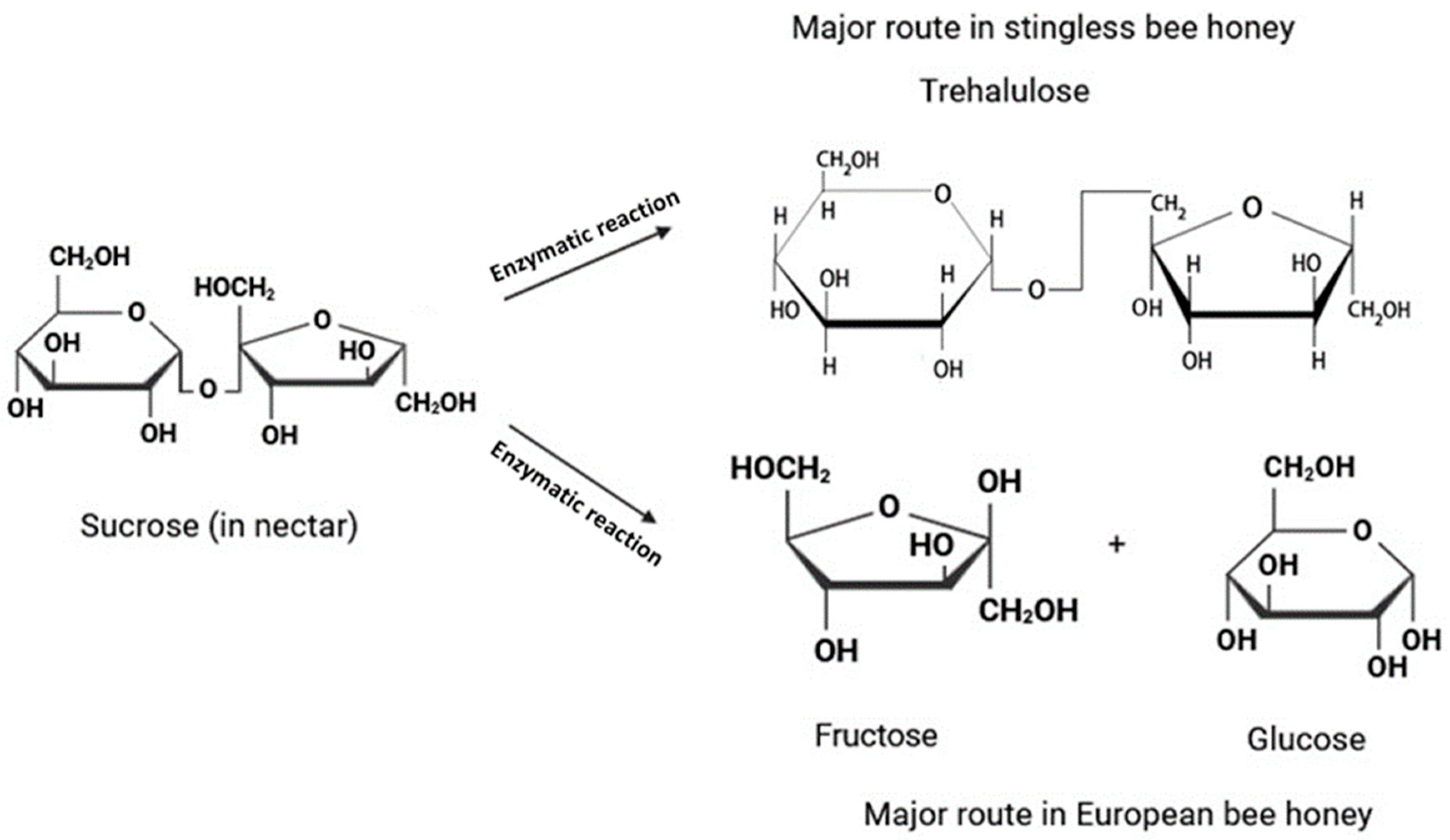
3.2. Sugar Analysis
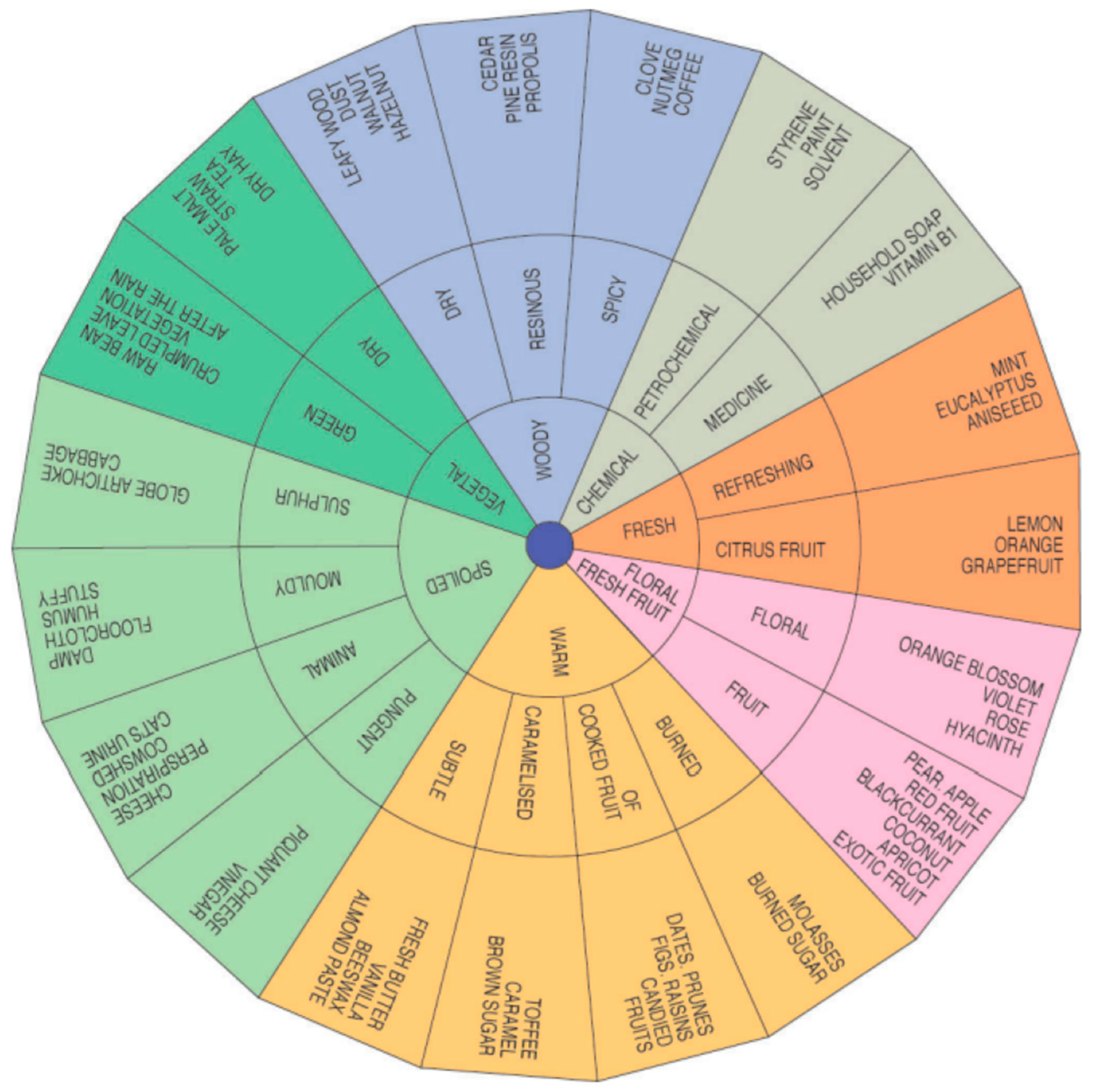
3.3. Consumer Evaluation
3.4. Total Phenolic Content (TPC)
3.5. Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abd Jalil, M.A.; Kasmuri, A.R.; Hadi, H. Stingless bee honey, the natural wound healer: A review. Skin Pharmacol. Physiol. 2017, 30, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Chuttong, B.; Chanbang, Y.; Sringarm, K.; Burgett, M. Physicochemical profiles of stingless bee (Apidae: Meliponini) honey from South East Asia (Thailand). Food Chem. 2016, 192, 149–155. [Google Scholar] [CrossRef]
- Perichon, S.; Heard, T.A.; Schouten, C. Perceptions of keepers of stingless bees (Tetragonula, Austroplebeia) regarding Aboriginal beliefs and practices in Australia. J. Apic. Res. 2021, 60, 665–677. [Google Scholar] [CrossRef]
- Al-Hatamleh, M.A.I.; Boer, J.C.; Wilson, K.L.; Plebanski, M.; Mohamud, R.; Mustafa, M.Z. Antioxidant-Based Medicinal Properties of Stingless Bee Products: Recent Progress and Future Directions. Biomolecules 2020, 10, 923. [Google Scholar] [CrossRef] [PubMed]
- Amin, F.A.Z.; Sabri, S.; Mohammad, S.M.; Ismail, M.; Chan, K.W.; Ismail, N.; Norhaizan, M.E.; Zawawi, N. Therapeutic Properties of Stingless Bee Honey in Comparison with European Bee Honey. Adv. Pharmacol. Sci. 2018, 2018, 6179596. [Google Scholar] [CrossRef] [PubMed]
- Esa, N.E.F.; Ansari, M.N.M.; Razak, S.I.A.; Ismail, N.I.; Jusoh, N.; Zawawi, N.A.; Jamaludin, M.I.; Sagadevan, S.; Nayan, N.H.M. A Review on Recent Progress of Stingless Bee Honey and Its Hydrogel-Based Compound for Wound Care Management. Molecules 2022, 27, 3080. [Google Scholar] [CrossRef] [PubMed]
- Ngaini, Z.; Hussain, H.; Kelabo, E.S.; Wahi, R.; Farooq, S. Chemical profiling, biological properties and environmental contaminants of stingless bee honey and propolis. J. Apic. Res. 2021, 62, 131–147. [Google Scholar] [CrossRef]
- Massaro, C.F.; Shelley, D.; Heard, T.A.; Brooks, P. In vitro antibacterial phenolic extracts from “sugarbag” pot-honeys of Australian stingless bees (Tetragonula carbonaria). J. Agric. Food Chem. 2014, 62, 12209–12217. [Google Scholar] [CrossRef]
- Haley, D. The Honey of Australian Native Stingless Bees; Self-Published Brisbane: Surrey Hills, VIC, Australia, 2021; ISBN 9780646838052. [Google Scholar]
- Shamsudin, S.; Selamat, J.; Sanny, M.; A.R., S.B.; Jambari, N.N.; Khatib, A. A Comparative Characterization of Physicochemical and Antioxidants Properties of Processed Heterotrigona itama Honey from Different Origins and Classification by Chemometrics Analysis. Molecules 2019, 24, 3898. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.M.; Giampieri, F.; Brenciani, A.; Mazzoni, L.; Gasparrini, M.; González-Paramás, A.M.; Santos-Buelga, C.; Morroni, G.; Simoni, S.; Forbes-Hernández, T.Y.; et al. Apis mellifera vs Melipona beecheii Cuban polifloral honeys: A comparison based on their physicochemical parameters, chemical composition and biological properties. LWT—Food Sci. Technol. 2018, 87, 272–279. [Google Scholar] [CrossRef]
- Costa Dos Santos, A.; Biluca, F.C.; Brugnerotto, P.; Valdemiro Gonzaga, L.; Oliveira Costa, A.C.; Fett, R. Brazilian stingless bee honey: Physicochemical properties and aliphatic organic acids content. Food Res. Int. 2022, 158, 111516. [Google Scholar] [CrossRef] [PubMed]
- Tuksitha, L.; Chen, Y.-L.S.; Chen, Y.-L.; Wong, K.-Y.; Peng, C.-C. Antioxidant and antibacterial capacity of stingless bee honey from Borneo (Sarawak). J. Asia Pac. Entomol. 2018, 21, 563–570. [Google Scholar] [CrossRef]
- Boorn, K.L.; Khor, Y.Y.; Sweetman, E.; Tan, F.; Heard, T.A.; Hammer, K.A. Antimicrobial activity of honey from the stingless bee Trigona carbonaria determined by agar diffusion, agar dilution, broth microdilution and time-kill methodology. J. Appl. Microbiol. 2010, 108, 1534–1543. [Google Scholar] [CrossRef] [PubMed]
- Mat Ramlan, N.A.F.; Md Zin, A.S.; Safari, N.F.; Chan, K.W.; Zawawi, N. Application of heating on the antioxidant and antibacterial properties of malaysian and australian stingless bee honey. Antibiotics 2021, 10, 1365. [Google Scholar] [CrossRef]
- Fletcher, M.T.; Hungerford, N.L.; Webber, D.; Carpinelli de Jesus, M.; Zhang, J.; Stone, I.S.J.; Blanchfield, J.T.; Zawawi, N. Stingless bee honey, a novel source of trehalulose: A biologically active disaccharide with health benefits. Sci. Rep. 2020, 10, 12128. [Google Scholar] [CrossRef]
- Zawawi, N.; Zhang, J.; Hungerford, N.L.; Yates, H.S.A.; Webber, D.C.; Farrell, M.; Tinggi, U.; Bhandari, B.; Fletcher, M.T. Unique physicochemical properties and rare reducing sugar trehalulose mandate new international regulation for stingless bee honey. Food Chem. 2022, 373, 131566. [Google Scholar] [CrossRef]
- Islam, M.K.; Lawag, I.L.; Sostaric, T.; Ulrich, E.; Ulrich, D.; Dewar, T.; Lim, L.Y.; Locher, C. Australian Honeypot Ant (Camponotus inflatus) Honey-A Comprehensive Analysis of the Physiochemical Characteristics, Bioactivity, and HPTLC Profile of a Traditional Indigenous Australian Food. Molecules 2022, 27, 2154. [Google Scholar] [CrossRef] [PubMed]
- Adaškevičiūtė, V.; Kaškonienė, V.; Kaškonas, P.; Barčauskaitė, K.; Maruška, A. Comparison of Physicochemical Properties of Bee Pollen with Other Bee Products. Biomolecules 2019, 9, 819. [Google Scholar] [CrossRef]
- Islam, M.K.; Sostaric, T.; Lim, L.Y.; Hammer, K.; Locher, C. A validated method for the quantitative determination of sugars in honey using high-performance thin-layer chromatography. JPC-J. Planar Chromatogr. 2020, 33, 489–499. [Google Scholar] [CrossRef]
- Mello dos Santos, M.; Jacobs, C.; Islam, M.K.; Lim, L.Y.; Locher, C. Validation of a high-performance thin-layer chromatography method for the quantitative determination of trehalulose. JPC-J. Planar Chromatogr. 2023, 36, 201–210. [Google Scholar] [CrossRef]
- Piana, M.L.; Persano Oddo, L.; Bentabol, A.; Bruneau, E.; Bogdanov, S.; Guyot Declerck, C. Sensory analysis applied to honey: State of the art. Apidologie 2004, 35, S26–S37. [Google Scholar] [CrossRef]
- González, M.M.; de Lorenzo, C.; Pérez, R.A. Development of a structured sensory honey analysis: Application to artisanal Madrid honeys. Food Sci. Technol. Int. 2010, 16, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Hunter, M.; Kellett, J.; Toohey, K.; Naumovski, N. Sensory and compositional properties affecting the likeability of commercially available australian honeys. Foods 2021, 10, 1842. [Google Scholar] [CrossRef] [PubMed]
- Liberato, M.D.C.C.; de Morais, S.M.; Siqueira, S.M.C.; de Menezes, J.E.S.A.; Ramos, D.N.; Machado, L.K.A.; Magalhães, I.L. Phenolic content and antioxidant and antiacetylcholinesterase properties of honeys from different floral origins. J. Med. Food 2011, 14, 658–663. [Google Scholar] [CrossRef]
- Lawag, I.L.; Nolden, E.S.; Schaper, A.A.M.; Lim, L.Y.; Locher, C. A Modified Folin-Ciocalteu Assay for the Determination of Total Phenolics Content in Honey. Appl. Sci. 2023, 13, 2135. [Google Scholar] [CrossRef]
- de Almeida, A.M.M.; Oliveira, M.B.S.; da Costa, J.G.; Valentim, I.B.; Goulart, M.O.F. Antioxidant Capacity, Physicochemical and Floral Characterization of Honeys from the Northeast of Brazil. Rev. Virtual Quim. 2016, 8, 57–77. [Google Scholar] [CrossRef]
- Lawag, I.L.; Islam, M.K.; Sostaric, T.; Lim, L.Y.; Hammer, K.; Locher, C. Antioxidant activity and phenolic compound identification and quantification in western australian honeys. Antioxidants 2023, 12, 189. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. Codex. 2022. Available online: https://www.fao.org/fao-who-codexalimentarius/codex-texts/list-standards/en/ (accessed on 24 March 2024).
- Ávila, S.; Beux, M.R.; Ribani, R.H.; Zambiazi, R.C. Stingless bee honey: Quality parameters, bioactive compounds, health-promotion properties and modification detection strategies. Trends Food Sci. Technol. 2018, 81, 37–50. [Google Scholar] [CrossRef]
- Ramón-Sierra, J.M.; Ruiz-Ruiz, J.C.; de la Luz Ortiz-Vázquez, E. Electrophoresis characterisation of protein as a method to establish the entomological origin of stingless bee honeys. Food Chem. 2015, 183, 43–48. [Google Scholar] [CrossRef]
- Oddo, L.P.; Heard, T.A.; Rodríguez-Malaver, A.; Pérez, R.A.; Fernández-Muiño, M.; Sancho, M.T.; Sesta, G.; Lusco, L.; Vit, P. Composition and antioxidant activity of Trigona carbonaria honey from Australia. J. Med. Food 2008, 11, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Biluca, F.C.; Braghini, F.; Campos Ferreira, G.; Costa dos Santos, A.; Baggio Ribeiro, D.H.; Valdemiro Gonzaga, L.; Vitali, L.; Amadeu Micke, G.; Carolina Oliveira Costa, A.; Fett, R. Physicochemical parameters, bioactive compounds, and antibacterial potential of stingless bee honey. J. Food Process. Preserv. 2021, 45, e15127. [Google Scholar] [CrossRef]
- Biluca, F.C.; Braghini, F.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Physicochemical profiles, minerals and bioactive compounds of stingless bee honey (Meliponinae). J. Food Compos. Anal. 2016, 50, 61–69. [Google Scholar] [CrossRef]
- Solayman, M.; Islam, M.A.; Paul, S.; Ali, Y.; Khalil, M.I.; Alam, N.; Gan, S.H. Physicochemical properties, minerals, trace elements, and heavy metals in honey of different origins: A comprehensive review. Comp. Rev. Food Sci. Food Safety 2016, 15, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Nordin, A.; Sainik, N.Q.A.V.; Chowdhury, S.R.; Saim, A.B.; Idrus, R.B.H. Physicochemical properties of stingless bee honey from around the globe: A comprehensive review. J. Food Compos. Anal. 2018, 73, 91–102. [Google Scholar] [CrossRef]
- da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Honey: Chemical composition, stability and authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Hungerford, N.L.; Zhang, J.; Smith, T.J.; Yates, H.S.A.; Chowdhury, S.A.; Carter, J.F.; Carpinelli de Jesus, M.; Fletcher, M.T. Feeding Sugars to Stingless Bees: Identifying the Origin of Trehalulose-Rich Honey Composition. J. Agric. Food Chem. 2021, 69, 10292–10300. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hungerford, N.L.; Yates, H.S.A.; Smith, T.J.; Fletcher, M.T. How is Trehalulose Formed by Australian Stingless Bees?—An Intermolecular Displacement of Nectar Sucrose. J. Agric. Food Chem. 2022, 70, 6530–6539. [Google Scholar] [CrossRef] [PubMed]
- El Sohaimy, S.A.; Masry, S.H.D.; Shehata, M.G. Physicochemical characteristics of honey from different origins. Ann. Agric. Sci. 2015, 60, 279–287. [Google Scholar] [CrossRef]
- de Sousa, J.M.B.; de Souza, E.L.; Marques, G.; de Toledo Benassi, M.; Gullón, B.; Pintado, M.M.; Magnani, M. Sugar profile, physicochemical and sensory aspects of monofloral honeys produced by different stingless bee species in Brazilian semi-arid region. LWT—Food Sci. Technol. 2016, 65, 645–651. [Google Scholar] [CrossRef]
- Fletcher, M.; Hungerford, N.; Smith, T. Optimising Bioactive Content of Australian Stingless Bee Honey; AgriFutures: Wagga Wagga, NSW, Australia, 2021. [Google Scholar]
- Julika, W.N.; Ajit, A.; Ismail, N.; Aqilah, N.; Naila, A.; Sulaiman, A.Z. Sugar profile and enzymatic analysis of stingless bee honey collected from local market in Malaysia. IOP Conf. Ser. Mater. Sci. Eng. 2020, 736, 062001. [Google Scholar] [CrossRef]
- Ferreira, E.L.; Lencioni, C.; Benassi, M.T.; Barth, M.O.; Bastos, D.H.M. Descriptive sensory analysis and acceptance of stingless bee honey. Food Sci. Technol. Int. 2009, 15, 251–258. [Google Scholar] [CrossRef]
- Shamsudin, S.; Selamat, J.; Sanny, M.; Razak, S.-B.A.; Jambari, N.N.; Mian, Z.; Khatib, A. Influence of origins and bee species on physicochemical, antioxidant properties and botanical discrimination of stingless bee honey. Int. J. Food Prop. 2019, 22, 239–264. [Google Scholar] [CrossRef]
- Ávila, S.; Hornung, P.S.; Teixeira, G.L.; Malunga, L.N.; Apea-Bah, F.B.; Beux, M.R.; Beta, T.; Ribani, R.H. Bioactive compounds and biological properties of Brazilian stingless bee honey have a strong relationship with the pollen floral origin. Food Res. Int. 2019, 123, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cianciosi, D.; Forbes-Hernández, T.Y.; Afrin, S.; Gasparrini, M.; Reboredo-Rodriguez, P.; Manna, P.P.; Zhang, J.; Bravo Lamas, L.; Martínez Flórez, S.; Agudo Toyos, P.; et al. Phenolic compounds in honey and their associated health benefits: A review. Molecules 2018, 23, 2322. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, T.C.; Rosset, M.; de Sousa, J.M.B.; de Oliveira, L.I.G.; Mafaldo, I.M.; Pintado, M.M.E.; de Souza, E.L.; Magnani, M. Stingless bee honey: An overview of health benefits and main market challenges. J. Food Biochem. 2022, 46, e13883. [Google Scholar] [CrossRef]
- da Silva, I.A.A.; da Silva, T.M.S.; Camara, C.A.; Queiroz, N.; Magnani, M.; de Novais, J.S.; Soledade, L.E.B.; Lima, E.d.O.; de Souza, A.L.; de Souza, A.G. Phenolic profile, antioxidant activity and palynological analysis of stingless bee honey from Amazonas, Northern Brazil. Food Chem. 2013, 141, 3552–3558. [Google Scholar] [CrossRef]
- Abu Bakar, M.F.; Sanusi, S.B.; Abu Bakar, F.I.; Cong, O.J.; Mian, Z. Physicochemical and antioxidant potential of raw unprocessed honey from malaysian stingless bees. Pak. J. Nutr. 2017, 16, 888–894. [Google Scholar] [CrossRef]

| Species | Sample | District Area | Harvest Date |
|---|---|---|---|
| Tetragonula carbonaria | C1 (n = 5) | Burpengary East, Property A | May 2022 |
| C2 (n = 5) | Burpengary East, Property B | May 2022 | |
| C3 (n = 5) | Burpengary East, Property A | September 2022 | |
| C4 (n = 3) | Burpengary East, Property B | September 2022 | |
| C5 (n = 6) | Burpengary East, Property A | November 2022 | |
| C6 (n = 2) | Burpengary East, Property B | November 2022 | |
| C7 (n = 1) | Brighton | September 2021 | |
| C8 (n = 1) | Tarragindi | March 2022 | |
| Tetragonula hockingsi | H1 (n = 2) | Burpengary East, Property A | May 2022 |
| H2 (n = 2) | Burpengary East, Property A | September 2022 | |
| H3 (n = 2) | Burpengary East, Property A | November 2022 | |
| H4 (n = 1) | Tarragindi | Not provided | |
| H5 (n = 1) | Brighton | September 2020 |
| Sample | Moisture (%w/w) | Soluble Solids (°Brix) | Electrical Conductivity (mS/cm) | pH |
|---|---|---|---|---|
| C1 | 26.7 ± 1.71 a,b | 73.3 ± 1.71 a,b | 1.34 ± 0.108 a | 3.85 ± 0.0619 a,b |
| C2 | 27.1 ± 0.868 a,b | 72.9 ± 0.868 a,b | 1.29 ± 0.275 a | 3.69 ± 0.0391 a,b |
| C3 | 27.5 ± 0.856 a,b | 72.5 ± 0.856 a,b | 1.62 ± 0.170 a | 4.64 ± 0.244 a,b,c,d |
| C4 | 28.3 ± 0.361 a | 71.7 ± 0.361 a | 1.41 ± 0.0961 a | 3.60 ± 0.0493 a |
| C5 | 27.1 ± 0.501 a,b | 72.9 ± 0.501 a,b | 1.41 ± 0.165 a | 4.91 ± 0.728 b,c,d |
| C6 | 27.3 ± 0.283 a,b | 72.7 ± 0.283 a,b | 1.24 ± 0.226 a | 3.89 ± 0.00 a,b |
| C7 | 27.9 ± 0.00 a,b | 72.1 ± 0.00 a,b | 1.52 ± 0.00 a | 3.73 ± 0.00 a,b |
| C8 | 27.3 ± 0.00 a,b | 72.7 ± 0.00 a,b | 2.15 ± 0.00 b | 3.72 ± 0.00 a,b |
| H1 | 29.9 ± 0.919 a | 70.2 ± 0.919 a | 1.53 ± 0.0283 a | 3.92 ± 0.0636 a,b |
| H2 | 29.4 ± 2.05 a | 70.7 ± 2.05 a | 1.63 ± 0.0212 a,b | 5.90 ± 0.912 d |
| H3 | 27.2 ± 1.98 a,b | 72.8 ± 1.98 a,b | 1.53 ± 0.247 a | 5.51 ± 0.785 c,d |
| H4 | 24.9 ± 0.00 b | 75.1 ± 0.00 b | 1.62 ± 0.00 a | 4.38 ± 0.00 a,b,c |
| H5 | 28.8 ± 0.00 a | 71.2 ± 0.00 a | 1.46 ± 0.00 a | 3.69 ± 0.00 a,b |
| Sample | Trehalulose (g/100 g) | Fructose (g/100 g) | Glucose (g/100 g) | Sucrose (g/100 g) | F/G | F/T | G/T |
|---|---|---|---|---|---|---|---|
| C1 | 26.4 ± 4.46 c,d | 15.7 ± 2.72 b | 8.37 ± 2.34 b | Not detected | 1.87 | 0.593 | 0.317 |
| C2 | 30.7 ± 5.04 d | 10.2 ± 2.64 a | 4.62 ± 1.43 a | Not detected | 2.20 | 0.331 | 0.150 |
| C3 | 16.2 ± 2.25 a,b | 26.3 ± 1.81 e,f,g | 19.7 ± 1.66 d,e | Not detected | 1.33 | 1.63 | 1.22 |
| C4 | 21.3 ± 2.83 b,c | 20.8 ± 2.12 c,d | 13.5 ± 1.07 c | Not detected | 1.54 | 0.977 | 0.636 |
| C5 | 11.4 ± 2.03 a | 28.6 ± 1.84 f,g,h | 23.4 ± 1.65 f | Not detected | 1.22 | 2.51 | 2.06 |
| C6 | 15.7 ± 0.493 a,b | 22.2 ± 0.331 c,d,e | 17.5 ± 1.16 d | Not detected | 1.27 | 1.42 | 1.12 |
| C7 | 15.8 ± 0.735 a,b | 24.2 ± 0.246 d,e,f | 16.8 ± 0.350 c,d | Not detected | 1.44 | 1.54 | 1.07 |
| C8 | 27.1 ± 0.786 c,d | 11.1 ± 0.370 a | 4.31 ± 0.175 a | Not detected | 2.56 | 0.408 | 0.159 |
| H1 | 25.5 ± 4.84 c,d | 17.7 ± 4.35 b,c | 9.86 ± 2.95 b | Not detected | 1.79 | 0.694 | 0.387 |
| H2 | 12.5 ± 2.67 a | 30.0 ± 1.04 g,h | 22.5 ± 0.526 e,f | Not detected | 1.34 | 2.39 | 1.79 |
| H3 | 10.0 ± 4.24 a | 31.1 ± 2.56 h | 24.0 ± 3.08 f | Not detected | 1.30 | 3.09 | 2.39 |
| H4 | 12.0 ± 0.257 a | 29.6 ± 0.882 g,h | 17.7 ± 0.379 d | Not detected | 1.67 | 2.48 | 1.48 |
| H5 | 11.2 ± 0.399 a | 26.7 ± 0.926 e,f,g,h | 16.4 ± 0.437 c,d | Not detected | 1.62 | 2.38 | 1.46 |
| Sample | TPC (mg GAE/100 g) | FRAP (mmol Fe2+/kg) | DPPH (mmol TE/kg) |
|---|---|---|---|
| C1 | 40.2 ± 5.17 d,e | 6.23 ± 0.656 g,h | 4.30 ± 1.06 e |
| C2 | 36.9 ± 7.81 c,d,e | 4.52 ± 0.639 d,e,f | 3.00 ± 0.543 c,d,e |
| C3 | 25.3 ± 2.36 a,b | 3.07 ± 0.284 a,b | 1.48 ± 0.353 a,b |
| C4 | 29.9 ± 1.05 a,b,c | 3.86 ± 0.517 b,c,d | 2.77 ± 0.243 b,c,d |
| C5 | 33.3 ± 4.70 b,c,d | 3.35 ± 0.649 b,c | 2.76 ± 0.754 b,c,d |
| C6 | 34.7 ± 1.47 c,d,e | 4.33 ± 0.333 c,d,e | 3.70 ± 0.378 d,e |
| C7 | 43.8 ± 0.363 e | 5.93 ± 0.211 g,h | 3.93 ± 0.125 d,e |
| C8 | 37.2 ± 0.201 c,d,e | 5.25 ± 0.0609 e,f,g | 3.78 ± 0.195 d,e |
| H1 | 53.7 ± 4.85 f | 9.10 ± 0.388 i | 6.67 ± 0.834 f |
| H2 | 22.1 ± 2.94 a | 2.07 ± 0.776 a | 0.704 ± 0.525 a |
| H3 | 31.2 ± 4.45 a,b,c,d | 3.02 ± 0.828 a,b | 2.03 ± 1.04 a,b,c |
| H4 | 63.8 ± 1.44 g | 6.42 ± 0.259 h | 5.95 ± 0.203 f |
| H5 | 39.4 ± 0.0644 d,e | 5.56 ± 0.0765 f,g,h | 4.04 ± 0.0865 d,e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mello dos Santos, M.; Khan, N.; Lim, L.Y.; Locher, C. Antioxidant Activity, Physicochemical and Sensory Properties of Stingless Bee Honey from Australia. Foods 2024, 13, 1657. https://doi.org/10.3390/foods13111657
Mello dos Santos M, Khan N, Lim LY, Locher C. Antioxidant Activity, Physicochemical and Sensory Properties of Stingless Bee Honey from Australia. Foods. 2024; 13(11):1657. https://doi.org/10.3390/foods13111657
Chicago/Turabian StyleMello dos Santos, Mariana, Nazim Khan, Lee Yong Lim, and Cornelia Locher. 2024. "Antioxidant Activity, Physicochemical and Sensory Properties of Stingless Bee Honey from Australia" Foods 13, no. 11: 1657. https://doi.org/10.3390/foods13111657
APA StyleMello dos Santos, M., Khan, N., Lim, L. Y., & Locher, C. (2024). Antioxidant Activity, Physicochemical and Sensory Properties of Stingless Bee Honey from Australia. Foods, 13(11), 1657. https://doi.org/10.3390/foods13111657








