The Metabolic and Antioxidant Activity Profiles of Aged Greek Grape Marc Spirits
Abstract
1. Introduction
2. Materials and Methods
2.1. Instruments
2.2. Samples and Chemicals
2.3. NMR-Based Metabolic Fingerprinting
2.3.1. Postprocessing of NMR Data
2.3.2. Multivariate Data Analysis
2.4. Antioxidant Activity Profile
2.4.1. Determination of Total Phenolic Content Using the Folin–Ciocalteau Assay
2.4.2. Assessment of Total O-Diphenolic Content
2.4.3. Assessment of Free Radical Scavenging Activity Using DPPH Assay
2.4.4. Assessment of Free Radical Scavenging Activity Using ABTS•+ Assay
2.4.5. Assessment of Reductive Capacity Using FRAP Assay
2.4.6. Assessment of Reductive Capacity Using CUPRAC Assay
2.5. Statistical Analysis
3. Results and Discussion
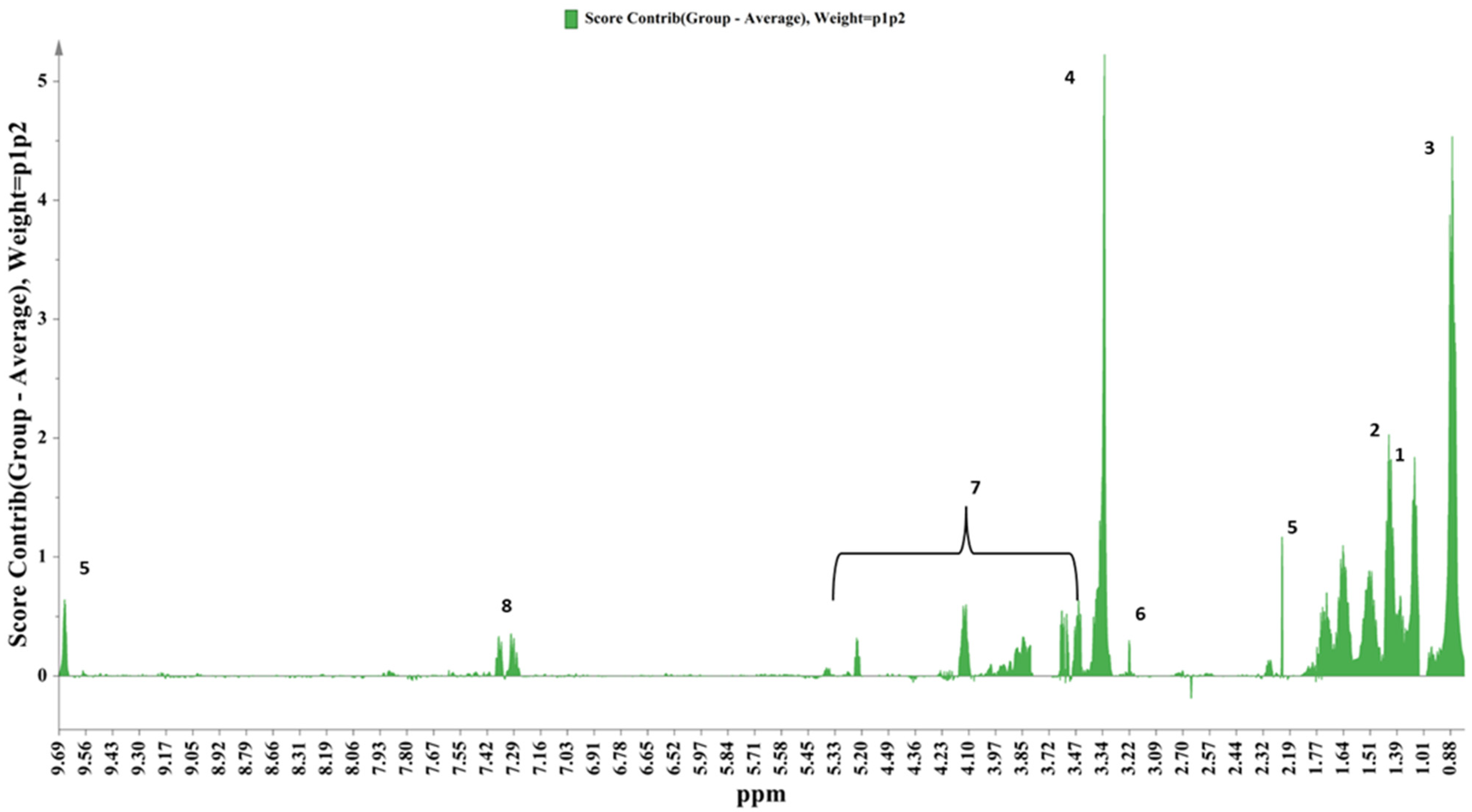
3.1. H NMR Metabolic Profile
3.2. Chemometrics Analysis on the NMR Metabolomics Data
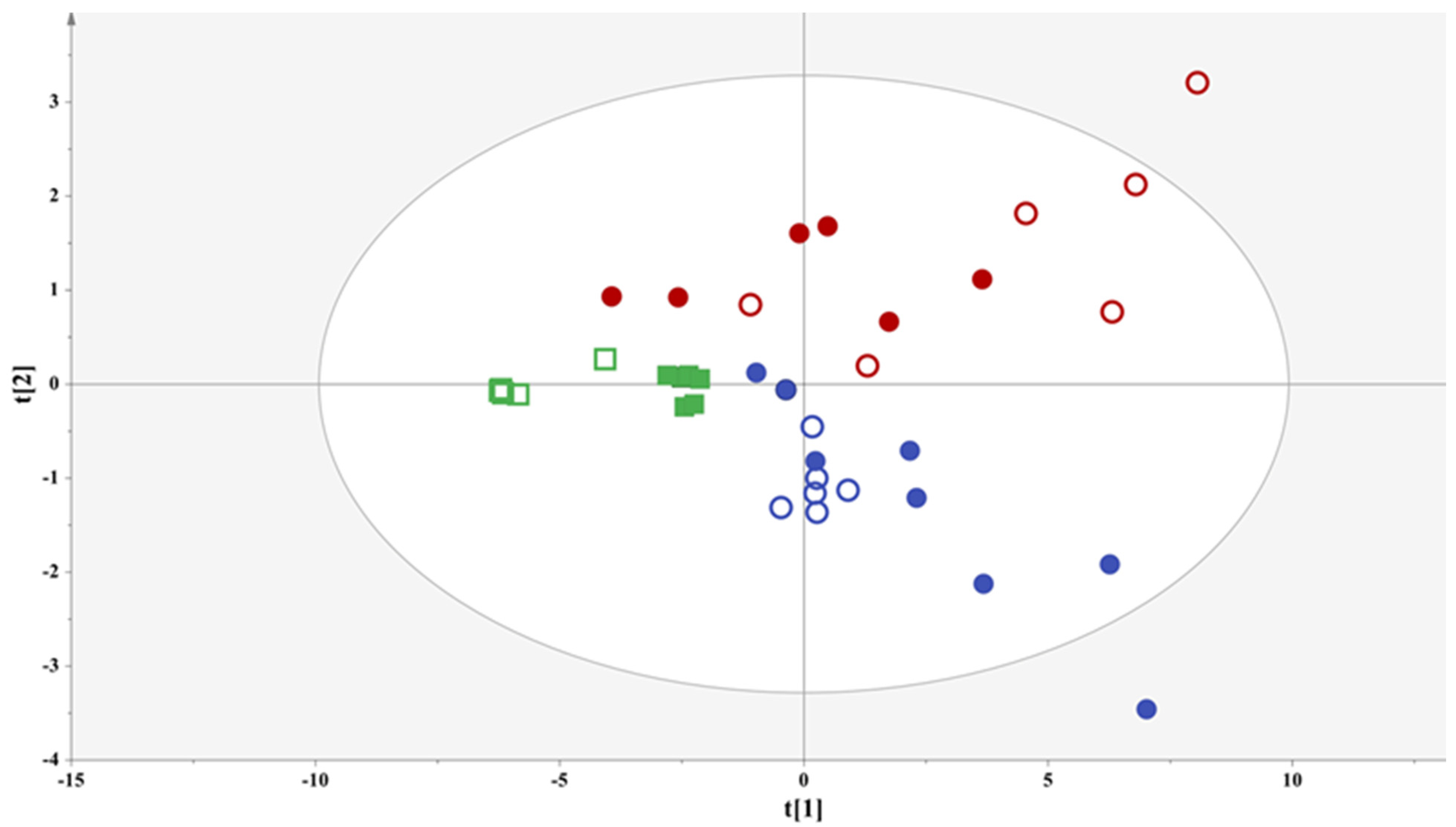
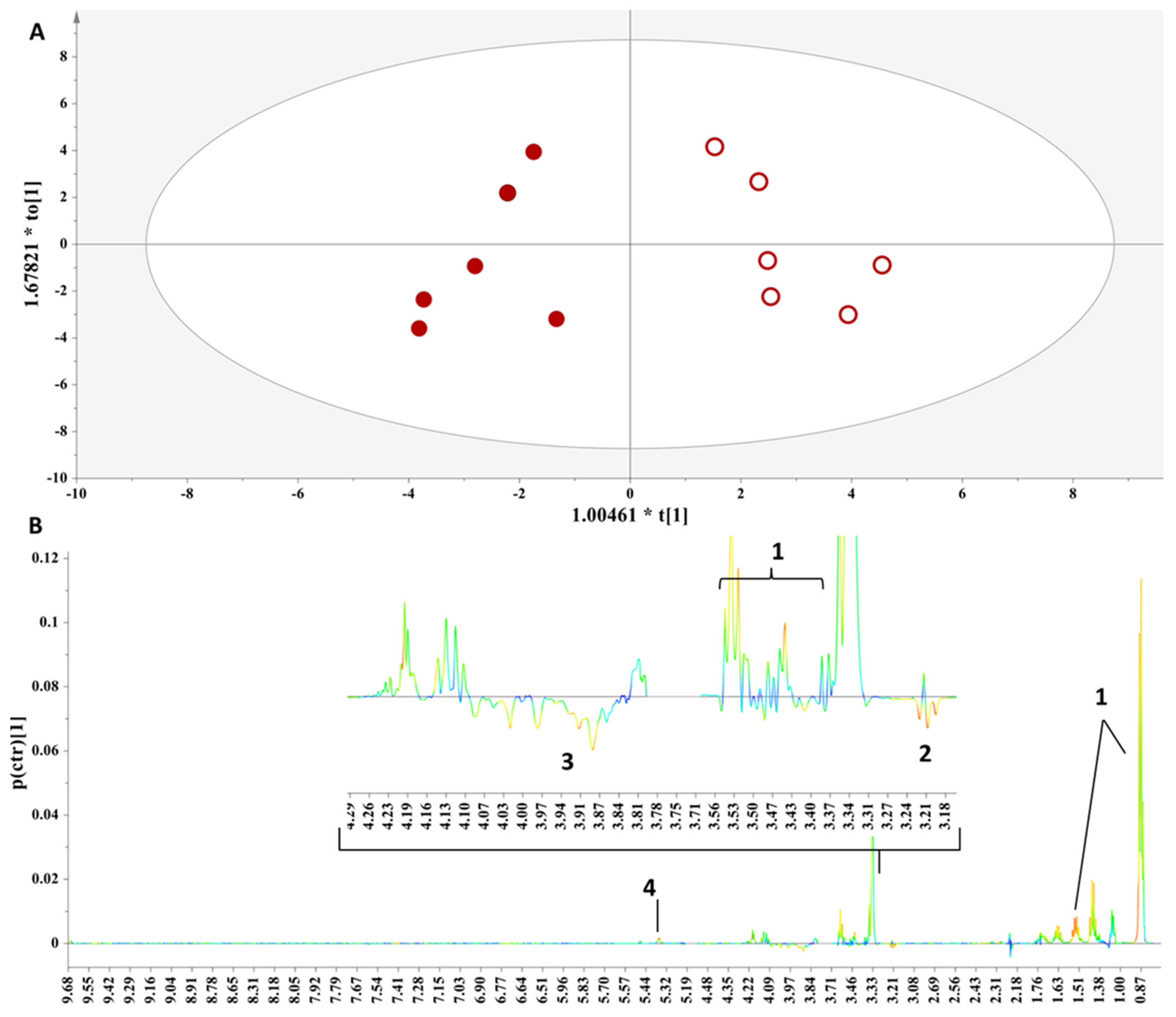
3.2.1. Comparing Samples of Different Geographic Origins
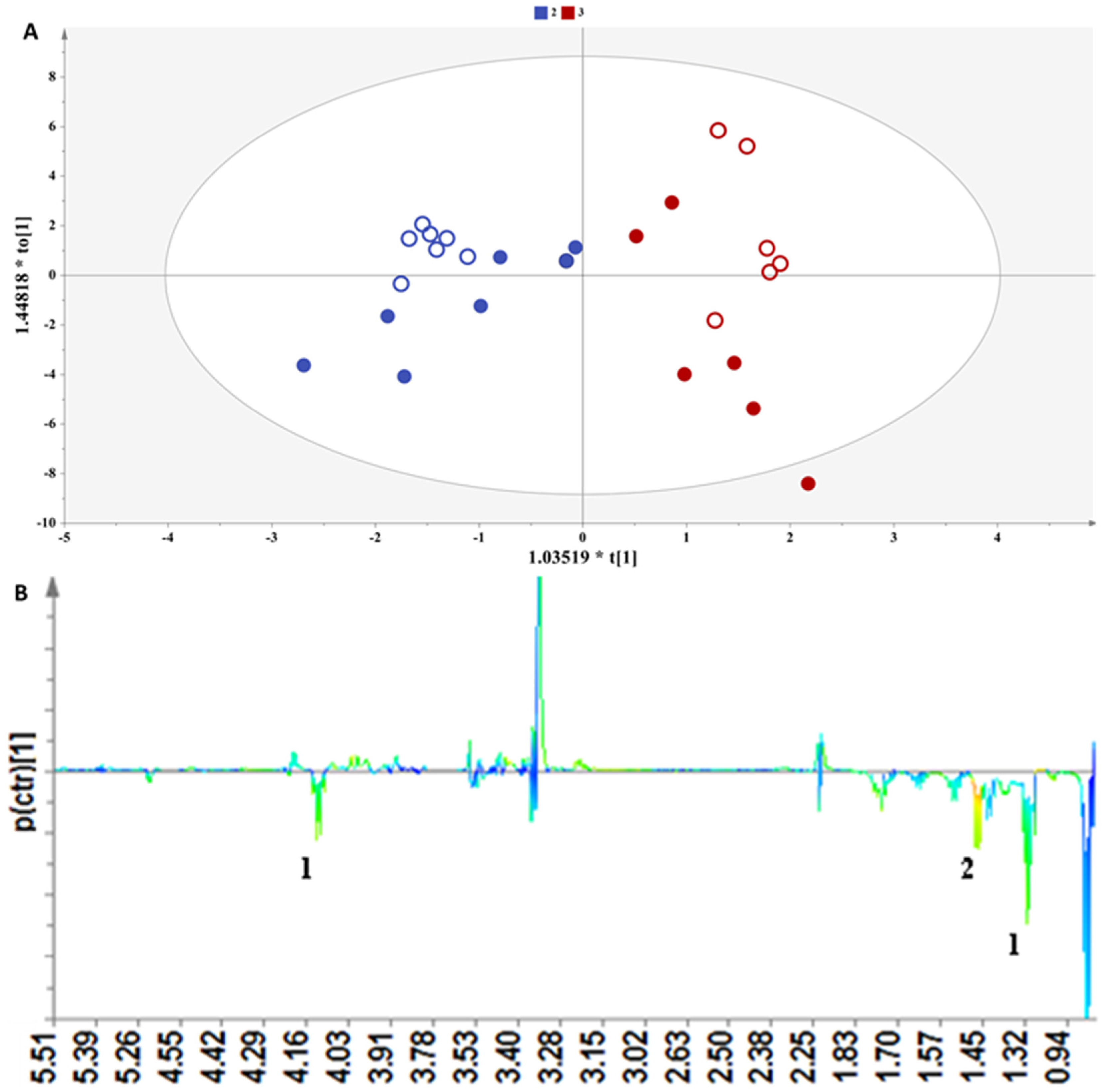
3.2.2. Comparing Samples from the Same Winery
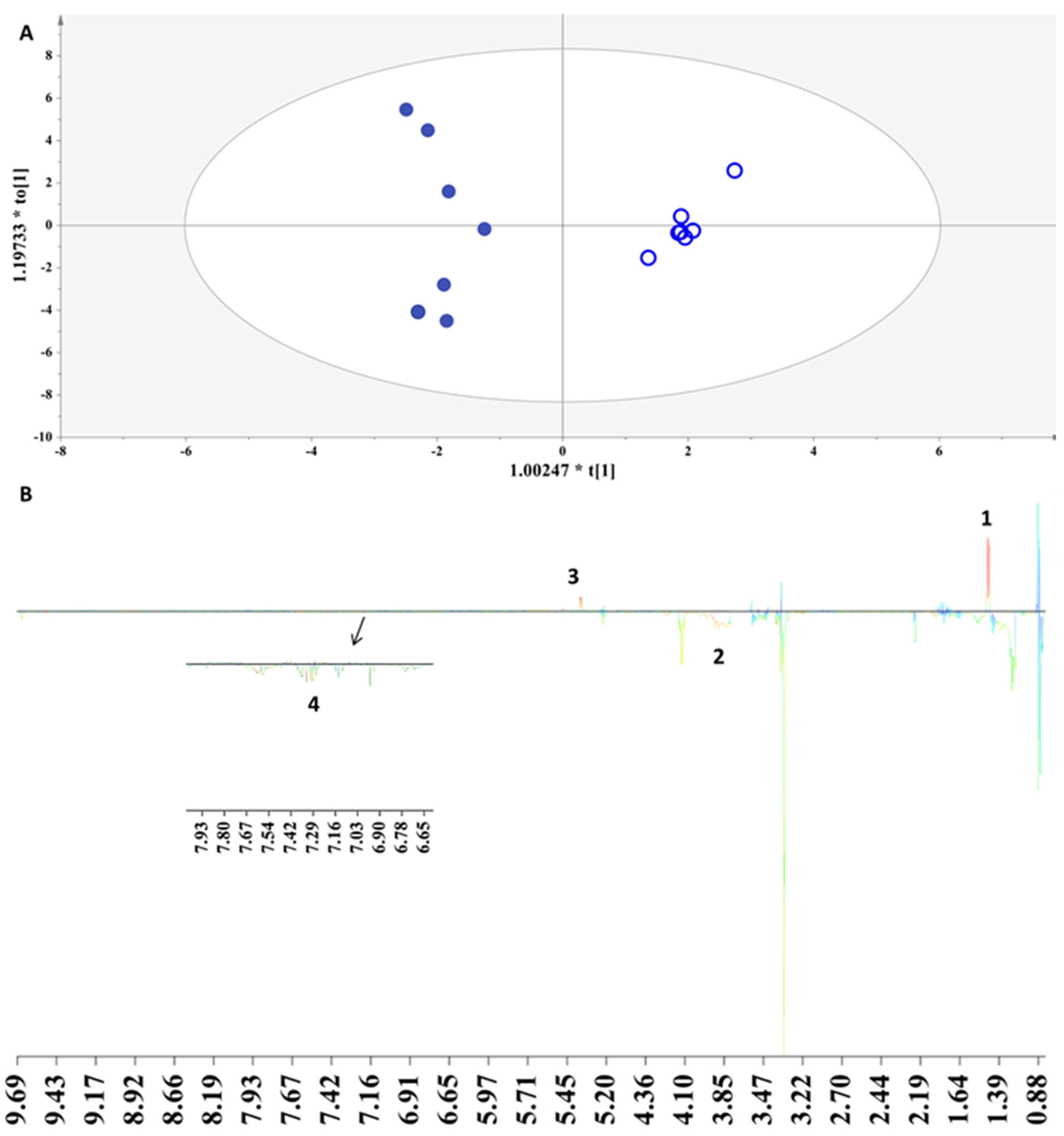
3.2.3. The Impact of Aging Procedure
3.3. Antioxidant Activity Profile
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miller, G.H. Maturation. In Whisky Science: A Condensed Distillation; Miller, G.H., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 259–322. ISBN 978-3-030-13732-8. [Google Scholar]
- Formation of Clusters in Whiskies during the Maturation Process—Morishima—2019—Journal of Food Science—Wiley Online Library. Available online: https://ift.onlinelibrary.wiley.com/doi/full/10.1111/1750-3841.14398 (accessed on 28 January 2024).
- Oliveira-Alves, S.; Lourenço, S.; Fernandes, T.A.; Canas, S. Coumarins in Spirit Beverages: Sources, Quantification, and Their Involvement in Quality, Authenticity and Food Safety. Appl. Sci. 2024, 14, 1010. [Google Scholar] [CrossRef]
- Rodríguez-Solana, R.; Rodríguez-Freigedo, S.; Salgado, J.M.; Domínguez, J.M.; Cortés-Diéguez, S. Optimisation of Accelerated Ageing of Grape Marc Distillate on a Micro-Scale Process Using a Box-Benhken Design: Influence of Oak Origin, Fragment Size and Toast Level on the Composition of the Final Product: Accelerated Ageing of Distillate. Aust. J. Grape Wine Res. 2017, 23, 5–14. [Google Scholar] [CrossRef]
- Belmonte-Sánchez, J.R.; Romero-González, R.; Martínez Vidal, J.L.; Arrebola, F.J.; Garrido Frenich, A. 1H NMR and Multi-Technique Data Fusion as Metabolomic Tool for the Classification of Golden Rums by Multivariate Statistical Analysis. Food Chem. 2020, 317, 126363. [Google Scholar] [CrossRef] [PubMed]
- Marinaki, M.; Sampsonidis, I.; Nakas, A.; Arapitsas, P.; Assimopoulou, A.N.; Theodoridis, G. Analysis of the Volatile Organic Compound Fingerprint of Greek Grape Marc Spirits of Various Origins and Traditional Production Styles. Beverages 2023, 9, 65. [Google Scholar] [CrossRef]
- Volatile Profile in Greek Grape Marc Spirits with HS-SPME-GC-MS and Chemometrics: Evaluation of Terroir Impact | ACS Omega. Available online: https://pubs.acs.org/doi/10.1021/acsomega.3c05686 (accessed on 28 January 2024).
- Fotakis, C.; Christodouleas, D.; Kokkotou, K.; Zervou, M.; Zoumpoulakis, P.; Moulos, P.; Liouni, M.; Calokerinos, A. NMR Metabolite Profiling of Greek Grape Marc Spirits. Food Chem. 2013, 138, 1837–1846. [Google Scholar] [CrossRef] [PubMed]
- Fotakis, C.; Zervou, M. NMR metabolic fingerprinting and chemometrics driven authentication of Greek grape marc spirits. Food Chem. 2013, 196, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Shu, N.; Wen, J.; Yang, Y.; Wang, Y.; Lu, W. Widely Targeted Metabolomics Was Used to Reveal the Differences between Non-Volatile Compounds in Different Wines and Their Associations with Sensory Properties. Foods 2023, 12, 290. [Google Scholar] [CrossRef] [PubMed]
- 1H-NMR Metabolomics for Wine Screening and Analysis | OENO One. Available online: https://oeno-one.eu/article/view/7134 (accessed on 28 January 2024).
- Tabago, M.K.A.G.; Calingacion, M.N.; Garcia, J. Recent Advances in NMR-Based Metabolomics of Alcoholic Beverages. Food Chem. Mol. Sci. 2021, 2, 100009. [Google Scholar] [CrossRef] [PubMed]
- Diamantidou, D.; Zotou, A.; Theodoridis, G. Wine and Grape Marc Spirits Metabolomics. Metabolomics 2018, 14, 159. [Google Scholar] [CrossRef]
- Le Mao, I.; Martin-Pernier, J.; Bautista, C.; Lacampagne, S.; Richard, T.; Da Costa, G. 1H-NMR Metabolomics as a Tool for Winemaking Monitoring. Molecules 2021, 26, 6771. [Google Scholar] [CrossRef]
- Fotakis, C.; Kokkotou, K.; Zoumpoulakis, P.; Zervou, M. NMR Metabolite Fingerprinting in Grape Derived Products: An Overview. Food Res. Int. 2013, 54, 1184–1194. [Google Scholar] [CrossRef]
- Trygg, J.; Holmes, E.; Lundstedt, T. Chemometrics in Metabonomics. J. Proteome Res. 2007, 6, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, L.; Johansson, E.; Kettaneh-Wold, N.; Trygg, J.; Wikstr, C.; Wold, S. Multi- and Megavariate Data Analysis. Part I Basic Principles and Applications. Second Revised and Enlarged Edition. Ume Swed. MKS Umetrics AB 2006, 1–103. [Google Scholar]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.-M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed Minimum Reporting Standards for Chemical Analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics Off. J. Metabolomic Soc. 2007, 3, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Andreou, V.; Strati, I.F.; Fotakis, C.; Liouni, M.; Zoumpoulakis, P.; Sinanoglou, V.J. Herbal distillates: A new era of grape marc distillates with enriched antioxidant profile. Food Chem. 2018, 253, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Fotakis, C.; Christodouleas, D.; Zervou, M.; Papadopoulos, K.; Calokerinos, A.C. Classification of Wines Based on Different Antioxidant Responses to Spectrophotometric Analytical Methods. Anal. Lett. 2012, 45, 581–591. [Google Scholar] [CrossRef]
- Tafulo, P.A.R.; Queirós, R.B.; Delerue-Matos, C.M.; Sales, M.G.F. Control and comparison of the antioxidant capacity of beers. Food Res. Int. 2010, 43, 1702–1709. [Google Scholar] [CrossRef]
- Alañón, M.E.; Díaz-Maroto, M.C.; Díaz-Maroto, I.J.; Vila-Lameiro, P.; Pérez-Coello, M.S. Cyclic Polyalcohols: Fingerprints to Identify the Botanical Origin of Natural Woods Used in Wine Aging. J. Agric. Food Chem. 2011, 59, 1269–1274. [Google Scholar] [CrossRef] [PubMed]
- Martínez Montero, C.; Rodríguez Dodero, M.d.C.; Guillén Sánchez, D.A.; García Barroso, C. Sugar Contents of Brandy de Jerez during Its Aging. J. Agric. Food Chem. 2005, 53, 1058–1064. [Google Scholar] [CrossRef]
- Goldberg, D.M.; Hoffman, B.; Yang, J.; Soleas, G.J. Phenolic Constituents, Furans, and Total Antioxidant Status of Distilled Spirits. J. Agric. Food Chem. 1999, 47, 3978–3985. [Google Scholar] [CrossRef]
- González, E.A.; Agrasar, A.T.; Castro, L.M.P.; Fernández, I.O.; Guerra, N.P. Solid-State Fermentation of Red Raspberry (Rubus ideaus L.) and Arbutus Berry (Arbutus unedo, L.) and Characterization of Their Distillates. Food Res. Int. 2011, 44, 1419–1426. [Google Scholar] [CrossRef]
- Moreno-Arribas, M.V.; Polo, M.C. (Eds.) Wine Chemistry and Biochemistry; Springer: New York, NY, USA, 2009; ISBN 978-0-387-74116-1. [Google Scholar]
- Pereira, G.E.; Gaudillere, J.-P.; Pieri, P.; Hilbert, G.; Maucourt, M.; Deborde, C.; Moing, A.; Rolin, D. Microclimate Influence on Mineral and Metabolic Profiles of Grape Berries. J. Agric. Food Chem. 2006, 54, 6765–6775. [Google Scholar] [CrossRef] [PubMed]
- Cortés, S.; Rodríguez, R.; Salgado, J.M.; Domínguez, J.M. Comparative Study between Italian and Spanish Grape Marc Spirits in Terms of Major Volatile Compounds. Food Control 2011, 22, 673–680. [Google Scholar] [CrossRef]
- Son, H.-S.; Hwang, G.-S.; Kim, K.M.; Ahn, H.-J.; Park, W.-M.; Van Den Berg, F.; Hong, Y.-S.; Lee, C.-H. Metabolomic Studies on Geographical Grapes and Their Wines Using 1H NMR Analysis Coupled with Multivariate Statistics. J. Agric. Food Chem. 2009, 57, 1481–1490. [Google Scholar] [CrossRef] [PubMed]
- Ali, K.; Maltese, F.; Toepfer, R.; Choi, Y.H.; Verpoorte, R. Metabolic Characterization of Palatinate German White Wines According to Sensory Attributes, Varieties, and Vintages Using NMR Spectroscopy and Multivariate Data Analyses. J. Biomol. NMR 2011, 49, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Mayr Marangon, C.; De Rosso, M.; Carraro, R.; Flamini, R. Changes in Volatile Compounds of Grape Pomace Distillate (Italian Grappa) during One-Year Ageing in Oak and Cherry Barrels. Food Chem. 2021, 344, 128658. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, R.; Salgado, J.; Domínguez, J.; Cortés-Diéguez, S. First Approach to the Analytical Characterization of Barrel-Aged Grape Marc Distillates Using Phenolic Compounds and Colour Parameters. Food Technol. Biotechnol. 2014, 52, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Gómez García-Carpintero, E.; Gómez Gallego, M.A.; Sánchez-Palomo, E.; González Viñas, M.A. Impact of Alternative Technique to Ageing Using Oak Chips in Alcoholic or in Malolactic Fermentation on Volatile and Sensory Composition of Red Wines. Food Chem. 2012, 134, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Olszowy-Tomczyk, M. How to express the antioxidant properties of substances properly? Chem. Pap. 2021, 75, 6157–6167. [Google Scholar] [CrossRef]
- Apak, R.; Çapanŏglu, E.; Shahidi, F. Measurement of Antioxidant Activity and Capacity—Recent Trends and Applications; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2018; pp. 1–283. [Google Scholar]
- Suzuki, K.; Nemoto, A.; Tanaka, I.; Koshimizu, S.; Suwa, Y.; Ishihara, H. Induction of Heme Oxygenase-1 by Whisky Congeners in Human Endothelial Cells. J. Food Sci. 2010, 75, H163–H166. [Google Scholar] [CrossRef]

| Assay | VR | VAG | ST |
|---|---|---|---|
| DPPH (mg GA/L) | 33.6 ± 0.5 * | 22.1 ± 0.1 * | 29.5 ± 1.1 * |
| ABTS•+ (mg GA/L) | 36.4 ± 0.6 * | 24.1 ± 0.1 * | 32.0 ± 1.1 * |
| CURPAC(mg GA/L) | 121.7 ± 1.2 *,a | 92.3 ± 4.6 *,b | 101.4 ± 10.8 a,b |
| FRAP (mg GA/L) | 96.1 ± 4.3 *,# | 66.4 ± 0.2 *,c | 68.4 ± 7.3 #,c |
| FC (mg GA/L) | 105.9 ± 3.5 * | 69.3 ± 0.6 * | 89.5 ± 2.4 * |
| O-D (mg CA/L) | 52.6 ± 2.8 *,d | 30.9 ± 0.5 *,$ | 49.4 ± 7.9 d,$ |
| N = 19 | Pearson Correlation | |||||
|---|---|---|---|---|---|---|
| DPPH | ABTS•+ | CUPRAC | FRAP | FC | OD | |
| DPPH | 1.0 | |||||
| ABTS•+ | 0.997 ** | 1.0 | ||||
| CUPRAC | 0.393 | 0.420 | 1.0 | |||
| FRAP | 0.488 * | 0.513 * | 0.869 ** | 1.0 | ||
| FC | 0.826 ** | 0.827 * | 0.711 ** | 0.815 ** | 1.0 | |
| OD | 0.484 * | 0.487 * | 0.608 ** | 0.529 * | 0.697 ** | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fotakis, C.; Andreou, V.; Christodouleas, D.C.; Zervou, M. The Metabolic and Antioxidant Activity Profiles of Aged Greek Grape Marc Spirits. Foods 2024, 13, 1664. https://doi.org/10.3390/foods13111664
Fotakis C, Andreou V, Christodouleas DC, Zervou M. The Metabolic and Antioxidant Activity Profiles of Aged Greek Grape Marc Spirits. Foods. 2024; 13(11):1664. https://doi.org/10.3390/foods13111664
Chicago/Turabian StyleFotakis, Charalambos, Vasiliki Andreou, Dionysios C. Christodouleas, and Maria Zervou. 2024. "The Metabolic and Antioxidant Activity Profiles of Aged Greek Grape Marc Spirits" Foods 13, no. 11: 1664. https://doi.org/10.3390/foods13111664
APA StyleFotakis, C., Andreou, V., Christodouleas, D. C., & Zervou, M. (2024). The Metabolic and Antioxidant Activity Profiles of Aged Greek Grape Marc Spirits. Foods, 13(11), 1664. https://doi.org/10.3390/foods13111664





