Analysis of Fatty Acid Composition and Volatile Profile of Powder from Edible Crickets (Acheta domesticus) Reared on Apple By-Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Cricket Powder
2.2. Fatty Acids
2.3. Volatile Compounds
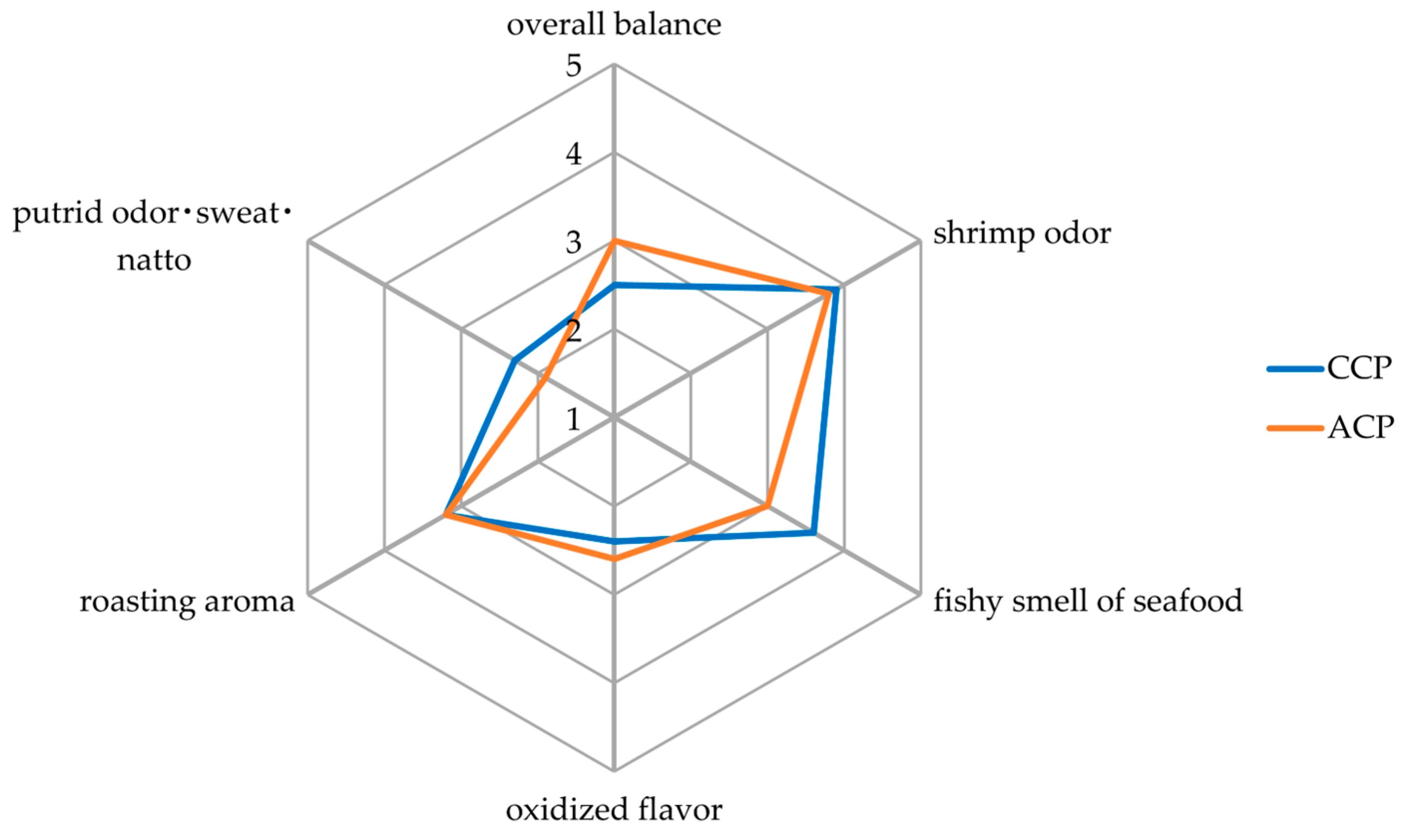
2.4. Sensory Analysis
2.5. Color Measurement
2.6. Sugar Content
2.7. Total Phenolic Content
2.8. Statistical Analysis
3. Results
3.1. Cricket Powder Fatty Acids Content
3.2. Cricket Powder Volatile Profiles
3.3. Sensory Analysis
3.4. Cricket Powder Color Characteristics
3.5. Cricket Powder Sugar Content
3.6. Cricket Powder Total Phenolic Content
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liceaga, A.M.; Toala, J.E.A.; Cordoba, B.V.; Cordova, A.F.C.; Mendoza, A.H. Insects as an Alternative Protein Source. Annu. Rev. Food Sci. Technol. 2022, 13, 19–34. [Google Scholar] [CrossRef]
- Hasnan, F.F.B.; Feng, Y.; Sun, T.; Parraga, K.; Schwarz, M.; Zarei, M. Insects as Valuable Sources of Protein and Peptides: Production, Functional Properties, and Challenges. Foods 2023, 12, 4243. [Google Scholar] [CrossRef] [PubMed]
- Azzollini, D.; Derossi, A.; Fogliano, V.; Lakemond, C.M.M.; Severini, C. Effects of formulation and process conditions on microstructure, texture and digestibility of extruded insect-riched snacks. Innov. Food Sci. Emerg. Technol. 2018, 45, 344–353. [Google Scholar] [CrossRef]
- Aidoo, O.F.; Osei-Owusu, J.; Asante, K.; Dofuor, A.K.; Boateng, B.O.; Debrah, S.K.; Ninsin, K.D.; Siddiqui, S.A.; Chia, S.Y. Insects as food and medicine: A sustainable solution for global health and environmental challenges. Front. Nutr. 2023, 10, 1113219. [Google Scholar] [CrossRef]
- Aiello, D.; Barbera, M.; Bongiorno, D.; Cammarata, M.; Censi, V.; Indelicato, S.; Mazzotti, F.; Napoli, A.; Piazzese, D.; Saiano, F. Edible Insects an Alternative Nutritional Source of Bioactive Compounds: A Review. Molecules 2023, 28, 699. [Google Scholar] [CrossRef] [PubMed]
- Van Huis, A. Edible insects are the future? Proc. Nutr. Soc. 2016, 75, 294–305. [Google Scholar] [CrossRef]
- Morales-Ramos, J.A.; Rojas, M.G.; Dossey, A.T.; Berhow, M. Self-selection of food ingredients and agricultural by-products by the house cricket, Acheta domesticus (Orthoptera: Gryllidae): A holistic approach to develop optimized diets. PLoS ONE 2020, 15, e0227400. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, D.; Kaewplik, T.; Fujisawa, T.; Kurosu, T.; Sasaki, Y. Crickets (Gryllus Bimaculatus) using food waste usefulness of self-selection feed design method through each growth stage. J. Insects Food Feed. 2023, 10, 247–258. [Google Scholar] [CrossRef]
- Kemsawasd, V.; Inthachat, W.; Suttisansanee, U.; Temviriyanukul, P. Road to the Red Carpet of Edible Crickets through Integration into the Human Food Chain with Biofunctions and Sustainability: A Review. Int. J. Mol. Sci. 2022, 23, 1801. [Google Scholar] [CrossRef]
- Ghosh, S.; Lee, S.M.; Jung, C.; Meyer-Rochow, V.B. Nutritional composition of five commercial edible insects in South Korea. J. Asia-Pac. Entomol. 2017, 20, 686–694. [Google Scholar] [CrossRef]
- Udomsil, N.; Imsoonthornruksa, S.; Gosalawit, C.; Ketudat-Cairns, M. Nutritional Values and Functional Properties of House Cricket (Acheta domesticus) and Field Cricket (Gryllus bimaculatus). Food Sci. Technol. Res. 2019, 25, 597–605. [Google Scholar] [CrossRef]
- Starčević, K.; Gavrilović, A.; Gottstein, Ž.; Mašek, T. Influence of substitution of sunflower oil by different oils on the growth, survival rate and fatty acid composition of Jamaican field cricket (Gryllus assimilis). Anim. Feed. Sci. Technol. 2017, 288, 66–71. [Google Scholar] [CrossRef]
- Oonincx, D.; Laurent, S.; Veenenbos, M.E.; van Loon, J.J.A. Dietary enrichment of edible insects with omega 3 fatty acids. Insect Sci. 2020, 27, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Dobermann, D.; Field, L.M.; Michaelson, L.V. Impact of heat processing on the nutritional content of Gryllus bimaculatus (black cricket). Nutr. Bull. 2019, 44, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Bawa, M.; Songsermpong, S.; Kaewtapee, C.; Chanput, W. Effect of Diet on the Growth Performance, Feed Conversion, and Nutrient Content of the House Cricket. J. Insect Sci. 2020, 20, 10. [Google Scholar] [CrossRef] [PubMed]
- Oonincx, D.G.; van Broekhoven, S.; van Huis, A.; van Loon, J.J. Feed Conversion, Survival and Development, and Composition of Four Insect Species on Diets Composed of Food By-Products. PLoS ONE 2015, 10, e0144601. [Google Scholar] [CrossRef] [PubMed]
- Perez-Santaescolastica, C.; De Winne, A.; Devaere, J.; Fraeye, I. The flavour of edible insects: A comprehensive review on volatile compounds and their analytical assessment. Trends Food Sci. Technol. 2022, 127, 352–367. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.Z.; He, J.; Liang, Y.Z.; Yuan, D.L.; Chau, F.T. Plasma fatty acid metabolic profiling and biomarkers of type 2 diabetes mellitus based on GC/MS and PLS-LDA. FEBS Lett. 2006, 580, 6837–6845. [Google Scholar] [CrossRef]
- Cialiè Rosso, M.; Stilo, F.; Mascrez, S.; Bicchi, C.; Purcaro, G.; Cordero, C. Shelf-Life Evolution of the Fatty Acid Fingerprint in High-Quality Hazelnuts (Corylus avellana L.) Harvested in Different Geographical Regions. Foods 2021, 10, 685. [Google Scholar] [CrossRef]
- Ogilvy, V.; Fidgett, A.L.; Preziosi, R.F. Differences in carotenoid accumulation among three feeder-cricket species: Implications for carotenoid delivery to captive insectivores. Zoo Biol. 2012, 31, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.J.P.T.; Pickova, J.; Ahmad, T.; Liaquat, M.; Farid, A.; Jahangir, M. Oxidation of lipids in foods. Sarhad J. Agric. 2016, 32, 230–238. [Google Scholar] [CrossRef]
- Grebenteuch, S.; Kanzler, C.; Klaußnitzer, S.; Kroh, L.W.; Rohn, S. The Formation of Methyl Ketones during Lipid Oxidation at Elevated Temperatures. Molecules 2021, 26, 1104. [Google Scholar] [CrossRef] [PubMed]
- Seong, H.Y.; Shin, E.C.; Lee, Y.; Kim, M. Characterization of Odor-Active Compounds from Gryllus bimaculatus Using Gas Chromatography-Mass Spectrometry-Olfactometry. Foods 2023, 12, 2328. [Google Scholar] [CrossRef] [PubMed]
- Reale, S.; Biancolillo, A.; Foschi, M.; D′Archivio, A.A. Characterization of the Volatile Profiles of Insect Flours by (HS)-SPME/GC-MS: A Preliminary Study. Molecules 2023, 28, 3075. [Google Scholar] [CrossRef]
- Bartkiene, E.; Zokaityte, E.; Starkute, V.; Zokaityte, G.; Kaminskaite, A.; Mockus, E.; Klupsaite, D.; Cernauskas, D.; Rocha, J.M.; Özogul, F.; et al. Crickets (Acheta domesticus) as Wheat Bread Ingredient: Influence on Bread Quality and Safety Characteristics. Foods 2023, 12, 325. [Google Scholar] [CrossRef] [PubMed]
- Chambers, E.T.; Koppel, K. Associations of volatile compounds with sensory aroma and flavor: The complex nature of flavor. Molecules 2013, 18, 4887–4905. [Google Scholar] [CrossRef] [PubMed]
- Skotnicka, M.; Karwowska, K.; Kłobukowski, F.; Borkowska, A.; Pieszko, M. Possibilities of the Development of Edible Insect-Based Foods in Europe. Foods 2021, 10, 766. [Google Scholar] [CrossRef]
- Kowalczewski, P.; Gumienna, M.; Rybicka, I.; Górna, B.; Sarbak, P.; Dziedzic, K.; Kmiecik, D. Nutritional Value and Biological Activity of Gluten-Free Bread Enriched with Cricket Powder. Molecules 2021, 26, 1184. [Google Scholar] [CrossRef]
- Mellican, R.I.; Li, J.; Mehansho, H.; Nielsen, S.S. The role of iron and the factors affecting off-color development of polyphenols. J. Agric. Food Chem. 2003, 51, 2304–2316. [Google Scholar] [CrossRef]
- Janssen, R.H.; Canelli, G.; Sanders, M.G.; Bakx, E.J.; Lakemond, C.M.M.; Fogliano, V.; Vincken, J.P. Iron-polyphenol complexes cause blackening upon grinding Hermetia illucens (black soldier fly) larvae. Sci. Rep. 2019, 9, 2967. [Google Scholar] [CrossRef] [PubMed]
- Pathare, P.; Opara, U.; Al-Said, F. Colour Measurement and Analysis in Fresh and Processed Foods: A Review. Food Bioprocess Technol. 2012, 6, 36–60. [Google Scholar] [CrossRef]
- De la Peña-Armada, R.; Mateos-Aparicio, I. Sustainable Approaches Using Green Technologies for Apple By-Product Valorisation as A New Perspective into the History of the Apple. Molecules 2022, 27, 6937. [Google Scholar] [CrossRef] [PubMed]
- Tsoupras, A.; Moran, D.; Byrne, T.; Ryan, J.; Barrett, L.; Traas, C.; Zabetakis, I. Anti-Inflammatory and Anti-Platelet Properties of Lipid Bioactives from Apple Cider By-Products. Molecules 2021, 26, 2869. [Google Scholar] [CrossRef]
- Fărcaș, A.C.; Socaci, S.A.; Chiș, M.S.; Dulf, F.V.; Podea, P.; Tofană, M. Analysis of Fatty Acids, Amino Acids and Volatile Profile of Apple By-Products by Gas Chromatography-Mass Spectrometry. Molecules 2022, 27, 1987. [Google Scholar] [CrossRef]

| Shorthand Nomenclature | Fatty Acid Name | Type | CCP | ACP |
|---|---|---|---|---|
| 12:0 | Lauric acid | SFA | 171.3 ± 14.1 | 226.9 ± 33.6 |
| 14:0 | Myristic acid | SFA | 290.1 ± 27.5 | 299.1 ± 41.5 |
| 14:1 (n-5) | Myristoleic acid | MUFA | 23.7 ± 3.6 | 40.4 ± 18.0 |
| 15:0 | Pentadecanoic acid | SFA | 39.1 ± 5.0 | 32.9 ± 2.7 |
| 15:1 (n-5) | Pentadecenoic acid | MUFA | 13.8 ± 1.5 | 13.0 ± 1.4 |
| 16:0 | Palmitic acid | SFA | 11,247.4 ± 1172.4 | 12,356.6 ± 811.7 |
| 16:1 (n-7) | Palmitoleic acid | MUFA | 499.5 ± 30.5 | 543.2 ± 23.3 |
| 17:0 | Margaric acid | SFA | 119.6 ± 11.0 | 118.8 ± 16.2 |
| 18:0 | Stearic acid | SFA | 4685.4 ± 324.3 | 4676.8 ± 238.6 |
| 18:1 (n-9) | Oleic acid | MUFA | 11,711.0 ± 1007.3 | 12,262.9 ± 1146.4 |
| 18:2 (n-6) | Linoleic acid | PUFA | 18,886.9 ± 1432.0 | 20,999.5 ± 1091.7 |
| 18:3 (n-6) | γ-Linolenic acid | PUFA | 443.9 ± 88.4 | 204.3 ± 28.9 * |
| 18:3 (n-3) | α-Linolenic acid | PUFA | 53.4 ± 7.1 | 34.5 ± 2.0 * |
| 20:1 (n-9) | Eicosenoic acid | MUFA | 80.9 ± 8.6 | 95.6 ± 11.5 |
| 20:2 (n-6) | Eicosadienoic acid | PUFA | 106.5 ± 14.2 | 141.7 ± 17.8 |
| 20:3 (n-6) | Eicosatrienoic acid | PUFA | 144.5 ± 8.5 | 142.5 ± 12.3 |
| 20:3 (n-3) | Eicosatrienoic acid | PUFA | 10.4 ± 1.9 | 9.1 ± 1.8 |
| 20:4 (n-6) | Arachidonic acid | PUFA | 595.5 ± 60.8 | 448.2 ± 19.4 * |
| 20:5 (n-3) | Eicosapentaenoic acid | PUFA | 12.4 ± 1.6 | 11.2 ± 0.6 |
| 21:0 | Heneicosanoic acid | SFA | 37.4 ± 11.2 | 33.0 ± 2.7 |
| 22:0 | Behenic acid | SFA | 17.4 ± 2.1 | 16.5 ± 3.4 |
| 22:1 (n-9) | Erucic acid | MUFA | 8.8 ± 1.5 | 4.9 ± 8.4 |
| 22:6 (n-3) | Docosahexaenoic acid | PUFA | 78.1 ± 7.2 | 15.6 ± 5.1 * |
| 24:1 (n-9) | Nervonic acid | MUFA | 37.6 ± 4.0 | 33.5 ± 2.1 |
| Total | 49,314.6 ± 4150.2 | 52,760.6 ± 3404.7 | ||
| Σ SFAs | 16,607.6 ± 1527.5 | 17,760.5 ± 1121.6 | ||
| Σ MUFAs | 12,375.3 ± 1041.5 | 12,993.5 ± 1158.1 | ||
| Σ PUFAs | 20,331.7 ± 1593.7 | 22,006.6 ± 1151.7 | ||
| Σ n-3 PUFAs | 154.3 ± 15.0 | 70.4 ± 9.0 * | ||
| Σ n-6 PUFAs | 20,177.4 ± 1579.4 | 21,936.2 ± 1142.7 | ||
| Σ n-6/n-3 | 130.9 ± 4.2 | 313.9 ± 25.2 * | ||
| Volatile Compounds | CCP | ACP |
|---|---|---|
| Aldehydes | ||
| Propanal, 2-methyl- a | 3.83 ± 0.45 | 5.15 ± 0.71 |
| Benzeneacetaldehyde a | n.d. | 0.01 ± 0.00 |
| 2-Butenal, 2-methyl- | 0.10 ± 0.02 | n.d. |
| Acetaldehyde | n.d. | 0.83 ± 0.12 |
| Butanal, 2-methyl- a | 6.82 ± 1.22 | 8.33 ± 1.40 |
| Butanal, 3-methyl- a | 10.22 ± 1.46 | 11.34 ± 1.12 |
| Heptanal | 0.33 ± 0.07 | 0.45 ± 0.04 |
| Hexanal b | 11.24 ± 2.79 | 14.97 ± 3.44 |
| Nonanal b | 0.42 ± 0.04 | 0.43 ± 0.04 |
| Pentanal b | 2.22 ± 0.43 | 2.78 ± 0.12 |
| Total | 35.17 ± 4.50 | 44.30 ± 1.04 |
| Ketones | ||
| 2-Butanone | n.d. | 3.27 ± 0.33 |
| 2-Decanone | 0.28 ± 0.05 | 0.18 ± 0.02 * |
| 2-Heptanone | 2.47 ± 0.90 | 2.06 ± 0.17 |
| 2-Hexanone | 0.15 ± 0.02 | n.d. |
| Acetone | 3.29 ± 0.85 | 3.89 ± 0.64 |
| Cyclohexanone | 0.14 ± 0.04 | n.d. |
| Ethanone, 1-(2,3-dihydro-1H-inden-5-yl)- | n.d. | 0.02 ± 0.01 |
| Total | 6.32 ± 0.81 | 9.42 ± 1.08 * |
| Hydrocarbons | ||
| 3-Ethyl-3-methylheptane | 0.03 ± 0.01 | n.d. |
| 4,4-Dimethyl octane | 0.26 ± 0.14 | n.d. |
| 5-Ethyldecane | 0.19 ± 0.01 | 0.16 ± 0.03 |
| Decane | 11.56 ± 1.89 | 10.34 ± 0.90 |
| Decane, 5-methyl-6-methylene- | 0.18 ± 0.01 | 0.12 ± 0.00 * |
| Dodecane | 5.28 ± 0.24 | n.d. |
| Dodecane, 2,7,10-trimethyl- | 3.74 ± 0.78 | 3.14 ± 0.56 |
| Dodecane, 4,6-dimethyl- | 0.31 ± 0.14 | 0.17 ± 0.02 |
| Dodecane, 4-methyl- | 0.06 ± 0.03 | n.d. |
| Heptane, 2,2,4,6,6-pentamethyl- | 0.73 ± 0.21 | 0.47 ± 0.06 |
| Heptane, 2,4-dimethyl- | 0.31 ± 0.15 | 0.32 ± 0.02 |
| Nonane, 2-methyl- | 0.19 ± 0.10 | 0.20 ± 0.04 |
| Octane, 3,5-dimethyl- | 0.17 ± 0.11 | 0.17 ± 0.04 |
| Tetradecane | 0.06 ± 0.10 | 0.00 ± 0.00 |
| Tridecane, 6-methyl- | 0.11 ± 0.01 | n.d. |
| Undecane, 5,7-dimethyl- | 0.22 ± 0.09 | 0.17 ± 0.03 |
| Undecane, 5-methyl- | 0.27 ± 0.01 | 0.20 ± 0.02 * |
| 2,4-Dimethyl-1-heptene | n.d. | 0.06 ± 0.01 |
| Octane, 4-ethyl- | n.d. | 0.20 ± 0.03 |
| Octane, 4-methyl- | n.d. | 0.09 ± 0.01 |
| Total | 23.67 ± 2.67 | 15.81 ± 1.71 * |
| Benzenoids | ||
| Benzene, 1,2,4-trimethyl- | 0.08 ± 0.02 | 0.07 ± 0.01 |
| Benzene, 1,3-bis(1-methylethenyl)- | 0.02 ± 0.01 | 0.02 ± 0.00 |
| Benzene, 1,3-dimethyl- | 0.23 ± 0.10 | 0.13 ± 0.02 |
| Benzene, 1,4-diethyl- | 0.05 ± 0.01 | 0.04 ± 0.01 |
| Benzene, 1-ethynyl-4-methyl- | 0.04 ± 0.01 | 0.04 ± 0.00 |
| Ethylbenzene | 0.37 ± 0.22 | 0.19 ± 0.05 |
| Total | 0.80 ± 0.36 | 0.48 ± 0.09 |
| Pyrazines | ||
| Pyrazine a | 0.12 ± 0.02 | 0.12 ± 0.02 |
| Pyrazine, 2,5-dimethyl- a | 0.68 ± 0.22 | 0.60 ± 0.08 |
| Pyrazine, 3-ethyl-2,5-dimethyl- a | 0.26 ± 0.03 | 0.29 ± 0.02 |
| Pyrazine, methyl- a | 0.71 ± 0.27 | 0.56 ± 0.06 |
| 2-(3-Methylbutyl)-3,5-dimethylpyrazine a | n.d. | 0.03 ± 0.01 |
| Total | 1.77 ± 0.53 | 1.59 ± 0.18 |
| Sulfur compounds | ||
| Dimethyl sulfide | n.d. | 0.30 ± 0.16 |
| Dimethyl sulfone | 0.18 ± 0.04 | n.d. |
| Disulfide, dimethyl | 0.59 ± 0.19 | 0.29 ± 0.11 |
| Total | 0.77 ± 0.21 | 0.60 ± 0.15 |
| Acids | ||
| Acetic acid | 22.14 ± 6.97 | 22.87 ± 1.80 |
| Butanoic acid, 3-methyl- | 0.10 ± 0.01 | 0.10 ± 0.03 |
| Total | 22.24 ± 6.97 | 22.97 ± 1.82 |
| Amines | ||
| (2-Aziridinylethyl)amine | 3.23 ± 1.13 | n.d. |
| Methylamine, N,N-dimethyl- | 1.95 ± 0.14 | 1.65 ± 0.42 |
| Total | 5.18 ± 1.10 | 1.65 ± 0.42 * |
| Terpenes | ||
| D-Limonene | 0.16 ± 0.03 | 0.12 ± 0.02 |
| 3-Carene | n.d. | 0.09 ± 0.01 |
| Total | 0.16 ± 0.03 | 0.21 ± 0.02 |
| Ethers | ||
| 3,5-Dimethyldihydropyran-2,6-dione | n.d. | 0.19 ± 0.02 |
| Furan, 2-pentyl- a | 3.33 ± 0.54 | 1.91 ± 0.23 * |
| Total | 3.33 ± 0.54 | 2.10 ± 0.24 * |
| Esters | ||
| Pentanoic acid, 5-hydroxy-, 2,4-di-t-butylphenyl esters | 0.03 ± 0.01 | n.d. |
| Total | 0.03 ± 0.01 | n.d. |
| Alcohols | ||
| 1-Pentanol | 0.56 ± 0.06 | 0.88 ± 0.30 |
| Total | 0.56 ± 0.06 | 0.88 ± 0.30 |
| Sample Powders | Color Parameters | |||
|---|---|---|---|---|
| L* | a* | b* | ΔE* | |
| CCP | 42.23 ± 0.87 | 5.57 ± 0.55 | 20.2 ± 0.87 | - |
| ACP | 34.40 ± 0.79 * | 6.90 ± 0.22 * | 19.21 ± 0.15 | 8.01 |
| Sugar | CCP | ACP |
|---|---|---|
| Glucose a | 0.42 ± 0.02 | 0.37 ± 0.06 |
| Fructose a | n.d. | 0.39 ± 0.11 |
| Sucrose | n.d. | 0.25 ± 0.04 |
| Maltose a | n.d. | n.d. |
| Trehalose | 0.18 ± 0.04 | 0.20 ± 0.05 |
| Total | 0.70 ± 0.05 | 1.22 ± 0.20 * |
| Samples | CCP | ACP |
|---|---|---|
| Total phenolic contents | 0.42 ± 0.02 | 0.40 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umebara, I.; Akutsu, K.; Kubo, M.; Iijima, A.; Sakurai, R.; Masutomi, H.; Ishihara, K. Analysis of Fatty Acid Composition and Volatile Profile of Powder from Edible Crickets (Acheta domesticus) Reared on Apple By-Products. Foods 2024, 13, 1668. https://doi.org/10.3390/foods13111668
Umebara I, Akutsu K, Kubo M, Iijima A, Sakurai R, Masutomi H, Ishihara K. Analysis of Fatty Acid Composition and Volatile Profile of Powder from Edible Crickets (Acheta domesticus) Reared on Apple By-Products. Foods. 2024; 13(11):1668. https://doi.org/10.3390/foods13111668
Chicago/Turabian StyleUmebara, Io, Keiko Akutsu, Misako Kubo, Akihiro Iijima, Ren Sakurai, Hirofumi Masutomi, and Katsuyuki Ishihara. 2024. "Analysis of Fatty Acid Composition and Volatile Profile of Powder from Edible Crickets (Acheta domesticus) Reared on Apple By-Products" Foods 13, no. 11: 1668. https://doi.org/10.3390/foods13111668





