Polyphenols from Foxtail Millet Improve Non-Alcoholic Fatty Liver Disease by Regulating Intestinal Microbiome in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Sample Collection
2.4. Histopathology and Immunohistochemistry Examination
2.5. Serum Biomarkers
2.6. RNA Isolation, cDNA Synthesis, and RT-qPCR
2.7. 16S rRNA Gene Sequencing Analysis of Microbiota in the Fecal Contents
2.8. Statistical Analysis
3. Results
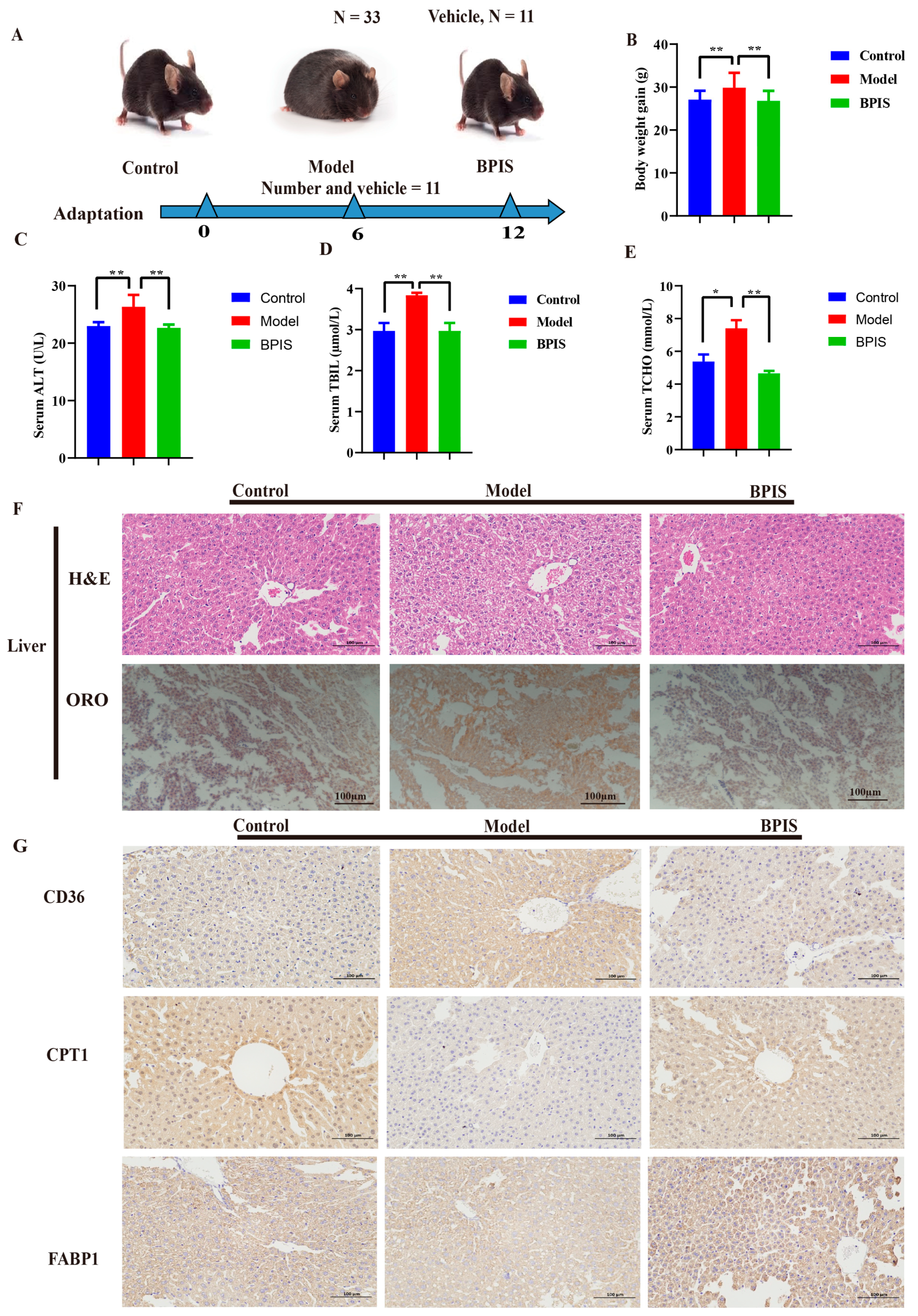
3.1. BPIS Attenuates NAFLD and Reduces Lipid Accumulation
3.2. Protection of Intestinal Epithelium by BPIS
3.3. BPIS Regulates the Gut Microbiome in C57BL/6N Mice
3.4. BPIS Modulates the Complete Structure of the Gut Microbiome
3.5. BPIS Regulates the Abundance of Certain Bacteria in Mice
4. Discussion
5. Possible Limitations of the Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harrison, S.A.; Gawrieh, S.; Roberts, K.; Lisanti, C.J.; Schwope, R.B.; Cebe, K.M.; Paradis, V.; Bedossa, P.; Whitehead, J.M.A.; Labourdette, A. Prospective evaluation of the prevalence of non-alcoholic fatty liver disease and steatohepatitis in a large middle-aged US cohort. J. Hepatol. 2021, 75, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Golabi, P.; de Avila, L.; Paik, J.M.; Srishord, M.; Fukui, N.; Qiu, Y.; Burns, L.; Afendy, A.; Nader, F. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J. Hepatol. 2019, 71, 793–801. [Google Scholar] [CrossRef]
- Riazi, K.; Azhari, H.; Charette, J.H.; Underwood, F.E.; King, J.A.; Afshar, E.E.; Swain, M.G.; Congly, S.E.; Kaplan, G.G.; Shaheen, A.-A. The prevalence and incidence of NAFLD worldwide: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2022, 7, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Anstee, Q.M.; Reeves, H.L.; Kotsiliti, E.; Govaere, O.; Heikenwalder, M. From NASH to HCC: Current concepts and future challenges. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 411–428. [Google Scholar] [CrossRef]
- Kokkorakis, M.; Boutari, C.; Hill, M.A.; Kotsis, V.; Loomba, R.; Sanyal, A.J.; Mantzoros, C.S. Resmetirom, the first approved drug for the management of metabolic dysfunction-associated steatohepatitis: Trials, opportunities, and challenges. Metab.-Clin. Exp. 2024, 154, 155835. [Google Scholar] [CrossRef] [PubMed]
- Pouwels, S.; Sakran, N.; Graham, Y.; Leal, A.; Pintar, T.; Yang, W.; Kassir, R.; Singhal, R.; Mahawar, K.; Ramnarain, D. Non-alcoholic fatty liver disease (NAFLD): A review of pathophysiology, clinical management and effects of weight loss. BMC Endocr. Disord. 2022, 22, 63. [Google Scholar] [CrossRef] [PubMed]
- Bäckhed, F.; Manchester, J.K.; Semenkovich, C.F.; Gordon, J.I. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc. Natl. Acad. Sci. USA 2007, 104, 979–984. [Google Scholar] [CrossRef]
- Le Roy, T.; Llopis, M.; Lepage, P.; Bruneau, A.; Rabot, S.; Bevilacqua, C.; Martin, P.; Philippe, C.; Walker, F.; Bado, A.; et al. Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice. Gut 2013, 62, 1787–1794. [Google Scholar] [CrossRef]
- Cianciosi, D.; Forbes-Hernández, T.Y.; Regolo, L.; Alvarez-Suarez, J.M.; Navarro-Hortal, M.D.; Xiao, J.; Quiles, J.L.; Battino, M.; Giampieri, F. The reciprocal interaction between polyphenols and other dietary compounds: Impact on bioavailability, antioxidant capacity and other physico-chemical and nutritional parameters. Food Chem. 2022, 375, 131904. [Google Scholar] [CrossRef]
- Nie, J.-Y.; Zhao, Q. Beverage consumption and risk of ulcerative colitis: Systematic review and meta-analysis of epidemiological studies. Medicine 2017, 96, e9070. [Google Scholar] [CrossRef]
- Jiang, Z.; Han, Z.; Zhu, M.; Wan, X.; Zhang, L. Effects of thermal processing on transformation of polyphenols and flavor quality. Curr. Opin. Food Sci. 2023, 51, 101014. [Google Scholar] [CrossRef]
- Golovinskaia, O.; Wang, C.-K. The hypoglycemic potential of phenolics from functional foods and their mechanisms. Food Sci. Hum. Wellness 2023, 12, 986–1007. [Google Scholar] [CrossRef]
- Echeverría, F.; Bustamante, A.; Sambra, V.; Videla, L.; Valenzuela, R. Beneficial effects of dietary polyphenols in the prevention and treatment of NAFLD: Cell-signaling pathways underlying health effects. Curr. Med. Chem. 2022, 29, 299–328. [Google Scholar] [CrossRef]
- Li, W.; Yang, H.; Zhao, Q.; Wang, X.; Zhang, J.; Zhao, X. Polyphenol-rich loquat fruit extract prevents fructose-induced nonalcoholic fatty liver disease by modulating glycometabolism, lipometabolism, oxidative stress, inflammation, intestinal barrier, and gut microbiota in mice. J. Agric. Food Chem. 2019, 67, 7726–7737. [Google Scholar] [CrossRef] [PubMed]
- Patel, S. Cereal bran: The next super food with significant antioxidant and anticancer potential. Mediterr. J. Nutr. Metab. 2012, 5, 91–104. [Google Scholar] [CrossRef]
- Suma, P.F.; Urooj, A. Antioxidant activity of extracts from foxtail millet (Setaria italica). J. Food Sci. Technol. 2012, 49, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Shan, S.; Lu, Y.; Zhang, X.; Shi, J.; Li, H.; Li, Z. Inhibitory effect of bound polyphenol from foxtail millet bran on miR-149 methylation increases the chemosensitivity of human colorectal cancer HCT-8/Fu cells. Mol. Cell. Biochem. 2021, 476, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Shan, S.; An, N.; Liu, F.; Cui, K.; Shi, J.; Li, H.; Li, Z. Polyphenols from foxtail millet bran ameliorate DSS-induced colitis by remodeling gut microbiome. Front. Nutr. 2022, 9, 1030744. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The human microbiome project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef]
- Spragge, F.; Bakkeren, E.; Jahn, M.T.; BN Araujo, E.; Pearson, C.F.; Wang, X.; Pankhurst, L.; Cunrath, O.; Foster, K.R. Microbiome diversity protects against pathogens by nutrient blocking. Science 2023, 382, eadj3502. [Google Scholar] [CrossRef]
- Lynch, S.V.; Pedersen, O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef] [PubMed]
- Cohen, L.J.; Esterhazy, D.; Kim, S.-H.; Lemetre, C.; Aguilar, R.R.; Gordon, E.A.; Pickard, A.J.; Cross, J.R.; Emiliano, A.B.; Han, S.M. Commensal bacteria make GPCR ligands that mimic human signalling molecules. Nature 2017, 549, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Kitabatake, M.; Kayano, S.-i.; Ito, T. Dietary Phenolic Compounds: Their Health Benefits and Association with the Gut Microbiota. Antioxidants 2023, 12, 880. [Google Scholar] [CrossRef] [PubMed]
- Fisette, A.; Sergi, D.; Breton-Morin, A.; Descôteaux, S.; Martinoli, M.-G. New Insights on the Role of Bioactive Food Derivatives in Neurodegeneration and Neuroprotection. Curr. Pharm. Des. 2022, 28, 3068–3081. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Xin, Z.; He, W.; Zhang, Y.; Xiong, J.; Wang, J.; Liao, Z.; Wang, L.; Zhong, Q.; Wei, H. Correlation between the regulation of intestinal bacteriophages by green tea polyphenols and the flora diversity in SPF mice. Food Funct. 2022, 13, 2952–2965. [Google Scholar] [CrossRef] [PubMed]
- Jansen, P.L. Consequences of non-alcoholic fatty liver disease still unclear. Ned. Tijdschr. Voor Geneeskd. 2012, 156, A4568. [Google Scholar]
- Kim, M.S.; Hwang, S.S.; Park, E.J.; Bae, J.W. Strict vegetarian diet improves the risk factors associated with metabolic diseases by modulating gut microbiota and reducing intestinal inflammation. Environ. Microbiol. Rep. 2013, 5, 765–775. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, Y.; Wang, Z.; Zhang, X.; Zhang, Y.; Lin, J.; Du, Y.; Wang, S.; Si, D.; Bao, J. Integration of Metabolomics, Lipidomics, and Proteomics Reveals the Metabolic Characterization of Nonalcoholic Steatohepatitis. J. Proteome Res. 2023, 22, 2577–2592. [Google Scholar] [CrossRef] [PubMed]
- Boccellino, M.; D’Angelo, S. Anti-obesity effects of polyphenol intake: Current status and future possibilities. Int. J. Mol. Sci. 2020, 21, 5642. [Google Scholar] [CrossRef]
- Minemura, M.; Shimizu, Y. Gut microbiota and liver diseases. World J. Gastroenterol. WJG 2015, 21, 1691. [Google Scholar] [CrossRef]
- Vasques-Monteiro, I.M.L.; Silva-Veiga, F.M.; Miranda, C.S.; de Andrade Gonçalves, É.C.B.; Daleprane, J.B.; Souza-Mello, V. A rise in Proteobacteria is an indicator of gut-liver axis-mediated nonalcoholic fatty liver disease in high-fructose-fed adult mice. Nutr. Res. 2021, 91, 26–35. [Google Scholar] [CrossRef]
- Porras, D.; Nistal, E.; Martínez-Flórez, S.; Pisonero-Vaquero, S.; Olcoz, J.L.; Jover, R.; González-Gallego, J.; García-Mediavilla, M.V.; Sánchez-Campos, S. Protective effect of quercetin on high-fat diet-induced non-alcoholic fatty liver disease in mice is mediated by modulating intestinal microbiota imbalance and related gut-liver axis activation. Free Radic. Biol. Med. 2017, 102, 188–202. [Google Scholar] [CrossRef]
- Sun, W.-L.; Li, X.-Y.; Dou, H.-Y.; Wang, X.-D.; Li, J.-D.; Shen, L.; Ji, H.-F. Myricetin supplementation decreases hepatic lipid synthesis and inflammation by modulating gut microbiota. Cell Rep. 2021, 36, 109641. [Google Scholar] [CrossRef] [PubMed]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The firmicutes/bacteroidetes ratio: A relevant marker of gut dysbiosis in obese patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Ding, Q.; Zhuge, H.; Lai, S.; Chang, K.; Le, C.; Yang, G.; Valencak, T.G.; Li, S.; Ren, D. Lactobacillus plantarum ZJUIDS14 alleviates non-alcoholic fatty liver disease in mice in association with modulation in the gut microbiota. Front. Nutr. 2023, 9, 1071284. [Google Scholar] [CrossRef]
- Hayashi, H.; Sawada, K.; Tanaka, H.; Muro, K.; Hasebe, T.; Nakajima, S.; Okumura, T.; Fujiya, M. The effect of heat-killed Lactobacillus brevis SBL88 on improving selective hepatic insulin resistance in non-alcoholic fatty liver disease mice without altering the gut microbiota. J. Gastroenterol. Hepatol. 2023, 38, 1847–1854. [Google Scholar] [CrossRef]
- Zhang, C.; Fang, T.; Shi, L.; Wang, Y.; Deng, X.; Wang, J.; Zhou, Y. The Synbiotic Combination of probiotics and inulin Improves NAFLD though Modulating Gut Microbiota. J. Nutr. Biochem. 2023, 125, 109546. [Google Scholar] [CrossRef]
- Yin, X.; Guo, X.; Liu, Z.; Wang, J. Advances in the diagnosis and treatment of non-alcoholic fatty liver disease. Int. J. Mol. Sci. 2023, 24, 2844. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Wang, Q.; Chang, R.; Zhou, X.; Xu, C. Intestinal barrier function–non-alcoholic fatty liver disease interactions and possible role of gut microbiota. J. Agric. Food Chem. 2019, 67, 2754–2762. [Google Scholar] [CrossRef]
- Landy, J.; Ronde, E.; English, N.; Clark, S.K.; Hart, A.L.; Knight, S.C.; Ciclitira, P.J.; Al-Hassi, H.O. Tight junctions in inflammatory bowel diseases and inflammatory bowel disease associated colorectal cancer. World J. Gastroenterol. 2016, 22, 3117. [Google Scholar] [CrossRef]
- Rau, M.; Rehman, A.; Dittrich, M.; Groen, A.K.; Hermanns, H.M.; Seyfried, F.; Beyersdorf, N.; Dandekar, T.; Rosenstiel, P.; Geier, A. Fecal SCFAs and SCFA-producing bacteria in gut microbiome of human NAFLD as a putative link to systemic T-cell activation and advanced disease. United Eur. Gastroenterol. J. 2018, 6, 1496–1507. [Google Scholar] [CrossRef] [PubMed]
- Saltzman, E.T.; Palacios, T.; Thomsen, M.; Vitetta, L. Intestinal microbiome shifts, dysbiosis, inflammation, and non-alcoholic fatty liver disease. Front. Microbiol. 2018, 9, 61. [Google Scholar] [CrossRef] [PubMed]




| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Cldn1 | TGGCTATGGAGGCGGCTATGG | CCTGAGCGGTCACGATGTTGTC |
| Ocld | TGGCTATGGAGGCGGCTATGG | AAGGAAGCGATGAAGCAGAAGG |
| Zo1 | CCACCTCGCACGCATCACAG | TGGTCCTTCACCTCTGAGCACTAC |
| GAPDH | ACCCACTCCTCCACCTTTGA | AAGGAAGCGATGAAGCAGAAGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghani, I.; An, Y.; Qiao, Q.; He, S.; Li, Z. Polyphenols from Foxtail Millet Improve Non-Alcoholic Fatty Liver Disease by Regulating Intestinal Microbiome in Mice. Foods 2024, 13, 1683. https://doi.org/10.3390/foods13111683
Ghani I, An Y, Qiao Q, He S, Li Z. Polyphenols from Foxtail Millet Improve Non-Alcoholic Fatty Liver Disease by Regulating Intestinal Microbiome in Mice. Foods. 2024; 13(11):1683. https://doi.org/10.3390/foods13111683
Chicago/Turabian StyleGhani, Israr, Yuxuan An, Qinqin Qiao, Shuiling He, and Zhuoyu Li. 2024. "Polyphenols from Foxtail Millet Improve Non-Alcoholic Fatty Liver Disease by Regulating Intestinal Microbiome in Mice" Foods 13, no. 11: 1683. https://doi.org/10.3390/foods13111683
APA StyleGhani, I., An, Y., Qiao, Q., He, S., & Li, Z. (2024). Polyphenols from Foxtail Millet Improve Non-Alcoholic Fatty Liver Disease by Regulating Intestinal Microbiome in Mice. Foods, 13(11), 1683. https://doi.org/10.3390/foods13111683





