Effect of Roasting on the Chemical Composition and Oxidative Stability of Tomato (Solanum lycopersicum L.) Seed Oil
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Raw Materials
2.1.2. Chemicals
2.2. Roasting and Oil Extraction
2.3. Determination of the Physicochemical Properties of TSO
2.4. Determination of the Fatty Acid Composition (FAC) of TSO
2.5. Determination of the Tocopherol Content of TSO
2.6. Determination of the Phytosterols and Squalene Contents of TSO
2.7. Determination of the Hydroxy Methyl Furfural (HMF) Content of TSO
2.8. Determination of the Non-Enzymatic Browning Index (BI) of TSO
2.9. Determination of the Total Polyphenol Content of TSO
2.10. Determination of the Antioxidant Capacity of TSO
2.11. Determination of the Oxidative Stability of TSO
2.12. Statistical Analysis
3. Results and Discussion
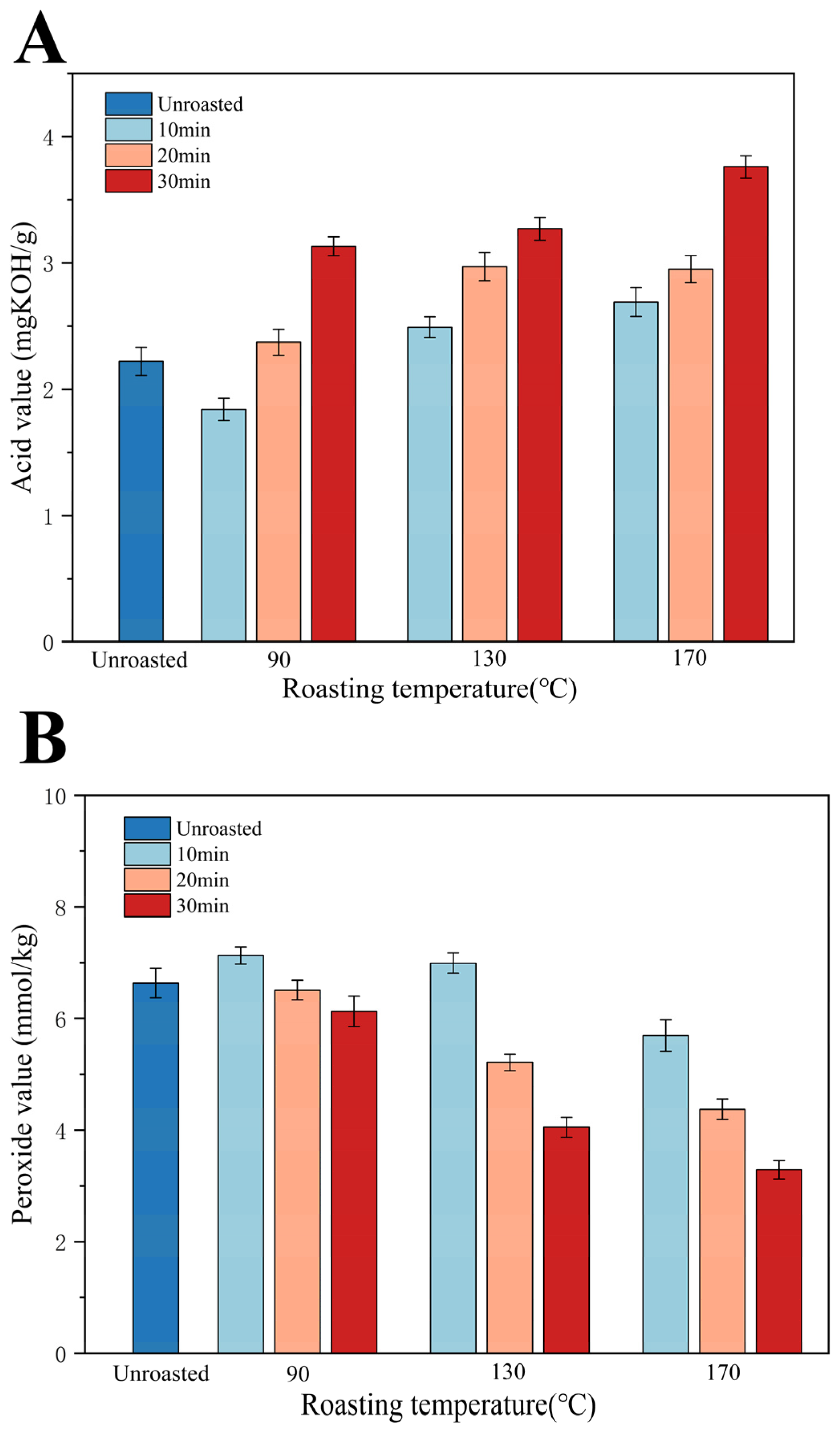
3.1. Acid Value (AV)
3.2. Peroxide Value (POV)
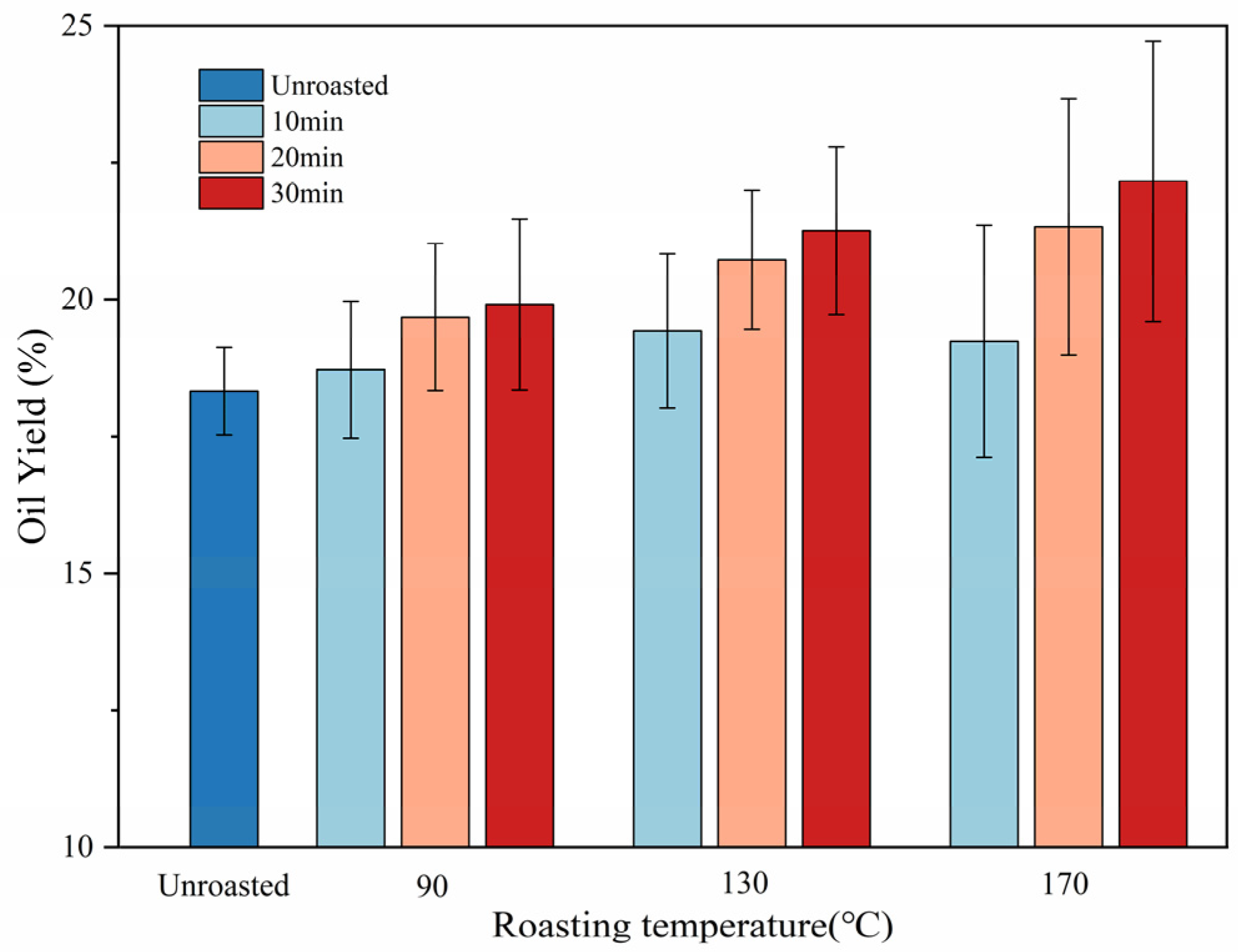
3.3. Oil Yield
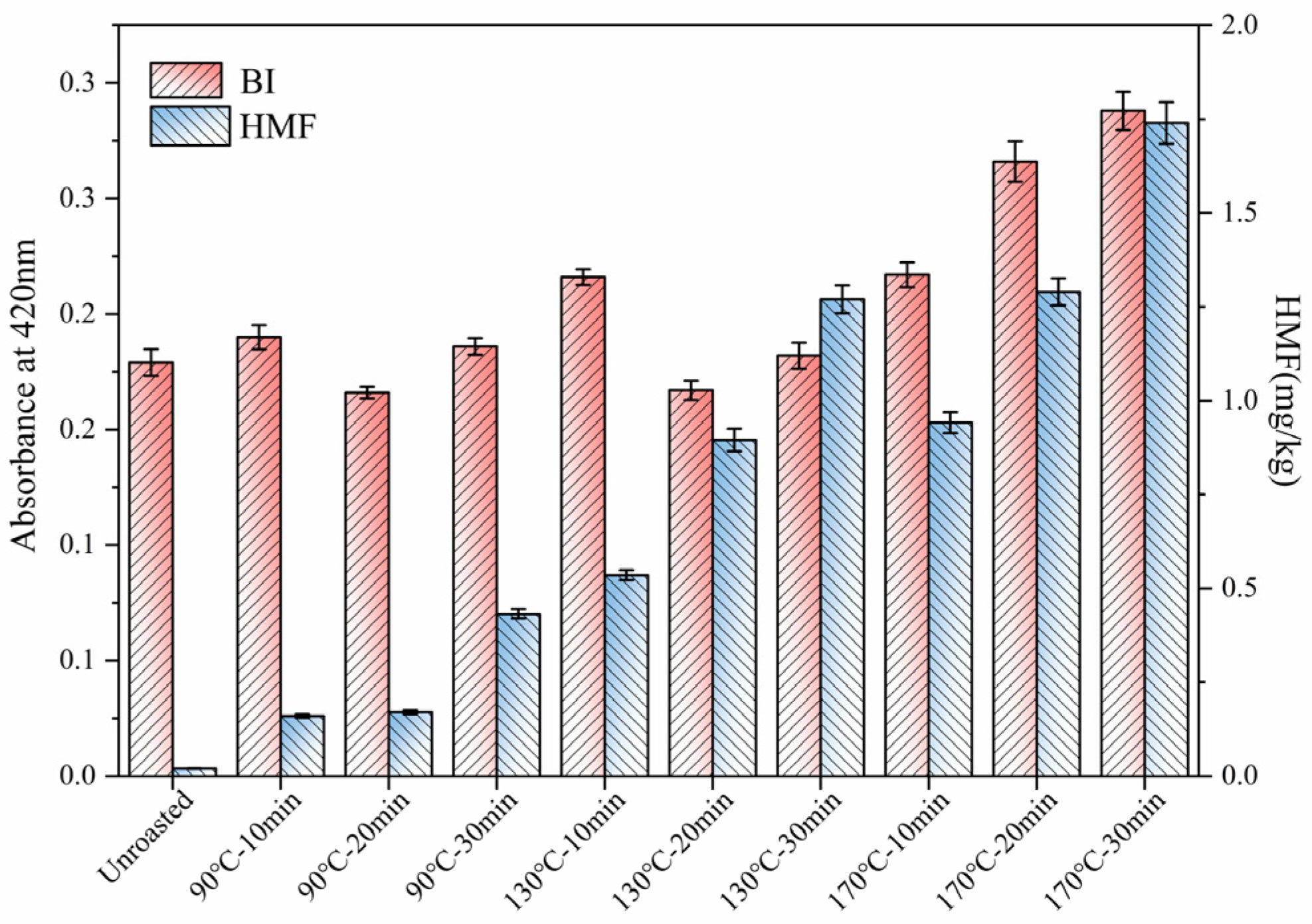
3.4. Maillard Reaction Products (MRPs)
3.5. Fatty Acid Composition (FAC)
3.6. Tocopherol Contents
3.7. Phytosterol Content
3.8. Squalene Content
3.9. Total Polyphenol Content (TPC)
3.10. Oxidative Stability Index (OSI)
3.11. Antioxidant Capacity
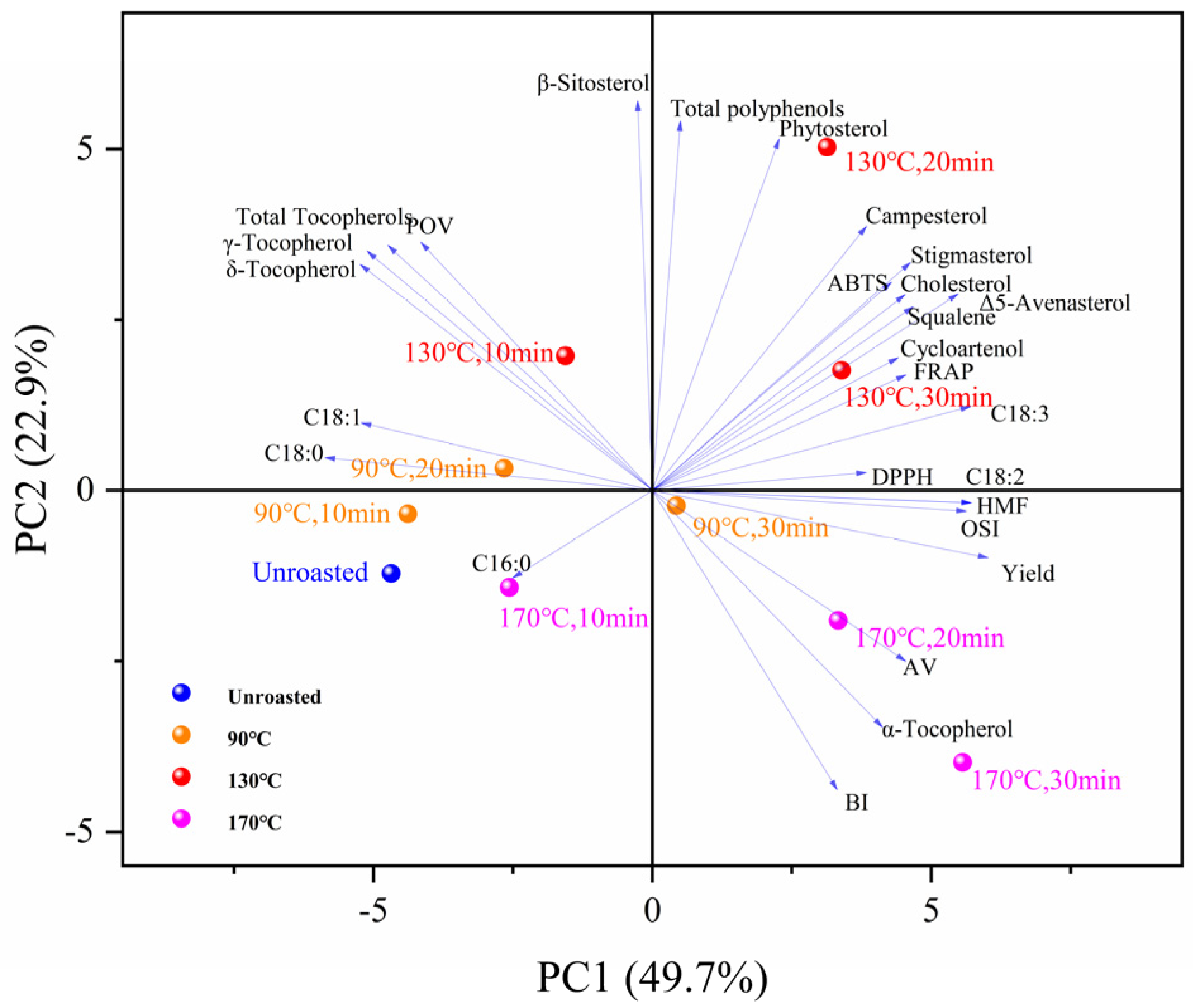
3.12. Principal Component Analysis (PCA)
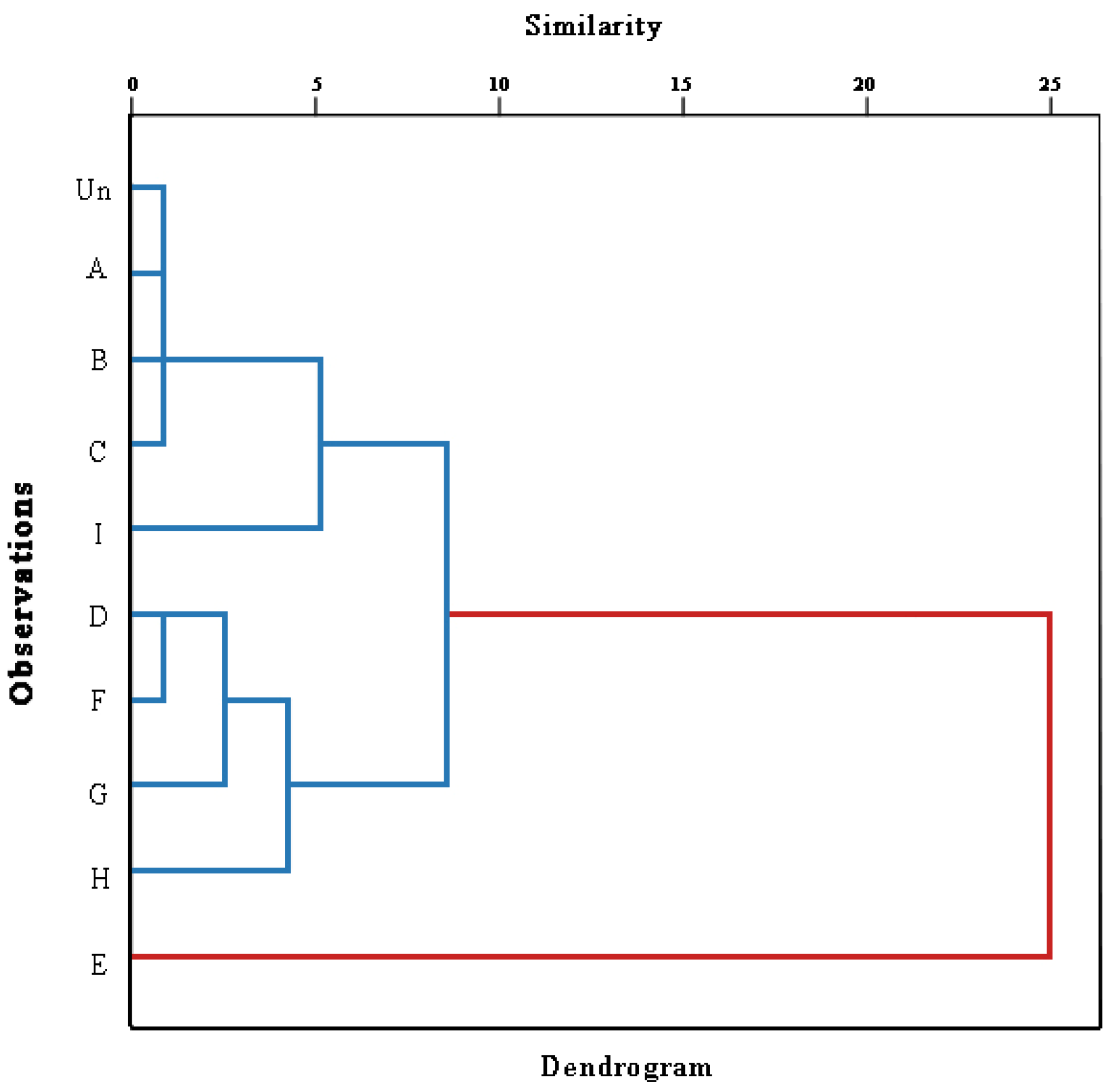
3.13. Hierarchical Cluster Analysis (HCA)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yuan, J.; Zhang, H.; Zeng, C.; Song, J.; Mu, Y.; Kang, S. Impact of Fermentation Conditions on Physicochemical Properties, Antioxidant Activity, and Sensory Properties of Apple–Tomato Pulp. Molecules 2023, 28, 4363. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, S.; Zhu, X.; Chang, Y.; Wang, C.; Ma, N.; Wang, J.; Zhang, X.; Lyu, J.; Xie, J. A comprehensive evaluation of tomato fruit quality and identification of volatile compounds. Plants 2023, 12, 2947. [Google Scholar] [CrossRef] [PubMed]
- Kaur, D.; Wani, A.A.; Oberoi, D.P.S.; Sogi, D.S.J. Effect of extraction conditions on lycopene extractions from tomato processing waste skin using response surface methodology. Food Chem. 2008, 108, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Wang, J.; Gao, R.; Ye, F.; Zhao, G.J. Sustainable valorisation of tomato pomace: A comprehensive review. Trends Food Sci. Technol. 2019, 86, 172–187. [Google Scholar] [CrossRef]
- Giuffre, A.M.; Capocasale, M.J. Policosanol in tomato (Solanum lycopersicum L.) seed oil: The effect of cultivar. J. Oleo Sci. 2015, 64, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Martins, Z.E.; Pinho, O.; Ferreira, I.M. Food industry by-products used as functional ingredients of bakery products. Trends Food Sci. Technol. 2017, 67, 106–128. [Google Scholar] [CrossRef]
- Gebeyew, K. The effect of feeding dried tomato pomace and concentrate on nutritional and growth parameters of hararghe highland sheep, eastern Ethiopia. Adv. Dairy Res. 2015, 3, 1000130. [Google Scholar] [CrossRef]
- Shao, D.Y.; Venkitasamy, C.; Li, X. Thermal and storage characteristics of tomato seed oil. LWT 2015, 63, 191–197. [Google Scholar] [CrossRef]
- Yilmaz, E.; Aydeniz, B.; Guneser, O.; Arsunar, E.S. Sensory and physico-chemical properties of cold press-produced tomato (Lycopersicon esculentum L.) seed oils. J. Am. Oil Chem. Soc. 2015, 92, 833–842. [Google Scholar] [CrossRef]
- Shao, D.; Bartley, G.E.; Yokoyama, W.; Pan, Z.; Zhang, H.; Zhang, A. Plasma and hepatic cholesterol-lowering effects of tomato pomace, tomato seed oil and defatted tomato seed in hamsters fed with high-fat diets. Food Chem. 2013, 139, 589–596. [Google Scholar] [CrossRef]
- Uquiche, E.; Jerez, M.; Ortiz, J. Effect of pretreatment with microwaves on mechanical extraction yield and quality of vegetable oil from Chilean hazelnuts (Gevuina avellana Mol). Innov. Food Sci. Emerg. Technol. 2008, 9, 495–500. [Google Scholar] [CrossRef]
- Sangeetha, K.; Ramyaa, R. Extraction, characterization, and application of tomato seed oil in the food industry: An updated review. J. Agric. Food Res. 2023, 11, 100529. [Google Scholar] [CrossRef]
- Giuffrè, A.M.; Capocasale, M.; Zappia, C. Tomato seed oil for edible use: Cold break, hot break, and harvest year effects. J. Food Process. Preserv. 2017, 41, e13309. [Google Scholar] [CrossRef]
- Oyinlola, A.; Ojo, A.; Adekoya, L.O. Development of a laboratory model screw press for peanut oil expression. J. Food Eng. 2004, 64, 221–227. [Google Scholar] [CrossRef]
- Alancay, M.M.; Lobo, M.O.; Samman, N.C. Physicochemical and structural characterization of whey protein concentrate–tomato pectin conjugates. J. Sci. Food Agric. 2023, 103, 5242–5252. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Oh, S.W.; Chang, J.; Kim, I. Chemical composition and oxidative stability of safflower oil prepared from safflower seed roasted with different temperatures. Food Chem. 2004, 84, 1–6. [Google Scholar]
- Cai, L.; Cao, A.; Aisikaer, G.; Ying, T.J. Influence of kernel roasting on bioactive components and oxidative stability of pine nut oil. Eur. J. Lipid Sci. Technol. 2013, 115, 556–563. [Google Scholar] [CrossRef]
- Stadler, R.H.; Blank, I.; Varga, N.; Robert, F.; Hau, J.; Guy, P.A.; Robert, M.; Riediker, S. Acrylamide from Maillard reaction products. Nature 2002, 419, 449–450. [Google Scholar] [CrossRef] [PubMed]
- Durmaz, G.; Gokmen, V. Determination of 5-hydroxymethyl-2-furfural and 2-furfural in oils as indicators of heat pre-treatment. Food Chem. 2010, 123, 912–916. [Google Scholar] [CrossRef]
- AOCS. Official Methods and Recommended Practices of the AOCS; AOCS Press: Washington, DC, USA, 2009. [Google Scholar]
- Sun, X.; Wang, Y.; Li, H.; Zhou, J.; Han, J.; Wei, C. Changes in the volatile profile, fatty acid composition and oxidative stability of flaxseed oil during heating at different temperatures. LWT-Food Sci. Technol. 2021, 151, 112137. [Google Scholar] [CrossRef]
- Dun, Q.; Yao, L.; Deng, Z.; Li, H.; Li, J.; Fan, Y.; Zhang, B. Effects of hot and cold-pressed processes on volatile compounds of peanut oil and corresponding analysis of characteristic flavor components. LWT-Food Sci. Technol. 2019, 112, 107648. [Google Scholar] [CrossRef]
- Murkovic, M.; Piironen, V.; Lampi, A.M.; Kraushofer, T.; Sontag, G. Changes in chemical composition of pumpkin seeds during the roasting process for production of pumpkin seed oil. (Part 1: Non-volatile compounds). Food Chem. 2004, 84, 359–365. [Google Scholar]
- Yilmaz, C.; Gokmen, V. Compositional characteristics of sour cherry kernel and its oil as influenced by different extraction and roasting conditions. Ind. Crops Prod. 2013, 49, 130–135. [Google Scholar] [CrossRef]
- Aksoz, E.; Korkut, O.; Aksit, D.; Gokbulut, C. Vitamin E (α-, β + γ- and δ-tocopherol) levels in plant oils. Flavour Fragr. J. 2020, 35, 504–510. [Google Scholar] [CrossRef]
- Pirsa, S.; Banafshechin, E.; Amiri, S.; Rahimirad, A.; Ghafarzadeh, J. Detection of fraud of palm, sunflower, and corn oil in butter using HPLC profile of tocopherols and tocotrienols by response surface method. J. Iran. Chem. Soc. 2020, 18, 1167–1177. [Google Scholar] [CrossRef]
- Liu, R.; Xu, Y.; Chang, M.; Tang, L.; Lu, M.; Liu, R.; Jin, Q.; Wang, X. Antioxidant interaction of alpha-tocopherol, gamma-oryzanol and phytosterol in rice bran oil. Food Chem. 2021, 343, 128431. [Google Scholar] [CrossRef] [PubMed]
- Wroniak, M.; Ptaszek, A.; Ratusz, K. Assessing the effect of pressing conditions in expeller press on quality and chemical composition of rapeseed oil. Zywnosc Nauka Technol. Jakosc/Food Sci. Technol. Qual. 2013, 20, 92–104. [Google Scholar] [CrossRef]
- Zou, Y.; Gao, Y.; He, H.; Yang, T. Effect of roasting on physico-chemical properties, antioxidant capacity, and oxidative stability of wheat germ oil. LWT-Food Sci. Technol. 2018, 90, 246–253. [Google Scholar] [CrossRef]
- Rękas, A.; Ścibisz, I.; Siger, A.; Wroniak, M. The effect of microwave pretreatment of seeds on the stability and degradation kinetics of phenolic compounds in rapeseed oil during long-term storage. Food Chem. 2017, 222, 43–52. [Google Scholar] [CrossRef]
- Gao, P.; Jin, J.; Liu, R.; Jin, Q.; Wang, X.J. Chemical compositions of walnut (Juglans regia L.) oils from different cultivated regions in China. J. Am. Oil Chem. Soc. 2018, 95, 825–834. [Google Scholar] [CrossRef]
- Ballus, C.A.; Meinhart, A.D.; Campos, F.A.D.S.; Godoy, H.T.J. Total phenolics of virgin olive oils highly correlate with the hydrogen atom transfer mechanism of antioxidant capacity. J. Am. Oil Chem. Soc. 2015, 92, 843–851. [Google Scholar] [CrossRef]
- Suri, K.; Singh, B.; Kaur, A.; Yadav, M.P.; Singh, N. Impact of infrared and dry air roasting on the oxidative stability, fatty acid composition, Maillard reaction products and other chemical properties of black cumin (Nigella sativa L.) seed oil. Food Chem. 2019, 295, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Liu, Y.; Shi, L.; Wang, N.; Wang, X.J. Effect of roasting treatment on the chemical composition of sesame oil. LWT-Food Sci. Technol. 2019, 101, 191–200. [Google Scholar] [CrossRef]
- Chirinos, R.; Zorrilla, D.; Aguilargalvez, A.; Pedreschi, R.; Campos, D.J. Impact of roasting on fatty acids, tocopherols, phytosterols, and phenolic compounds present in Plukenetia huayllabambana seed. J. Chem. 2016, 2016, 6570935. [Google Scholar] [CrossRef]
- Ergönül, P.G.; Köseoğlu, O. Changes in α-, β-, γ- and δ-tocopherol contents of mostly consumed vegetable oils during refining process. CyTA-J. Food 2013, 12, 199–202. [Google Scholar] [CrossRef]
- Schmidt, S.; Pokorny, J. Potential application of oilseeds as sources of antioxidants for food lipids—A review. Czech J. Food Sci. 2011, 23, 93–102. [Google Scholar] [CrossRef]
- Farzaneh, V.; Gominho, J.; Pereira, H.; Carvalho, I.S.J. Screening of the antioxidant and enzyme inhibition potentials of portuguese Pimpinella anisum L. Seeds by GC-MS. Food Anal. Methods 2018, 11, 2645–2656. [Google Scholar] [CrossRef]
- Vujasinovic, V.; Djilas, S.; Dimic, E.; Basic, Z.; Radocaj, O. The effect of roasting on the chemical composition and oxidative stability of pumpkin oil. Eur. J. Lipid Sci. Technol. 2012, 114, 568–574. [Google Scholar] [CrossRef]
- Siger, A.; Kaczmarek, A.; Rudzinska, M.J. Antioxidant activity and phytochemical content of cold-pressed rapeseed oil obtained from roasted seeds. Eur. J. Lipid Sci. Technol. 2015, 117, 1225–1237. [Google Scholar] [CrossRef]
- Oracz, J.; Nebesny, E.; Żyżelewicz, D.J. Effect of roasting conditions on the fat, tocopherol, and phytosterol content and antioxidant capacity of the lipid fraction from cocoa beans of different Theobroma cacao L. cultivars. Eur. J. Lipid Sci. Technol. 2014, 116, 1002–1014. [Google Scholar] [CrossRef]
- Tikekar, R.V.; Ludescher, R.D.; Karwe, M.V. Processing stability of squalene in amaranth and antioxidant potential of amaranth extract. J. Agric. Food Chem. 2008, 56, 10675–10678. [Google Scholar] [CrossRef] [PubMed]
- Özbek, A.Z.; Çelik, K.; Ergönül, P.; Hepçimen, A.Z. A promising food waste for food fortification: Characterization of dried tomato pomace and its cold pressed oil. J. Food Chem Nanotec. 2020, 6, 9–17. [Google Scholar] [CrossRef]
- Özbek, Z.A.; Yıldız, K.; Ergönül, P.G.; İyilikeden, E.; Uzlaşır, T. Comparison of the effects of oven- and microwave-roasting on the physicochemical properties and bioactive compounds of tomato seeds and oils. Eur. J. Lipid Sci. Technol. 2023, 125, 1438–1456. [Google Scholar] [CrossRef]
| Compound | Relative Content (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Unroasted | 90 °C | 130 °C | 170 °C | |||||||
| 10 min | 20 min | 30 min | 10 min | 20 min | 30 min | 10 min | 20 min | 30 min | ||
| C16:0 | 13.26 ± 0.13 ef | 13.16 ± 0.13 f | 13.59 ± 0.16 bc | 13.45 ± 0.15 cd | 13.32 ± 0.17 e | 13.45 ± 0.16 cde | 13.53 ± 0.14 cd | 13.97 ± 0.11 ab | 13.93 ± 0.33 a | 13.79 ± 0.12 ab |
| C18:0 | 6.11 ± 0.08 c | 6.43 ± 0.16 bc | 7.08 ± 0.16 a | 7.23 ± 0.12 a | 6.22 ± 0.16 bc | 6.53 ± 0.16 b | 7.23 ± 0.12 a | 6.61 ± 0.01 bc | 7.08 ± 0.28 a | 7.17 ± 0.15 a |
| ΣSFA | 19.36 ± 0.20 c | 19.59 ± 0.21 bc | 20.67 ± 0.23 a | 20.67 ± 0.14 a | 19.55 ± 0.01 bc | 19.97 ± 0.14 b | 20.75 ± 0.3 a | 20.58 ± 0.1 b | 21.02 ± 0.16 a | 20.96 ± 0.3 a |
| C18:1 | 22.61 ± 0.13 b | 22.92 ± 0.12 ab | 23.07 ± 0.11 a | 23.07 ± 0.01 a | 22.70 ± 0.12 ab | 22.73 ± 0.14 ab | 23.02 ± 0.11 a | 22.58 ± 0.16 b | 22.80 ± 0.37 ab | 23.07 ± 0.11 a |
| ΣMUFA | 22.61 ± 0.13 b | 22.92 ± 0.12 ab | 23.07 ± 0.11 a | 23.07 ± 0.01 a | 22.70 ± 0.12 ab | 22.73 ± 0.14 ab | 23.02 ± 0.11 a | 22.58 ± 0.16 b | 22.80 ± 0.37 ab | 23.07 ± 0.11 a |
| C18:2 | 55.59 ± 0.32 a | 55.11 ± 0.24 bc | 54.04 ± 0.33 e | 54.12 ± 0.31 e | 55.31 ± 0.23 ab | 54.89 ± 0.19 cd | 54.00 ± 0.23 e | 54.59 ± 0.31 d | 54.01 ± 0.29 e | 53.80 ± 0.22 e |
| C18:3 | 2.44 ± 0.00 a | 2.38 ± 0.02 ab | 2.22 ± 0.01 bc | 2.14 ± 0.01 c | 2.44 ± 0.01 a | 2.41 ± 0.03 ab | 2.23 ± 0.01 bc | 2.26 ± 0.03 abc | 2.17 ± 0.16 c | 2.17 ± 0.02 c |
| ΣPUFA | 58.03 ± 0.36 a | 57.49 ± 0.27 b | 56.26 ± 0.34 d | 56.26 ± 0.33 d | 57.75 ± 0.31 ab | 57.30 ± 0.22 bc | 56.23 ± 0.25 d | 56.85 ± 0.3 c | 56.18 ± 0.3 d | 55.98 ± 0.26 d |
| MUFA/PUFA | 0.39 ± 0.04 c | 0.40 ± 0.04 b | 0.41 ± 0.07 a | 0.41 ± 0.08 a | 0.39 ± 0.06 c | 0.40 ± 0.07 b | 0.41 ± 0.03 a | 0.40 ± 0.06 b | 0.41 ± 0.03 a | 0.41 ± 0.08 a |
| Compound | Absolute Content (mg/kg) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Unroasted | 90 °C | 130 °C | 170 °C | |||||||
| 10 min | 20 min | 30 min | 10 min | 20 min | 30 min | 10 min | 20 min | 30 min | ||
| α-Tocopherol | 7.76 ± 1.28 f | 7.89 ± 0.49 f | 8.38 ± 0.03 f | 11.44 ± 1.43 e | 14.53 ± 0.20 c | 13.37 ± 0.15 d | 12.49 ± 1.30 de | 23.42 ± 1.95 b | 31.2 ± 1.05 a | 31.82 ± 0.43 a |
| γ-Tocopherol | 535.32 ± 1.78 a | 526.27 ± 6.98 ab | 518.06 ± 7.56 b | 498.35 ± 5.27 cd | 513.15 ± 4.29 bcd | 512.59 ± 4.86 bcd | 498.73 ± 2.76 de | 485.12 ± 3.12 e | 453.66 ± 5.08 f | 446.89 ± 0.28 f |
| δ-Tocopherol | 213.52 ± 0.16 a | 210.74 ± 2.71 ab | 209.33 ± 4.61 abc | 196.01 ± 0.91 e | 205.68 ± 1.39 bcd | 201.81 ± 0.63 cd | 190.16 ± 2.41 fg | 194.35 ± 1.49 ef | 185.69 ± 0.74 g | 163.71 ± 0.82 h |
| Total-Tocopherol | 758.53 ± 0.89 a | 748.16 ± 8.41 ab | 738.06 ± 5.72 ab | 707.54 ± 4.61 d | 734.59 ± 0.26 bc | 729.50 ± 0.91 bc | 702.45 ± 3.24 d | 704.93 ± 1.68 d | 672.11 ± 4.22 e | 643.72 ± 1.39 f |
| β-Sitosterol | 1600.66 ± 23.75 de | 1610.56 ± 27.19 de | 1626.30 ± 33.64 d | 1584.94 ± 14.26 e | 1687.46 ± 12.58 bc | 1788.64 ± 4.90 a | 1708.11 ± 7.56 b | 1679.44 ± 13.81 c | 1586.95 ± 22.63 e | 1590.79 ± 4.20 e |
| Stigmasterol | 369.21 ± 1.87 d | 390.48 ± 9.61 c | 411.12 ± 2.77 a | 365.13 ± 0.51 e | 359.04 ± 7.94 e | 408.52 ± 1.08 ab | 404.52 ± 1.08 b | 361.95 ± 7.55 e | 343.38 ± 5.61 fg | 345.96 ± 3.98 f |
| Δ5-Avenasterol | 216.00 ± 3.25 f | 216.84 ± 1.56 f | 218.03 ± 0.89 ef | 221.12 ± 1.39 de | 230.88 ± 0.82 b | 244.42 ± 0.48 a | 223.63 ± 2.93 d | 226.82 ± 1.26 c | 234.02 ± 4.10 b | 221.83 ± 0.66 d |
| Cholesterol | 451.11 ± 2.15 b | 439.97 ± 0.89 c | 421.43 ± 3.19 d | 413.26 ± 0.80 e | 389.52 ± 4.34 f | 450.01 ± 4.68 b | 456.93 ± 2.20 a | 413.09 ± 1.43 e | 394.90 ± 2.28 f | 390.74 ± 2.96 f |
| Campesterol | 93.25 ± 1.36 a | 91.76 ± 3.16 ab | 84.95 ± 3.54 c | 81.21 ± 1.81 d | 81.21 ± 1.81 d | 88.57 ± 6.02 c | 89.37 ± 4.29 bc | 81.31 ± 6.61 d | 80.04 ± 0.84 de | 81.08 ± 2.11 d |
| Cycloartenol | 59.33 ± 6.52 b | 56.21 ± 0.79 de | 56.84 ± 0.48 cd | 55.42 ± 2.03 de | 58.73 ± 0.62 bc | 57.15 ± 2.29 cd | 61.46 ± 0.76 a | 59.15 ± 2.24 b | 54.32 ± 1.22 e | 58.41 ± 4.80 bc |
| Phytosterol | 2789.56 ± 19.34 b | 2805.82 ± 23.69 b | 2818.67 ± 21.15 b | 2721.08 ± 17.58 c | 2806.84 ± 20.73 b | 3037.31 ± 28.57 a | 2487.09 ± 17.65 e | 2821.76 ± 19.41 b | 2693.61 ± 30.94 d | 2216.99 ± 8.27 f |
| Squalene | 5.06 ± 0.51 g | 5.94 ± 0.22 g | 7.36 ± 0.08 ef | 10.11 ± 0.34 c | 8.12 ± 0.04 de | 13.10 ± 0.72 a | 11.48 ± 1.11 b | 6.82 ± 0.35 f | 8.32 ± 0.37 d | 10.62 ± 0.24 bc |
| Total polyphenols A | 22.95 ± 1.13 d | 25.43 ± 3.22 bc | 25.14 ± 0.19 c | 23.71 ± 2.48 d | 24.66 ± 1.36 c | 26.67 ± 0.41 a | 26.52 ± 1.53 ab | 22.90 ± 1.25 d | 23.61 ± 2.09 d | 22.37 ± 3.10 d |
| Roasting Temperature | Roasting Time | OSI (h) | DPPH (μmol TE/100 g) | ABTS (μmol TE/100 g) | FRAP (μmol TE/100 g) |
|---|---|---|---|---|---|
| Unroasted | — | 5.35 ± 0.03 de | 43.17 ± 1.96 f | 51.261 ± 0.19 de | 36.39 ± 0.33 e |
| 90 °C | 10 min | 4.82 ± 0.04 e | 56.96 ± 2.43 c | 49.791 ± 0.21 e | 36.90 ± 0.83 e |
| 20 min | 5.71 ± 0.19 d | 59.87 ± 1.61 a | 52.60 ± 3.84 d | 44.78 ± 1.97 a | |
| 30 min | 6.66 ± 0.11 c | 57.99 ± 3.11 b | 53.48 ± 0.09 cd | 43.17 ± 0.95 b | |
| 130 °C | 10 min | 4.83 ± 0.06 e | 46.79 ± 0.57 e | 54.87 ± 3.11 c | 43.17 ± 2.16 b |
| 20 min | 6.73 ± 0.20 c | 59.78 ± 2.68 a | 59.95 ± 1.85 a | 43.25 ± 0.42 b | |
| 30 min | 7.12 ± 0.09 b | 55.40 ± 1.12 cd | 55.03 ± 0.07 bc | 44.65 ± 2.25 a | |
| 170 °C | 10 min | 4.67 ± 0.16 e | 48.17 ± 2.96 d | 48.23 ± 0.14 f | 37.36 ± 1.44 d |
| 20 min | 7.36 ± 0.28 a | 59.38 ± 1.50 a | 52.92 ± 2.70 d | 42.41 ± 2.68 c | |
| 30 min | 7.07 ± 0.27 b | 59.69 ± 3.75 a | 56.16 ± 2.72 b | 44.06 ± 0.93 ab |
| Variable | Components | F1 | F2 | F3 | F4 | F5 |
|---|---|---|---|---|---|---|
| X1 | Total polyphenols | 0.085 | 0.868 | −0.235 | 0.243 | −0.071 |
| X2 | Oil yield | 0.947 | −0.019 | −0.068 | 0.013 | −0.02 |
| X3 | POV | 0.752 | −0.573 | 0.175 | 0.266 | 0.031 |
| X4 | AV | −0.925 | −0.153 | −0.128 | −0.133 | 0.164 |
| X5 | ABTS | 0.007 | 0.641 | −0.075 | 0.647 | 0.276 |
| X6 | FRAP | −0.511 | 0.402 | −0.382 | 0.396 | 0.296 |
| X7 | DPPH | 0.251 | −0.392 | −0.488 | 0.634 | −0.307 |
| X8 | BI | 0.663 | −0.589 | 0.296 | 0.105 | 0.149 |
| X9 | HMF | 0.913 | −0.012 | 0.029 | 0.012 | −0.109 |
| X10 | C16:0 | −0.273 | −0.242 | 0.792 | 0.001 | 0.163 |
| X11 | C18:0 | −0.835 | −0.047 | 0.477 | 0.155 | −0.11 |
| X12 | C18:1 | −0.788 | 0.03 | −0.004 | 0.3 | −0.384 |
| X13 | C18:2 | 0.785 | 0.07 | −0.574 | −0.165 | 0.117 |
| X14 | C18:3 | 0.779 | 0.307 | −0.49 | −0.179 | 0.033 |
| X15 | Stigmasterol | 0.636 | 0.602 | 0.382 | 0.061 | 0.233 |
| X16 | β- sitosterol | −0.087 | 0.879 | 0.331 | −0.049 | −0.319 |
| X17 | Δ5-Avenasterol | 0.709 | 0.494 | 0.017 | −0.28 | −0.376 |
| X18 | Cholesterol | 0.681 | 0.577 | 0.362 | 0.063 | 0.184 |
| X19 | Campesterol | 0.475 | 0.725 | 0.14 | 0.101 | 0.446 |
| X20 | Cycloartenol | 0.721 | 0.385 | 0.24 | 0.077 | −0.455 |
| X21 | Squalene | 0.741 | 0.559 | −0.318 | −0.088 | −0.073 |
| X22 | Phytosterol | 0.308 | 0.862 | 0.372 | −0.018 | −0.122 |
| X23 | OSI | 0.96 | −0.059 | 0.071 | 0.152 | 0.011 |
| X24 | Total tocopherols | −0.884 | 0.426 | −0.001 | −0.181 | 0.029 |
| X25 | α-Tocopherol | 0.789 | −0.441 | 0.39 | 0.126 | −0.022 |
| X26 | γ-Tocopherol | −0.847 | 0.444 | −0.159 | −0.232 | −0.003 |
| X27 | δ-Tocopherol | −0.899 | 0.4 | 0.071 | −0.046 | 0.09 |
| Characteristic value | 13.928 | 6.5434 | 2.915 | 1.673 | 1.347 | |
| Variance contribution rate (%) | 49.744 | 23.369 | 10.409 | 5.976 | 4.811 | |
| Cumulative variance contribution rate (%) | 49.744 | 73.113 | 83.522 | 89.499 | 94.31 | |
| Samples | F1 | F2 | F3 | F4 | F5 | F-Total | Sequence |
|---|---|---|---|---|---|---|---|
| Unroasted | −1.25208 | −0.71214 | 0.37288 | −1.99261 | 0.80049 | −0.831 | 10 |
| 90 °C, 10 min | −1.18462 | −0.27328 | −0.49983 | 0.60111 | −0.25088 | −0.681 | 9 |
| 90 °C, 20 min | −0.96374 | −0.00551 | −0.68179 | 1.38102 | −0.17007 | −0.477 | 8 |
| 90 °C, 30 min | −0.14641 | −0.0935 | −1.43871 | −0.14616 | 0.61412 | −0.224 | 7 |
| 130 °C, 10 min | −0.3834 | 0.83876 | 1.00741 | 0.21699 | 0.70703 | 0.157 | 5 |
| 130 °C, 20 min | 0.62083 | 2.021 | 0.30154 | −0.97784 | −0.50309 | 0.730 | 1 |
| 130 °C, 30 min | 0.73695 | 0.88904 | −0.97687 | 0.30729 | −0.30051 | 0.477 | 3 |
| 170 °C, 10 min | −0.16318 | −0.74519 | 1.29621 | 0.16185 | −2.14675 | −0.214 | 6 |
| 170 °C, 20 min | 1.02529 | −0.49369 | 1.34507 | 1.09458 | 1.5352 | 0.674 | 2 |
| 170 °C, 30 min | 1.71037 | −1.42549 | −0.7259 | −0.64624 | −0.28554 | 0.390 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, Z.; Zhu, Z.; Zhou, J.; Xu, C.; Wei, C.; Liu, W.; Liu, Z.; Wang, T.; Xiao, H. Effect of Roasting on the Chemical Composition and Oxidative Stability of Tomato (Solanum lycopersicum L.) Seed Oil. Foods 2024, 13, 1682. https://doi.org/10.3390/foods13111682
Niu Z, Zhu Z, Zhou J, Xu C, Wei C, Liu W, Liu Z, Wang T, Xiao H. Effect of Roasting on the Chemical Composition and Oxidative Stability of Tomato (Solanum lycopersicum L.) Seed Oil. Foods. 2024; 13(11):1682. https://doi.org/10.3390/foods13111682
Chicago/Turabian StyleNiu, Zhiya, Zhongyan Zhu, Jing Zhou, Chengjian Xu, Changqing Wei, Wenyu Liu, Zhanxia Liu, Ting Wang, and Hang Xiao. 2024. "Effect of Roasting on the Chemical Composition and Oxidative Stability of Tomato (Solanum lycopersicum L.) Seed Oil" Foods 13, no. 11: 1682. https://doi.org/10.3390/foods13111682
APA StyleNiu, Z., Zhu, Z., Zhou, J., Xu, C., Wei, C., Liu, W., Liu, Z., Wang, T., & Xiao, H. (2024). Effect of Roasting on the Chemical Composition and Oxidative Stability of Tomato (Solanum lycopersicum L.) Seed Oil. Foods, 13(11), 1682. https://doi.org/10.3390/foods13111682







