Prevention of the Quality Degradation of Antarctic Krill (Euphausia superba) Meal through Two-Stage Drying
Abstract
:1. Introduction
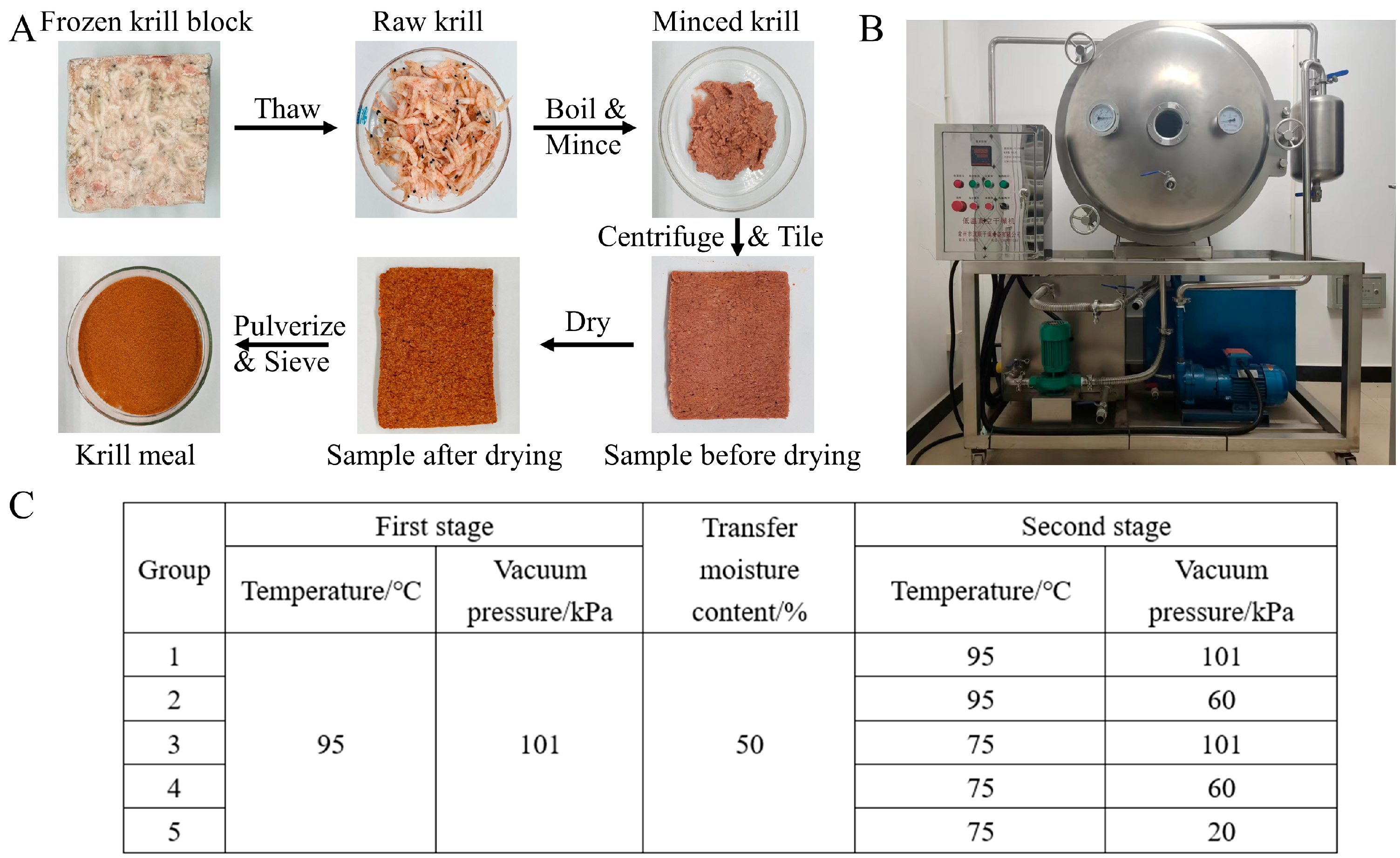
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Drying Characteristics
2.4. Low-Field Nuclear Magnetic Resonance (LF-NMR)
2.5. Color Analysis
2.6. Headspace Gas Chromatography–Ion Mobility Spectrometry (HS-GC-IMS)
2.7. Thiobarbituric Acid-Reactive Substances (TBARS)
2.8. Phospholipid
2.9. Astaxanthin and the Geometrical Isomers
2.10. Fatty Acid
2.11. Statistical Analysis
3. Results and Discussion
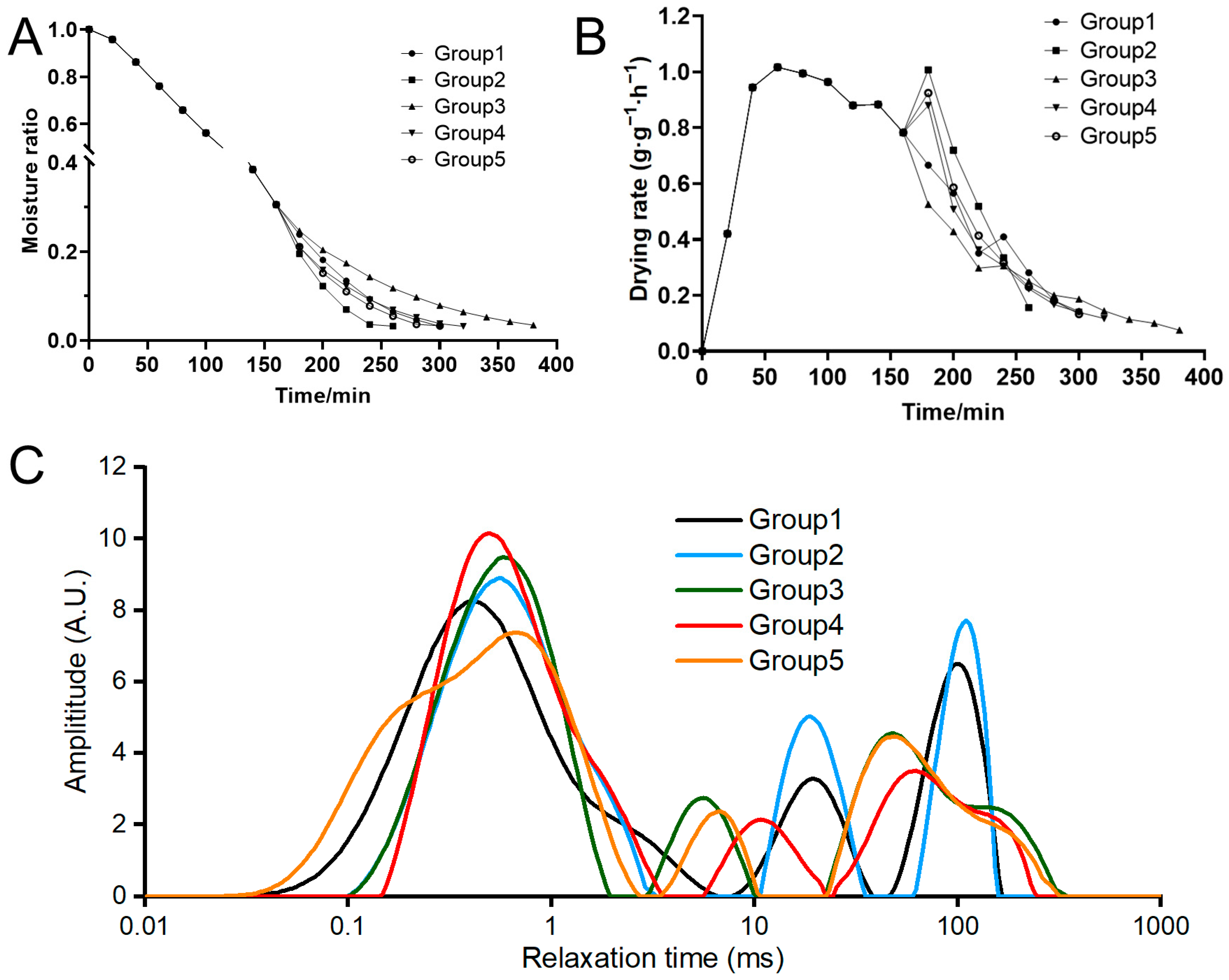
3.1. Drying Characteristics
3.1.1. Moisture Ratio and Drying Rate
3.1.2. Water Distribution
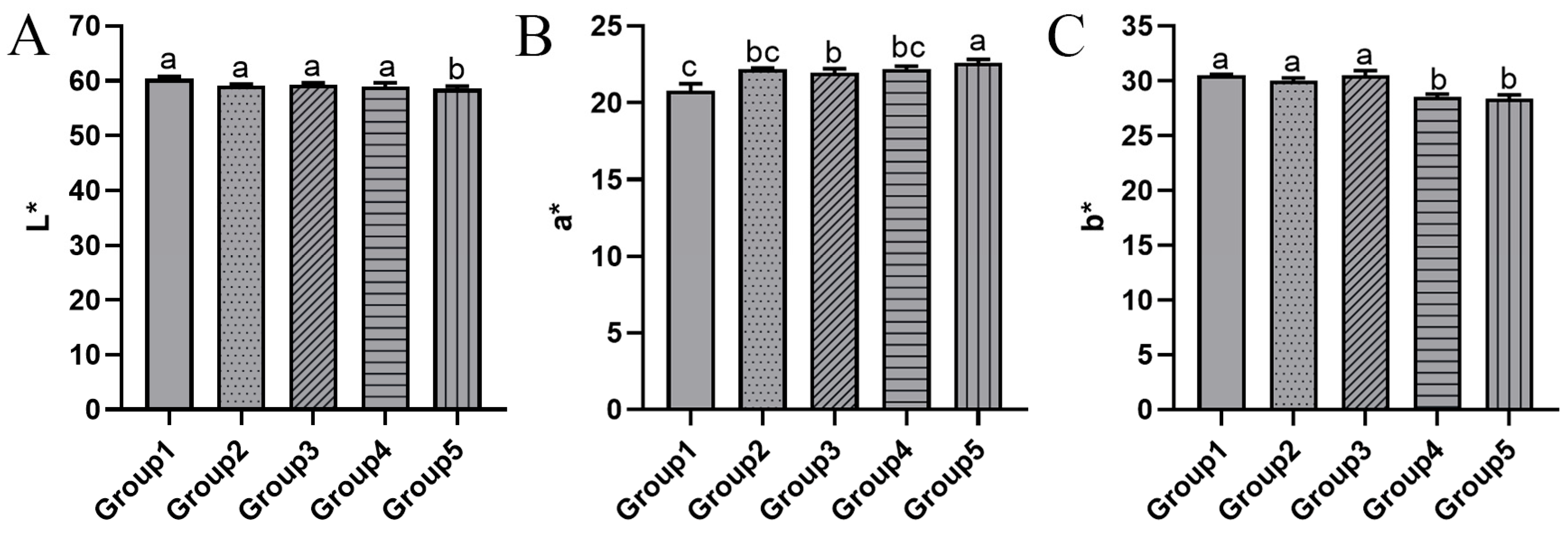
3.2. Sensory Quality
3.2.1. Color
3.2.2. Volatile Odor
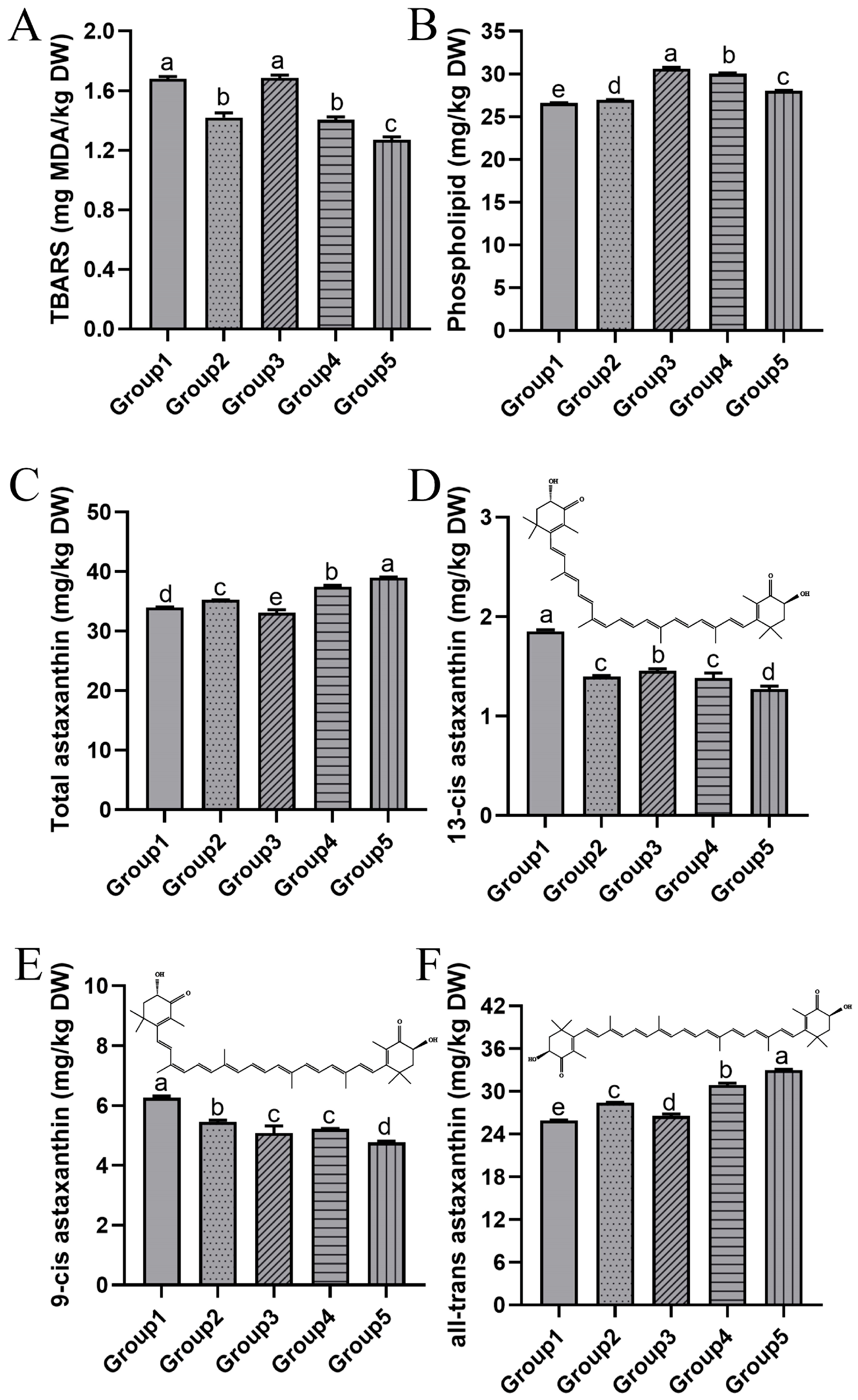
3.3. Bioactive Compounds
3.3.1. Lipid Oxidation and Phospholipid Content
3.3.2. Astaxanthin and Geometrical Isomers
3.3.3. Fatty Acid Composition
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Atkinson, A.; Siegel, V.; Pakhomov, E.A.; Jessopp, M.J.; Loeb, V. A re-appraisal of the total biomass and annual production of Antarctic krill. Deep-Sea Res. Part I-Oceanogr. Res. Pap. 2009, 56, 727–740. [Google Scholar] [CrossRef]
- Kol’akowski, E.; Sikorski, Z.E. Endogenous Enzymes in Antarctic Krill: Control of Activity during Storage and Utilization; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Morkore, T.; Moreno, H.M.; Borderias, J.; Larsson, T.; Hellberg, H.; Haden, B.; Romarheim, O.H.; Ruyter, B.; Lazado, C.C.; Jimenez-Guerrero, R.; et al. Dietary inclusion of Antarctic krill meal during the finishing feed period improves health and fillet quality of Atlantic salmon (Salmo salar L.). Br. J. Nutr. 2020, 124, 418–431. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Shibata, N. The utilization of antarctic krill for human food. Food Rev. Int. 1990, 6, 119–147. [Google Scholar] [CrossRef]
- Higuera-Ciapara, I.; Felix-Valenzuela, L.; Goycoolea, F.M. Astaxanthin: A review of its chemistry and applications. Crit. Rev. Food Sci. Nutr. 2006, 46, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Peng, X.; Yuan, T.; Yang, J.; Li, X.; Zhang, H.; Zhang, Y.; Zhang, Z.; Jia, X. Effect of vacuum drying on drying kinetics and quality of the aqueous extracts of Callicarpa nudiflora Hook. et Arn. LWT-Food Sci. Technol. 2021, 152, 112305. [Google Scholar] [CrossRef]
- Cong, X.-Y.; Miao, J.-K.; Zhang, H.-Z.; Sun, W.-H.; Xing, L.-H.; Sun, L.-R.; Zu, L.; Gao, Y.; Leng, K.-L. Effects of Drying Methods on the Content, Structural Isomers, and Composition of Astaxanthin in Antarctic Krill. ACS Omega 2019, 4, 17972–17980. [Google Scholar] [CrossRef]
- Richter Reis, F.; Marques, C.; Moraes, A.C.S.d.; Masson, M.L. Trends in quality assessment and drying methods used for fruits and vegetables. Food Control 2022, 142, 109254. [Google Scholar] [CrossRef]
- Bozkir, H. Effects of hot air, vacuum infrared, and vacuum microwave dryers on the drying kinetics and quality characteristics of orange slices. J. Food Process Eng. 2020, 43, e13485. [Google Scholar] [CrossRef]
- Xu, P.; Zhang, Z.; Peng, X.; Yang, J.; Li, X.; Yuan, T.; Jia, X.; Liu, Y.; Abdullaev, O.; Jenis, J. Study on vacuum drying kinetics and processing of the Lonicera japonica Thunb. aqueous extracts. LWT-Food Sci. Technol. 2022, 167, 113868. [Google Scholar] [CrossRef]
- Zhu, W.K.; Wang, L.; Duan, K.; Chen, L.Y.; Li, B. Experimental and Numerical Investigation of the Heat and Mass Transfer for Cut Tobacco during Two-Stage Convective Drying. Dry. Technol. 2015, 33, 907–914. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, M.; Tu, D.; Sun, J.; Zhou, L.; Mujumdar, A.S. A two-stage convective air and vacuum freeze-drying technique for bamboo shoots. Int. J. Food Sci. Technol. 2005, 40, 589–595. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, M.; Mujumdar, A.S.; Duan, X.; Sun, J.-C. A two-stage vacuum freeze and convective air drying method for strawberries. Dry. Technol. 2006, 24, 1019–1023. [Google Scholar] [CrossRef]
- Phoungchandang, S.; Saentaweesuk, S. Effect of two stage, tray and heat pump assisted-dehumidified drying on drying characteristics and qualities of dried ginger. Food Bioprod. Process. 2011, 89, 429–437. [Google Scholar] [CrossRef]
- Pei, F.; Shi, Y.; Gao, X.; Wu, F.; Mariga, A.M.; Yang, W.; Zhao, L.; An, X.; Xin, Z.; Yang, F.; et al. Changes in non-volatile taste components of button mushroom (Agaricus bisporus) during different stages of freeze drying and freeze drying combined with microwave vacuum drying. Food Chem. 2014, 165, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Chen, X.; Pan, N.; Liu, S.; Su, Y.; Xiao, M.; Shi, W.; Liu, Z. The effects of five different drying methods on the quality of semi-dried Takifugu obscurus fillets. LWT 2022, 161, 113340. [Google Scholar] [CrossRef]
- Zhang, Q.; Ding, Y.; Gu, S.; Zhu, S.; Zhou, X.; Ding, Y. Identification of changes in volatile compounds in dry-cured fish during storage using HS-GC-IMS. Food Res. Int. 2020, 137, 109339. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Qiu, Z.; Wang, X. Protein oxidation and tandem mass tag-based proteomic analysis in the dorsal muscle of farmed obscure pufferfish subjected to multiple freeze-thaw cycles. J. Food Process. Preserv. 2020, 44, e14721. [Google Scholar] [CrossRef]
- Oliveira, M.d.S.; Feddern, V.; Kupski, L.; Cipolatti, E.P.; Badiale-Furlong, E.; de Souza-Soares, L.A. Changes in lipid, fatty acids and phospholipids composition of whole rice bran after solid-state fungal fermentation. Bioresour. Technol. 2011, 102, 8335–8338. [Google Scholar] [CrossRef] [PubMed]
- Gigliotti, J.C.; Davenport, M.P.; Beamer, S.K.; Tou, J.C.; Jaczynski, J. Extraction and characterisation of lipids from Antarctic krill (Euphausia superba). Food Chem. 2011, 125, 1028–1036. [Google Scholar] [CrossRef]
- García-Moreira, D.P.; Hernández-Guzmán, H.; Pacheco, N.; Cuevas-Bernardino, J.C.; Herrera-Pool, E.; Moreno, I.; López-Vidaña, E.C. Solar and Convective Drying: Modeling, Color, Texture, Total Phenolic Content, and Antioxidant Activity of Peach (Prunus persica (L.) Batsch) Slices. Processes 2023, 11, 1280. [Google Scholar] [CrossRef]
- Bao, X.; Min, R.; Zhou, K.; Traffano-Schiffo, M.V.; Dong, Q.; Luo, W. Effects of vacuum drying assisted with condensation on drying characteristics and quality of apple slices. J. Food Eng. 2023, 340, 111286. [Google Scholar] [CrossRef]
- Wang, J.; Law, C.-L.; Nema, P.K.; Zhao, J.-H.; Liu, Z.-L.; Deng, L.-Z.; Gao, Z.-J.; Xiao, H.-W. Pulsed vacuum drying enhances drying kinetics and quality of lemon slices. J. Food Eng. 2018, 224, 129–138. [Google Scholar] [CrossRef]
- Li, M.; Wang, H.; Zhao, G.; Qiao, M.; Li, M.; Sun, L.; Gao, X.; Zhang, J. Determining the drying degree and quality of chicken jerky by LF-NMR. J. Food Eng. 2014, 139, 43–49. [Google Scholar] [CrossRef]
- Li, M.; Chen, Y.; Geng, Y.; Liu, F.; Guo, L.; Wang, X. Convenient use of low field nuclear magnetic resonance to determine the drying kinetics and predict the quality properties of mulberries dried in hot-blast air. LWT-Food Sci. Technol. 2021, 137, 110402. [Google Scholar] [CrossRef]
- Han, B.; Ding, C.; Jia, Y.; Wang, H.; Bao, Y.; Zhang, J.; Duan, S.; Song, Z.; Chen, H.; Lu, J. Influence of electrohydrodynamics on the drying characteristics and physicochemical properties of garlic. Food Chem.-X 2023, 19, 100818. [Google Scholar] [CrossRef]
- Namsanguan, Y.; Tia, W.; Devahastin, S.; Soponronnarit, S. Drying kinetics and quality of shrimp undergoing different two-stage drying processes. Dry. Technol. 2004, 22, 759–778. [Google Scholar] [CrossRef]
- Zeng, X.-B.; Yin, F.-W.; Zhao, G.-H.; Guo, C.; Li, D.-Y.; Liu, H.-L.; Qin, L.; Shahidi, F.; Zhou, D.-Y. Mechanism of color change in Antarctic krill oil during storage. Food Chem. 2024, 444, 138583. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Zhang, L.; Li, Q.; Wang, Y.; Chen, Q.; Kong, B. Impacts of different altitudes and natural drying times on lipolysis, lipid oxidation and flavour profile of traditional Tibetan yak jerky. Meat Sci. 2020, 162, 108030. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Chen, D.; Dong, Y.F.; Ju, H.P.; Wu, C.; Lin, S.Y. Characteristic volatiles fingerprints and changes of volatile compounds in fresh and dried Tricholoma matsutake Singer by HS-GC-IMS and HS-SPME-GC-MS. J. Chromatogr. B-Anal. Technol. Biomed. Life Sci. 2018, 1099, 46–55. [Google Scholar] [CrossRef]
- Fan, Y.; Li, Z.; Xue, Y.; Hou, H.; Xue, C. Identification of volatile compounds in Antarctic krill (Euphausia superba) using headspace solid-phase microextraction and GC-MS. Int. J. Food Prop. 2017, 20, S820–S829. [Google Scholar] [CrossRef]
- Aykin-Dincer, E.; Erbas, M. A comparative study of cold drying conditions on the physicochemical, microbial and sensory properties of dried chicken slices. Int. J. Food Sci. Technol. 2024, 59, 1979–1989. [Google Scholar] [CrossRef]
- Castro-Gomez, M.P.; Holgado, F.; Rodriguez-Alcala, L.M.; Montero, O.; Fontecha, J. Comprehensive Study of the Lipid Classes of Krill Oil by Fractionation and Identification of Triacylglycerols, Diacylglycerols, and Phospholipid Molecular Species by Using UPLC/QToF-MS. Food Anal. Methods 2015, 8, 2568–2580. [Google Scholar] [CrossRef]
- Li, D.-Y.; Zhou, D.-Y.; Yin, F.-W.; Dong, X.-P.; Xie, H.-K.; Liu, Z.-Y.; Li, A.; Li, J.-X.; Rakariyatham, K.; Shahidi, F. Impact of different drying processes on the lipid deterioration and color characteristics of Penaeus vannamei. J. Sci. Food Agric. 2020, 100, 2544–2553. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.S.H.; Bruheim, I.; Ale, M.T.; Jacobsen, C. The effect of thermal treatment on the quality changes of Antartic krill meal during the manufacturing process: High processing temperatures decrease product quality. Eur. J. Lipid Sci. Technol. 2015, 117, 411–420. [Google Scholar] [CrossRef]
- Ahmed, F.; Li, Y.; Fanning, K.; Netzel, M.; Schenk, P.M. Effect of drying, storage temperature and air exposure on astaxanthin stability from Haematococcus pluvialis. Food Res. Int. 2015, 74, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Anahi Martinez-Delgado, A.; Khandual, S.; Josefina Villanueva-Rodriguez, S. Chemical stability of astaxanthin integrated into a food matrix: Effects of food processing and methods for preservation. Food Chem. 2017, 225, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Osawa, T. Cis astaxanthin and especially 9-cis astaxanthin exhibits a higher antioxidant activity in vitro compared to the all-trans isomer. Biochem. Biophys. Res. Commun. 2007, 357, 187–193. [Google Scholar] [CrossRef]
- Connor, W.E. Importance of n-3 fatty acids in health and disease. Am. J. Clin. Nutr. 2000, 71, 171S–175S. [Google Scholar] [CrossRef]
| Group 1 1 | Group 2 | Group 3 | Group 4 | Group 5 | |
|---|---|---|---|---|---|
| Isoterpinene | 855.90 ± 37.80 a | 738.92 ± 5.69 c | 714.52 ± 4.77 c | 801.92 ± 13.64 b | 834.57 ± 16.48 ab |
| (Z)-4-Heptenal | 1009.52 ± 192.24 b | 1443.57 ± 61.42 a | 624.74 ± 56.90 c | 1479.70 ± 55.92 a | 1414.23 ± 118.57 a |
| 3-Methyl-1-butanol | 483.13 ± 78.14 b | 585.61 ± 49.56 a | 304.79 ± 14.96 c | 578.99 ± 19.64 a | 505.20 ± 20.41 ab |
| Dipentene | 333.93 ± 14.08 a | 239.21 ± 2.92 c | 174.44 ± 8.86 d | 295.05 ± 4.25 b | 231.35 ± 8.19 c |
| Ethyl pyruvate | 4355.27 ± 254.52 a | 1920.54 ± 297.64 b | 837.80 ± 33.54 c | 1794.61 ± 125.21 b | 902.98 ± 95.78 c |
| 2-Heptanone | 87.04 ± 3.40 a | 49.79 ± 3.14 c | 26.14 ± 2.78 e | 64.89 ± 2.97 b | 42.02 ± 4.80 d |
| Cyclopentanone | 905.99 ± 172.49 c | 712.11 ± 42.15 cd | 625.54 ± 93.29 d | 1425.16 ± 206.51 a | 1163.76 ± 36.88 b |
| (E)-2-Hexenal | 1110.55 ± 114.23 a | 492.55 ± 56.71 b | 260.48 ± 5.65 c | 449.55 ± 22.67 b | 287.10 ± 25.99 c |
| 1,4-Dimethylbenzene | 190.58 ± 18.00 a | 152.83 ± 10.81 b | 99.10 ± 2.39 d | 151.58 ± 13.06 b | 126.25 ± 9.39 c |
| 1-Butanol | 1080.13 ± 27.95 a | 629.69 ± 43.73 c | 432.41 ± 19.72 e | 696.39 ± 12.33 b | 483.42 ± 7.88 d |
| 3-Pentanol-D | 271.40 ± 29.71 c | 176.73 ± 14.33 d | 190.60 ± 28.09 d | 558.30 ± 77.09 a | 373.16 ± 35.60 b |
| 3-pentanol-M | 1234.49 ± 58.02 c | 992.25 ± 2.98 d | 1059.99 ± 81.58 d | 1630.35 ± 86.05 a | 1477.30 ± 52.50 b |
| Hexanal | 462.53 ± 2.71 b | 467.78 ± 27.39 b | 367.05 ± 20.92 c | 514.24 ± 28.66 a | 433.66 ± 17.68 b |
| Valeraldehyde | 4415.02 ± 206.50 b | 3734.59 ± 138.59 c | 3665.15 ± 56.64 c | 4762.56 ± 79.13 a | 4551.34 ± 40.84 ab |
| Ethyl 3-methylbutanoate | 3023.46 ± 41.55 a | 2610.81 ± 33.00 c | 2313.58 ± 22.93 d | 2772.85 ± 45.67 b | 2568.92 ± 30.23 c |
| 2-Butanol | 4672.94 ± 164.85 d | 9381.98 ± 81.96 a | 8302.70 ± 64.61 b | 3807.24 ± 64.77 e | 4868.30 ± 72.52 c |
| Propyl acetate | 1491.92 ± 40.23 a | 1428.60 ± 7.25 b | 1471.18 ± 7.89 ab | 1458.11 ± 23.00 ab | 1478.96 ± 26.23 a |
| 2,3-Butanedione | 675.95 ± 11.33 d | 765.77 ± 26.62 c | 972.39 ± 9.19 a | 750.76 ± 21.88 c | 923.63 ± 9.78 b |
| 3-Methylbutyraldehyde | 1135.22 ± 30.08 d | 1451.21 ± 78.33 c | 1466.66 ± 67.44 c | 1721.58 ± 32.08 b | 1875.72 ± 19.12 a |
| Ethyl butyrate | 771.71 ± 12.38 a | 573.87 ± 42.49 b | 415.06 ± 19.00 d | 588.59 ± 11.85 b | 472.96 ± 20.70 c |
| Butyraldehyde-D | 239.14 ± 12.05 c | 403.45 ± 52.14 b | 418.12 ± 35.51 b | 615.52 ± 34.72 a | 666.28 ± 20.67 a |
| Butyraldehyde-M | 3402.20 ± 51.56 d | 3564.81 ± 43.11 c | 5169.60 ± 125.41 a | 2844.71 ± 46.93 e | 3970.59 ± 113.30 b |
| 1-Octene | 38,295.01 ± 647.40 a | 20,848.17 ± 2509.28 b | 10,536.48 ± 293.09 c | 22,992.20 ± 1185.91 b | 12,301.65 ± 670.54 c |
| Butylcyclohexane | 2133.51 ± 41.50 d | 3672.78 ± 111.23 a | 3739.73 ± 18.39 a | 2399.09 ± 54.49 c | 2844.88 ± 66.76 b |
| 2-Pentanone | 350.30 ± 24.46 c | 359.85 ± 32.83 c | 460.76 ± 7.83 b | 449.34 ± 21.30 b | 566.55 ± 10.35 a |
| Ethyl 2-methylbutyrate | 235.26 ± 11.46 b | 249.22 ± 3.68 b | 357.30 ± 1.04 a | 250.88 ± 7.86 b | 360.95 ± 15.86 a |
| Tetrahydrofuran | 543.02 ± 16.58 d | 864.71 ± 90.58 c | 1652.08 ± 15.32 a | 852.56 ± 59.94 c | 1515.79 ± 23.49 b |
| 2-Methylpropanal | 490.48 ± 23.08 c | 975.51 ± 7.42 a | 506.19 ± 7.24 c | 720.19 ± 121.01 b | 549.83 ± 7.03 c |
| Methyl acetate | 196.88 ± 7.36 a | 50.29 ± 4.42 e | 61.45 ± 6.36 d | 133.91 ± 5.66 c | 164.28 ± 3.78 b |
| Ethyl formate-D | 59.21 ± 7.14 b | 58.12 ± 21.87 b | 34.64 ± 1.52 c | 185.00 ± 8.15 a | 180.06 ± 0.44 a |
| Ethyl formate-M | 739.14 ± 48.19 b | 623.60 ± 115.93 c | 433.65 ± 26.49 d | 1108.99 ± 15.72 a | 1097.49 ± 30.99 a |
| Ethyl 2-methylpropanoate | 179.98 ± 5.56 b | 156.14 ± 8.47 c | 151.80 ± 3.67 c | 178.80 ± 11.52 b | 208.12 ± 8.83 a |
| 4-Methyl-3-penten-2-one | 126.50 ± 13.34 d | 130.76 ± 0.63 cd | 149.63 ± 3.06 ab | 141.47 ± 7.70 bc | 161.68 ± 4.85 a |
| (E)-2-Pentenal | 243.51 ± 7.90 b | 222.87 ± 6.69 c | 258.84 ± 3.44 ab | 245.67 ± 16.64 b | 275.55 ± 15.13 a |
| Ethyl 2-hydroxypropanoate | 349.17 ± 8.98 a | 304.17 ± 11.89 c | 297.52 ± 3.02 c | 326.76 ± 3.31 b | 334.94 ± 11.10 ab |
| Butyl acetate | 257.81 ± 35.74 a | 163.34 ± 0.79 b | 92.82 ± 4.46 c | 174.91 ± 2.64 b | 110.68 ± 7.02 c |
| 4-Isopropyltoluene | 41.58 ± 4.91 a | 40.92 ± 1.53 a | 38.48 ± 0.91 a | 40.80 ± 3.67 a | 44.19 ± 2.33 a |
| Group 1 1 | Group 2 | Group 3 | Group 4 | Group 5 | |
|---|---|---|---|---|---|
| c12:0 | 0.02 ± 0.00 a | 0.02 ± 0.00 a | 0.02 ± 0.00 a | 0.01 ± 0.00 c | 0.02 ± 0.00 b |
| c13:0 | 0.01 ± 0.00 b | 0.01 ± 0.00 b | 0.01 ± 0.00 a | 0.01 ± 0.00 c | 0.01 ± 0.00 b |
| c14:0 | 1.18 ± 0.00 b | 1.15 ± 0.00 b | 1.25 ± 0.03 a | 0.92 ± 0.01 d | 1.09 ± 0.00 c |
| c15:0 | 0.04 ± 0.00 a | 0.04 ± 0.00 b | 0.04 ± 0.00 a | 0.03 ± 0.00 d | 0.04 ± 0.00 c |
| c16:0 | 2.17 ± 0.01 b | 2.12 ± 0.01 c | 2.28 ± 0.02 a | 1.72 ± 0.01 e | 1.98 ± 0.01 d |
| c17:0 | 0.18 ± 0.00 b | 0.18 ± 0.00 c | 0.22 ± 0.00 a | 0.14 ± 0.00 d | 0.16 ± 0.00 a |
| c18:0 | 0.14 ± 0.00 b | 0.13 ± 0.00 c | 0.14 ± 0.00 a | 0.11 ± 0.00 e | 0.13 ± 0.00 d |
| c20:0 | 0.01 ± 0.00 b | 0.01 ± 0.00 b | 0.00 ± 0.00 a | 0.00 ± 0.00 c | 0.00 ± 0.00 b |
| c21:0 | 0.00 ± 0.00 ab | 0.00 ± 0.00 bc | 0.01 ± 0.00 c | 0.00 ± 0.00 d | 0.00 ± 0.00 a |
| c22:0 | 0.20 ± 0.02 a | 0.25 ± 0.02 a | 0.22 ± 0.07 a | 0.25 ± 0.02 a | 0.28 ± 0.02 a |
| c23:0 | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a |
| c24:0 | 0.02 ± 0.00 b | 0.02 ± 0.00 c | 0.02 ± 0.00 a | 0.02 ± 0.00 e | 0.02 ± 0.00 d |
| c14:1n5 | 0.04 ± 0.00 b | 0.04 ± 0.00 c | 0.04 ± 0.00 a | 0.03 ± 0.00 e | 0.04 ± 0.00 d |
| c15:1n5 | 0.01 ± 0.00 b | 0.01 ± 0.00 c | 0.01 ± 0.00 a | 0.01 ± 0.00 e | 0.01 ± 0.00 d |
| c16:1n7 | 0.71 ± 0.00 b | 0.69 ± 0.00 b | 0.77 ± 0.00 a | 0.55 ± 0.00 c | 0.65 ± 0.00 d |
| c17:1n7 | 0.03 ± 0.00 b | 0.03 ± 0.00 b | 0.03 ± 0.00 a | 0.02 ± 0.00 d | 0.03 ± 0.00 c |
| c18:1n9t | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.00 c | 0.01 ± 0.00 b |
| c18:1n9c | 1.16 ± 0.00 ab | 1.14 ± 0.00 a | 1.24 ± 0.01 bc | 0.93 ± 0.00 c | 1.08 ± 0.00 bc |
| c20:1n9 | 0.06 ± 0.00 a | 0.06 ± 0.00 b | 0.07 ± 0.00 a | 0.05 ± 0.00 d | 0.06 ± 0.00 c |
| c22:1n9 | 0.04 ± 0.00 b | 0.03 ± 0.00 c | 0.04 ± 0.00 a | 0.03 ± 0.00 e | 0.04 ± 0.00 d |
| c18:2n6t | 0.02 ± 0.00 b | 0.02 ± 0.00 c | 0.02 ± 0.00 a | 0.01 ± 0.00 e | 0.02 ± 0.00 d |
| c18:2n6c | 0.18 ± 0.00 b | 0.18 ± 0.00 c | 0.19 ± 0.00 a | 0.14 ± 0.00 e | 0.17 ± 0.00 d |
| c20:2n6 | 0.01 ± 0.00 b | 0.01 ± 0.00 c | 0.01 ± 0.00 a | 0.01 ± 0.00 c | 0.01 ± 0.00 a |
| c18:3n6 | 0.00 ± 0.00 b | 0.00 ± 0.00 c | 0.00 ± 0.00 a | 0.00 ± 0.00 e | 0.00 ± 0.00 d |
| c18:3n3 | 0.02 ± 0.00 b | 0.02 ± 0.00 b | 0.02 ± 0.00 a | 0.01 ± 0.00 d | 0.01 ± 0.00 c |
| c20:3n6 | 0.07 ± 0.00 b | 0.07 ± 0.00 b | 0.07 ± 0.00 a | 0.05 ± 0.00 c | 0.06 ± 0.00 bc |
| c20:4n6 | 0.01 ± 0.00 a | 0.00 ± 0.00 ab | 0.01 ± 0.00 a | 0.00 ± 0.00 c | 0.00 ± 0.00 b |
| c20:3n3 | 0.03 ± 0.00 a | 0.03 ± 0.00 a | 0.03 ± 0.00 ab | 0.03 ± 0.00 c | 0.03 ± 0.00 b |
| c20:5n3 | 0.01 ± 0.00 b | 0.01 ± 0.00 ab | 0.01 ± 0.00 ab | 0.01 ± 0.00 ab | 0.01 ± 0.00 a |
| c22:2n6 | 1.29 ± 0.02 a | 1.21 ± 0.02 b | 1.31 ± 0.06 c | 1.00 ± 0.02 d | 1.12 ± 0.02 b |
| C22:6n3 | 0.40 ± 0.00 a | 0.39 ± 0.00 b | 0.40 ± 0.00 a | 0.34 ± 0.00 d | 0.38 ± 0.00 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Zhang, S.; Yang, L.; Wei, B.; Guo, Q. Prevention of the Quality Degradation of Antarctic Krill (Euphausia superba) Meal through Two-Stage Drying. Foods 2024, 13, 1706. https://doi.org/10.3390/foods13111706
Zheng Y, Zhang S, Yang L, Wei B, Guo Q. Prevention of the Quality Degradation of Antarctic Krill (Euphausia superba) Meal through Two-Stage Drying. Foods. 2024; 13(11):1706. https://doi.org/10.3390/foods13111706
Chicago/Turabian StyleZheng, Yao, Shuaishuai Zhang, Liu Yang, Banghong Wei, and Quanyou Guo. 2024. "Prevention of the Quality Degradation of Antarctic Krill (Euphausia superba) Meal through Two-Stage Drying" Foods 13, no. 11: 1706. https://doi.org/10.3390/foods13111706





