Preparation and Characterization of a Novel Longzhua mushroom Polysaccharide Hydrogel and Slow-Release Behavior of Encapsulated Rambutan Peel Polyphenols
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Longzhua mushroom Polysaccharide (LMP) Hydrogel
2.3. Texture Properties of LMPH
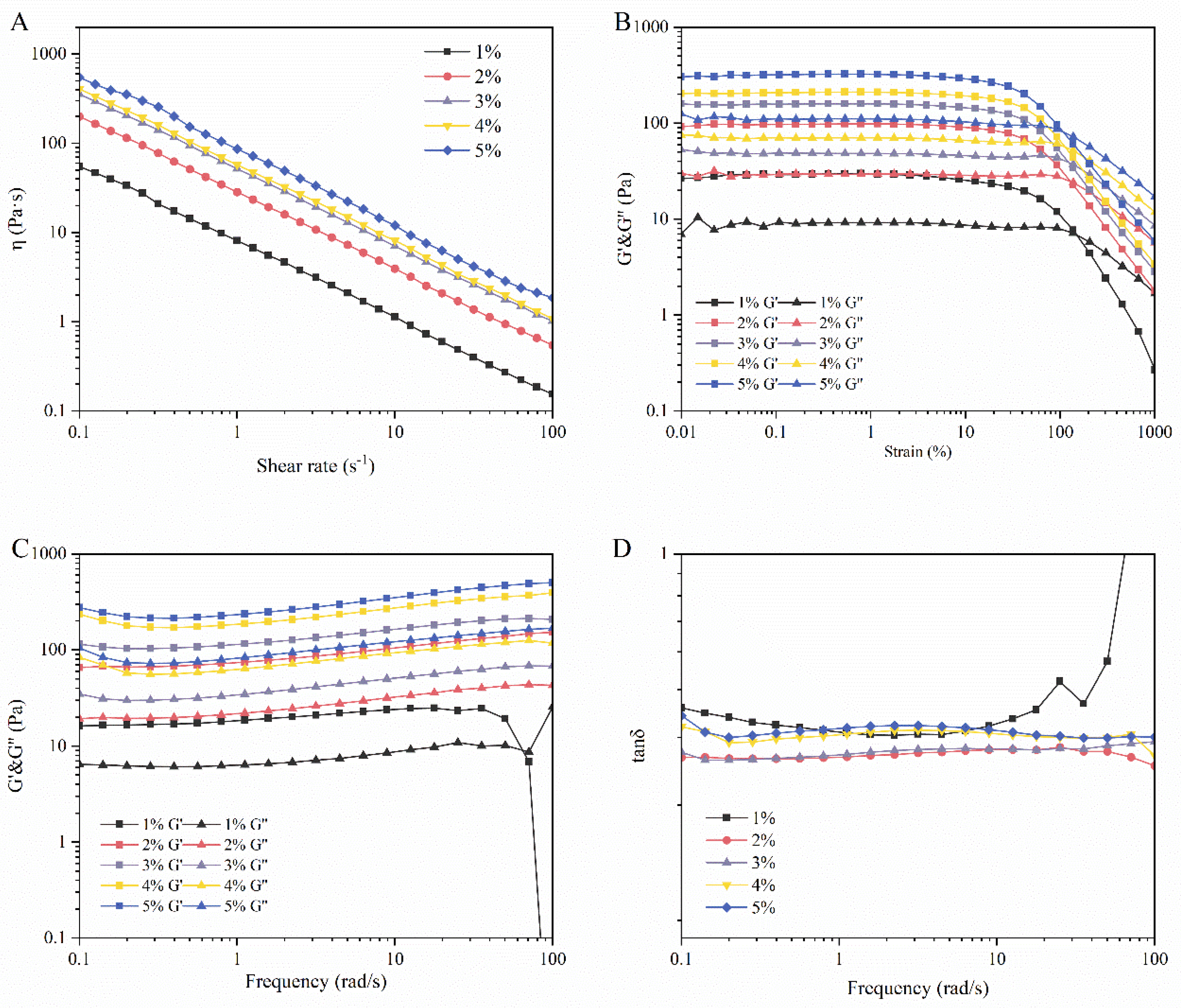
2.4. Rheological Properties of LMPH
2.4.1. Viscosity Sweep
2.4.2. Strain Sweep
2.4.3. Frequency Sweep
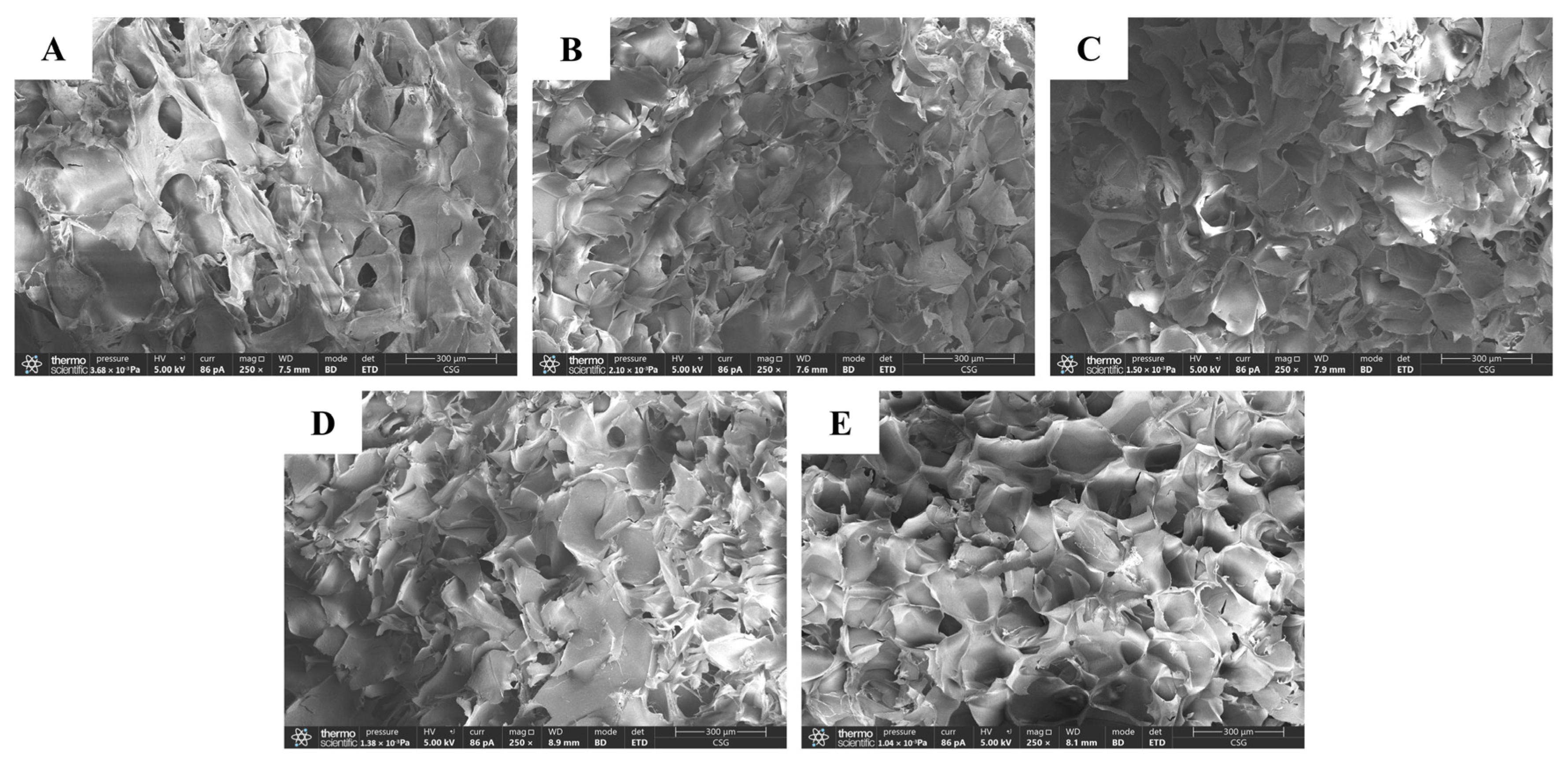
2.5. Scanning Electron Microscope (SEM)
2.6. Self-Restoring Characteristic
2.6.1. Macro Self-Recovery Performance
2.6.2. Micro Self-Recovery Performance
2.7. Stability of LMPH
2.7.1. Differential Scanning Calorimetry (DSC)
2.7.2. Freeze–Thaw Stability
2.8. Water Holding Capacity and Swelling Properties of LMPH
2.8.1. Swelling Property
2.8.2. Water Holding Capacity
2.9. In Vitro Sustained Release Behavior
2.9.1. Encapsulation Efficiency (EE)
2.9.2. In Vitro Release Behavior
2.10. Statistical Analysis
3. Results and Discussions
3.1. Texture Property
3.2. Rheology Analysis
3.3. The Self-Healing Ability of LMPH
3.3.1. Macroscopic Morphology of LMPH Self-Healing Process
3.3.2. Time Sweep
3.3.3. FT-IR
3.4. SEM
3.5. Water Hold Capacity and Swelling Rate
3.5.1. WHC
3.5.2. Swelling Rate
3.6. Stability
3.6.1. Differential Scanning Calorimetry (DSC)
3.6.2. Freeze–Thaw Stability
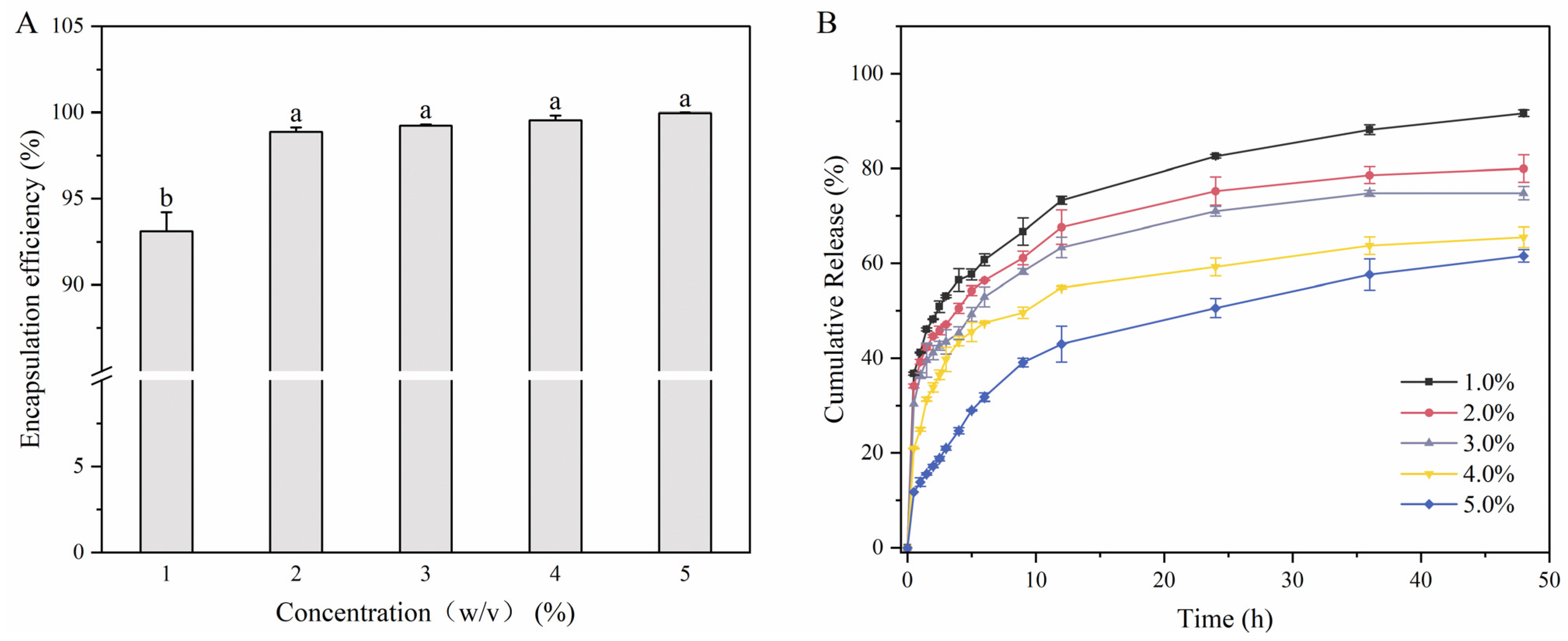
3.7. In Vitro Release Behavior
3.7.1. Encapsulation Efficiency (EE)
3.7.2. In Vitro Release Behavior of Polyphenols from Rambutan Peel Polyphenols
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Solís-Fuentes, J.A.; Camey-Ortíz, G.; Hernández-Medel, M.d.R.; Pérez-Mendoza, F.; Durán-de-Bazúa, C. Composition, phase behavior and thermal stability of natural edible fat from rambutan (Nephelium lappaceum L.) seed. Bioresour. Technol. 2010, 101, 799–803. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Hernández, C.; Aguilar, C.N.; Rodríguez-Herrera, R.; Flores-Gallegos, A.C.; Morlett-Chávez, J.; Govea-Salas, M.; Ascacio-Valdés, J.A. Rambutan (Nephelium lappaceum L.): Nutritional and functional properties. Trends Food Sci. Technol. 2019, 85, 201–210. [Google Scholar] [CrossRef]
- Phuong, N.N.M.; Le, T.T.; Dang, M.Q.; Van Camp, J.; Raes, K. Selection of extraction conditions of phenolic compounds from rambutan (Nephelium lappaceum L.) peel. Food Bioprod. Process. 2020, 122, 222–229. [Google Scholar] [CrossRef]
- Xiao, J.; Liu, B.; Zhuang, Y. Effects of rambutan (Nephelium lappaceum) peel phenolics and Leu-Ser-Gly-Tyr-Gly-Pro on hairless mice skin photoaging induced by ultraviolet irradiation. Food Chem. Toxicol. 2019, 129, 30–37. [Google Scholar] [CrossRef]
- Heleno, S.A.; Martins, A.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. Bioactivity of phenolic acids: Metabolites versus parent compounds: A review. Food Chem. 2015, 173, 501–513. [Google Scholar] [CrossRef]
- Hu, B.; Liu, X.; Zhang, C.; Zeng, X. Food macromolecule based nanodelivery systems for enhancing the bioavailability of polyphenols. J. Food Drug Anal. 2017, 25, 3–15. [Google Scholar] [CrossRef]
- Siddiqui, I.A.; Adhami, V.M.; Bharali, D.J.; Hafeez, B.B.; Asim, M.; Khwaja, S.I.; Ahmad, N.; Cui, H.; Mousa, S.A.; Mukhtar, H. Introducing Nanochemoprevention as a Novel Approach for Cancer Control: Proof of Principle with Green Tea Polyphenol Epigallocatechin-3-Gallate. Cancer Res. 2009, 69, 1712–1716. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, H.; Xu, C.; Gu, L. A review: Using nanoparticles to enhance absorption and bioavailability of phenolic phytochemicals. Food Hydrocoll. 2015, 43, 153–164. [Google Scholar] [CrossRef]
- Yang, M.; Zhao, S.; Zhao, C.; Cui, J.; Wang, Y.; Fang, X.; Zheng, J. Caseinate-reinforced pectin hydrogels: Efficient encapsulation, desirable release, and chemical stabilization of (−)-epigallocatechin. Int. J. Biol. Macromol. 2023, 230, 123298. [Google Scholar] [CrossRef]
- Yegappan, R.; Selvaprithiviraj, V.; Amirthalingam, S.; Jayakumar, R. Carrageenan based hydrogels for drug delivery, tissue engineering and wound healing. Carbohydr. Polym. 2018, 198, 385–400. [Google Scholar] [CrossRef] [PubMed]
- Münster, L.; Capáková, Z.; Fišera, M.; Kuřitka, I.; Vícha, J. Biocompatible dialdehyde cellulose/poly(vinyl alcohol) hydrogels with tunable properties. Carbohydr. Polym. 2019, 218, 333–342. [Google Scholar] [CrossRef]
- Yan, S.; Wu, S.; Zhang, J.; Zhang, S.; Huang, Y.; Zhu, H.; Li, Y.; Qi, B. Controlled release of curcumin from gelatin hydrogels by the molecular-weight modulation of an oxidized dextran cross-linker. Food Chem. 2023, 418, 135966. [Google Scholar] [CrossRef]
- Do, N.H.N.; Huynh, T.N.A.; Le, T.X.; Ha, A.C.; Le, P.K. Encapsulation of Triphasia trifolia extracts by pH and thermal dual-sensitive chitosan hydrogels for controlled release. Carbohydr. Polym. 2023, 320, 121264. [Google Scholar] [CrossRef]
- Samadian, H.; Maleki, H.; Allahyari, Z.; Jaymand, M. Natural polymers-based light-induced hydrogels: Promising biomaterials for biomedical applications. Coord. Chem. Rev. 2020, 420, 213432. [Google Scholar] [CrossRef]
- Hu, X.; Wang, Y.; Zhang, L.; Xu, M. Construction of self-assembled polyelectrolyte complex hydrogel based on oppositely charged polysaccharides for sustained delivery of green tea polyphenols. Food Chem. 2020, 306, 125632. [Google Scholar] [CrossRef]
- Yu, Q.; Chen, W.; Zhong, J.; Huang, D.; Shi, W.; Chen, H.; Yan, C. Purification, structural characterization, and bioactivities of a polysaccharide from Coreopsis tinctoria. Food Front. 2022, 3, 736–748. [Google Scholar] [CrossRef]
- Manzoor, A.; Dar, A.H.; Pandey, V.K.; Shams, R.; Khan, S.; Panesar, P.S.; Kennedy, J.F.; Fayaz, U.; Khan, S.A. Recent insights into polysaccharide-based hydrogels and their potential applications in food sector: A review. Int. J. Biol. Macromol. 2022, 213, 987–1006. [Google Scholar] [CrossRef]
- Peers, S.; Montembault, A.; Ladavière, C. Chitosan hydrogels incorporating colloids for sustained drug delivery. Carbohydr. Polym. 2022, 275, 118689. [Google Scholar] [CrossRef]
- Obaroakpo, J.U.; Liu, L.; Zhang, S.; Lu, J.; Liu, L.; Pang, X.; Lv, J. In vitro modulation of glucagon-like peptide release by DPP-IV inhibitory polyphenol-polysaccharide conjugates of sprouted quinoa yoghurt. Food Chem. 2020, 324, 126857. [Google Scholar] [CrossRef]
- Li, W.; Fang, K.; Yuan, H.; Li, D.; Li, H.; Chen, Y.; Luo, X.; Zhang, L.; Ye, X. Acid-induced Poria cocos alkali-soluble polysaccharide hydrogel: Gelation behaviour, characteristics, and potential application in drug delivery. Int. J. Biol. Macromol. 2023, 242, 124383. [Google Scholar] [CrossRef]
- Srivastava, N.; Richa; Roy Choudhury, A. Recent advances in composite hydrogels prepared solely from polysaccharides. Colloids Surf. B Biointerfaces 2021, 205, 111891. [Google Scholar] [CrossRef]
- Zhang, M.; Li, Y.; Wang, W.; Yang, Y.; Shi, X.; Sun, M.; Hao, Y.; Li, Y. Comparison of physicochemical and rheology properties of Shiitake stipes-derived chitin nanocrystals and nanofibers. Carbohydr. Polym. 2020, 244, 116468. [Google Scholar] [CrossRef]
- Wang, W.; Tan, J.; Nima, L.; Sang, Y.; Cai, X.; Xue, H. Polysaccharides from fungi: A review on their extraction, purification, structural features, and biological activities. Food Chem. X 2022, 15, 100414. [Google Scholar] [CrossRef]
- Zhang, R.; Tao, Y.; Xu, W.; Xiao, S.; Du, S.; Zhou, Y.; Hasan, A. Rheological and controlled release properties of hydrogels based on mushroom hyperbranched polysaccharide and xanthan gum. Int. J. Biol. Macromol. 2018, 120, 2399–2409. [Google Scholar] [CrossRef]
- Ma, X.; Yang, M.; He, Y.; Zhai, C.; Li, C. A review on the production, structure, bioactivities and applications of Tremella polysaccharides. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211000541. [Google Scholar] [CrossRef]
- Li, D.; Wei, Z.; Sun, J.; Xue, C. Tremella polysaccharides-coated zein nanoparticles for enhancing stability and bioaccessibility of curcumin. Curr. Res. Food Sci. 2022, 5, 611–618. [Google Scholar] [CrossRef]
- Menezes, T.M.F.; Campelo, M.d.S.; Lima, A.B.N.; Câmara Neto, J.F.; Saraiva, M.M.; de Sousa, J.A.C.; Gonzaga, M.L.d.C.; Leal, L.K.A.M.; Ribeiro, M.E.N.P.; Ricardo, N.M.P.S.; et al. Effects of polysaccharides isolated from mushrooms (Lentinus edodes Berk or Agaricus blazei Murill) on the gelation of Pluronic® F127. Colloids Surf. A Physicochem. Eng. Asp. 2022, 642, 128684. [Google Scholar] [CrossRef]
- Rodríguez-Seoane, P.; Torres, M.D.; González-Muñoz, M.J.; Sinde-Stompel, E.; Domínguez, H. Formulation of bio-hydrogels from Hericium erinaceus in Paulownia elongata x fortunei autohydrolysis aqueous extracts. Food Bioprod. Process. 2021, 128, 12–20. [Google Scholar] [CrossRef]
- Sun, L.; Su, X.; Zhuang, Y. Preparation, characterization and antiglycation activities of the novel polysaccharides from Boletus snicus. Int. J. Biol. Macromol. 2016, 92, 607–614. [Google Scholar] [CrossRef]
- Wang, M.; Yin, Z.; Sun, W.; Zhong, Q.; Zhang, Y.; Zeng, M. Microalgae play a structuring role in food: Effect of spirulina platensis on the rheological, gelling characteristics, and mechanical properties of soy protein isolate hydrogel. Food Hydrocoll. 2023, 136, 108244. [Google Scholar] [CrossRef]
- Hou, Y.; Zhao, J.; Yin, J.; Geng, F.; Nie, S. The synergistic gelation of Dendrobium officinale polysaccharide (Dendronans) with xanthan gum and its rheological and texture properties. Food Hydrocoll. 2023, 141, 108674. [Google Scholar] [CrossRef]
- Lin, R.; Xu, R.; Chen, H.; Liu, B.; Yuan, C.; Guo, L.; Cui, B.; Fang, Y. Dual cross-linked starch hydrogel for eugenol encapsulation and the formation of hydrogen bonds on textural hydrogel. Carbohydr. Polym. 2023, 316, 121044. [Google Scholar] [CrossRef]
- Wei, Z.; Yang, J.H.; Liu, Z.Q.; Xu, F.; Zhou, J.X.; Zrínyi, M.; Osada, Y.; Chen, Y.M. Novel Biocompatible Polysaccharide-Based Self-Healing Hydrogel. Adv. Funct. Mater. 2015, 25, 1352–1359. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Z.; Zhang, C.; Kang, W. Physicochemical properties and biological activities of Tremella hydrocolloids. Food Chem. 2023, 407, 135164. [Google Scholar] [CrossRef]
- Khalifa, I.; Li, M.; Mamet, T.; Li, C. Maltodextrin or gum Arabic with whey proteins as wall-material blends increased the stability and physiochemical characteristics of mulberry microparticles. Food Biosci. 2019, 31, 100445. [Google Scholar] [CrossRef]
- Tan, J.; Ma, Q.; Li, J.; Liu, Q.; Zhuang, Y. Bioavailability and Antioxidant Activity of Rambutan (Nephelium lappaceum) Peel Polyphenols during in Vitro Simulated Gastrointestinal Digestion, Caco-2 Monolayer Cell Model Application, and Colonic Fermentation. J. Agric. Food Chem. 2023, 71, 15829–15841. [Google Scholar] [CrossRef]
- Bhat, M.A.; Rather, R.A.; Shalla, A.H. Texture and rheological features of strain and pH sensitive chitosan-imine graphene-oxide composite hydrogel with fast self-healing nature. Int. J. Biol. Macromol. 2022, 222, 3129–3141. [Google Scholar] [CrossRef]
- Chang, A.; Ye, Z.; Ye, Z.; Deng, J.; Lin, J.; Wu, C.; Zhu, H. Citric acid crosslinked sphingan WL gum hydrogel films supported ciprofloxacin for potential wound dressing application. Carbohydr. Polym. 2022, 291, 119520. [Google Scholar] [CrossRef]
- Stenner, R.; Matubayasi, N.; Shimizu, S. Gelation of carrageenan: Effects of sugars and polyols. Food Hydrocoll. 2016, 54, 284–292. [Google Scholar] [CrossRef]
- Mahajan, P.; Bera, M.B.; Panesar, P.S. Modification of Kutki millet (Panicum sumatrense) starch properties by the addition of amino acids for the preparation of hydrogels and its characterization. Int. J. Biol. Macromol. 2021, 191, 9–18. [Google Scholar] [CrossRef]
- Teng, L.Y.; Chin, N.L.; Yusof, Y.A. Rheological and textural studies of fresh and freeze-thawed native sago starch–sugar gels. II. Comparisons with other starch sources and reheating effects. Food Hydrocoll. 2013, 31, 156–165. [Google Scholar] [CrossRef]
- Kongjaroen, A.; Methacanon, P.; Seetapan, N.; Fuongfuchat, A.; Gamonpilas, C.; Nishinari, K. Effects of dispersing media on the shear and extensional rheology of xanthan gum and guar gum-based thickeners used for dysphagia management. Food Hydrocoll. 2022, 132, 107857. [Google Scholar] [CrossRef]
- Li, M.; Cui, H.; Lou, W. Millettia speciosa Champ cellulose-based hydrogel as a novel delivery system for Lactobacillus paracasei: Its relationship to structure, encapsulation and controlled release. Carbohydr. Polym. 2023, 316, 121034. [Google Scholar] [CrossRef]
- Cui, T.; Wu, Y.; Ni, C.; Sun, Y.; Cheng, J. Rheology and texture analysis of gelatin/dialdehyde starch hydrogel carriers for curcumin controlled release. Carbohydr. Polym. 2022, 283, 119154. [Google Scholar] [CrossRef]
- Lin, X.; Zhao, X.; Xu, C.; Wang, L.; Xia, Y. Progress in the mechanical enhancement of hydrogels: Fabrication strategies and underlying mechanisms. J. Polym. Sci. 2022, 60, 2525–2542. [Google Scholar] [CrossRef]
- Xu, X.; Xu, J.; Zhang, Y.; Zhang, L. Rheology of triple helical Lentinan in solution: Steady shear viscosity and dynamic oscillatory behavior. Food Hydrocoll. 2008, 22, 735–741. [Google Scholar] [CrossRef]
- Feng, J.; Tian, H.; Chen, X.; Cai, X.; Shi, X.; Wang, S. Interaction between fish gelatin and tremella polysaccharides from aqueous solutions to complex coacervates: Structure and rheological properties. Food Hydrocoll. 2023, 138, 108439. [Google Scholar] [CrossRef]
- Zhu, H.; Xu, L.; Wang, J.; Zhang, Z.; Xu, X.; Yang, K.; Sun, P.; Liao, X.; Cai, M. Rheological behaviors of ethanol-fractional polysaccharides from Dendrobium officinale in aqueous solution: Effects of concentration, temperature, pH, and metal ions. Food Hydrocoll. 2023, 137, 108311. [Google Scholar] [CrossRef]
- Nair, R.; Roy Choudhury, A. Synthesis and rheological characterization of a novel shear thinning levan gellan hydrogel. Int. J. Biol. Macromol. 2020, 159, 922–930. [Google Scholar] [CrossRef]
- Hu, Y.; Li, C.; Tan, Y.; McClements, D.J.; Wang, L. Insight of rheology, water distribution and in vitro digestive behavior of starch based-emulsion gel: Impact of potato starch concentration. Food Hydrocoll. 2022, 132, 107859. [Google Scholar] [CrossRef]
- Lin, Y.; An, F.; He, H.; Geng, F.; Song, H.; Huang, Q. Structural and rheological characterization of pectin from passion fruit (Passiflora edulis f. flavicarpa) peel extracted by high-speed shearing. Food Hydrocoll. 2021, 114, 106555. [Google Scholar] [CrossRef]
- Wen, Y.; Che, Q.T.; Kim, H.W.; Park, H.J. Potato starch altered the rheological, printing, and melting properties of 3D-printable fat analogs based on inulin emulsion-filled gels. Carbohydr. Polym. 2021, 269, 118285. [Google Scholar] [CrossRef] [PubMed]
- Fitzsimons, S.M.; Tobin, J.T.; Morris, E.R. Synergistic binding of konjac glucomannan to xanthan on mixing at room temperature. Food Hydrocoll. 2008, 22, 36–46. [Google Scholar] [CrossRef]
- Ferraro, G.; Fratini, E.; Sacco, P.; Asaro, F.; Cuomo, F.; Donati, I.; Lopez, F. Structural characterization and physical ageing of mucilage from chia for food processing applications. Food Hydrocoll. 2022, 129, 107614. [Google Scholar] [CrossRef]
- Li, L.; Yan, J.-N.; Lai, B.; Wang, C.; Sun, J.-J.; Wu, H.-T. Rheological properties of chia seed gum extracted by high-speed shearing and its comparison with commercial polysaccharides. Food Hydrocoll. 2023, 144, 108936. [Google Scholar] [CrossRef]
- Shao, P.; Qin, M.; Han, L.; Sun, P. Rheology and characteristics of sulfated polysaccharides from chlorophytan seaweeds Ulva fasciata. Carbohydr. Polym. 2014, 113, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Chen, C.; Yang, K.; Wang, H.; Xia, H.; Zhao, Y.; Teng, Y.; Feng, G.; Chen, Y.M. Hyaluronic acid and chitosan-based injectable and self-healing hydrogel with inherent antibacterial and antioxidant bioactivities. Int. J. Biol. Macromol. 2023, 227, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Wen, L.; Ai, T.; Liang, H.; Li, J.; Li, B. A novel emulsion gel solely stabilized by the hot water extracted polysaccharide from psyllium husk: Self-healing plays a key role. Food Hydrocoll. 2022, 130, 107718. [Google Scholar] [CrossRef]
- Tong, C.; Jiang, S.; Ye, D.; Li, K.; Liu, J.; Zeng, X.; Wu, C.; Pang, J. Enhanced mechanical property and freeze-thaw stability of alkali-induced heat-set konjac glucomannan hydrogel through anchoring interface effects of carboxylated cellulose nanocrystals. Food Hydrocoll. 2023, 142, 108812. [Google Scholar] [CrossRef]
- Li, S.; Wang, L.; Zheng, W.; Yang, G.; Jiang, X. Rapid Fabrication of Self-Healing, Conductive, and Injectable Gel as Dressings for Healing Wounds in Stretchable Parts of the Body. Adv. Funct. Mater. 2020, 30, 2002370. [Google Scholar] [CrossRef]
- Ren, W.; Qiang, T.; Chen, L. Recyclable and biodegradable pectin-based film with high mechanical strength. Food Hydrocoll. 2022, 129, 107643. [Google Scholar] [CrossRef]
- Wang, C.; Jiang, X.; Kim, H.-J.; Zhang, S.; Zhou, X.; Chen, Y.; Ling, H.; Xue, Y.; Chen, Z.; Qu, M.; et al. Flexible patch with printable and antibacterial conductive hydrogel electrodes for accelerated wound healing. Biomaterials 2022, 285, 121479. [Google Scholar] [CrossRef] [PubMed]
- Needham, P. Hydrogen bonding: Homing in on a tricky chemical concept. Stud. Hist. Philos. Sci. Part A 2013, 44, 51–65. [Google Scholar] [CrossRef]
- Cao, J.; He, G.; Ning, X.; Wang, C.; Fan, L.; Yin, Y.; Cai, W. Hydroxypropyl chitosan-based dual self-healing hydrogel for adsorption of chromium ions. Int. J. Biol. Macromol. 2021, 174, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, G.; Peng, L.; Guo, J.; Tao, L.; Yuan, J.; Chang, C.; Wei, Y.; Zhang, L. Highly Efficient Self-Healable and Dual Responsive Cellulose-Based Hydrogels for Controlled Release and 3D Cell Culture. Adv. Funct. Mater. 2017, 27, 1703174. [Google Scholar] [CrossRef]
- Qi, X.; Tong, X.; You, S.; Mao, R.; Cai, E.; Pan, W.; Zhang, C.; Hu, R.; Shen, J. Mild Hyperthermia-Assisted ROS Scavenging Hydrogels Achieve Diabetic Wound Healing. ACS Macro Lett. 2022, 11, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Deka, R.; Sarmah, J.K.; Baruah, S.; Dutta, R.R. An okra polysaccharide (Abelmoschus esculentus) reinforced green hydrogel based on guar gum and poly-vinyl alcohol double network for controlled release of nanocurcumin. Int. J. Biol. Macromol. 2023, 234, 123618. [Google Scholar] [CrossRef] [PubMed]
- Ridolfo, R.; Tavakoli, S.; Junnuthula, V.; Williams, D.S.; Urtti, A.; van Hest, J.C.M. Exploring the Impact of Morphology on the Properties of Biodegradable Nanoparticles and Their Diffusion in Complex Biological Medium. Biomacromolecules 2020, 22, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Shi, D.; Zhang, Y.; Li, W.; Li, F.; Feng, H.; Ma, L.; Yang, C.; Peng, Z.; Song, G.; et al. An injectable hydrogel based on hyaluronic acid prepared by Schiff base for long-term controlled drug release. Int. J. Biol. Macromol. 2023, 245, 125341. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, M.; Zhao, Z. Facile fabrication of self-healing, injectable and antimicrobial cationic guar gum hydrogel dressings driven by hydrogen bonds. Carbohydr. Polym. 2023, 310, 120723. [Google Scholar] [CrossRef]
- He, X.; Zhao, H.; Xu, Y.; Yi, S.; Li, J.; Li, X. Synergistic effects of oat β-glucan combined with ultrasound treatment on gel properties of silver carp surimi. Ultrason. Sonochemistry 2023, 95, 106406. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Fu, Y.; Yang, F.; Chang, Z.; Zou, C.; Gao, H.; Jiang, D.; Jia, C. Effects of polysaccharides autoclave extracted from Flammulina velutipes mycelium on freeze-thaw stability of surimi gels. LWT 2022, 169, 113941. [Google Scholar] [CrossRef]
- Liu, M.; Chen, G.; Zhang, H.; Yu, Q.; Mei, X.; Kan, J. Heat-induced inulin-gluten gel: Insights into the influences of inulin molecular weight on the rheological and structural properties of gluten gel to molecular and physicochemical characteristics. Food Hydrocoll. 2021, 111, 106397. [Google Scholar] [CrossRef]
- Wan, L.; Yang, Z.; Cai, R.; Pan, S.; Liu, F.; Pan, S. Calcium-induced-gel properties for low methoxyl pectin in the presence of different sugar alcohols. Food Hydrocoll. 2021, 112, 106252. [Google Scholar] [CrossRef]
- Ma, Z.; Yang, X.; Ma, J.; Lv, J.; He, J.; Jia, D.; Qu, Y.; Chen, G.; Yan, H.; Zeng, R. Development of the mussel-inspired pH-responsive hydrogel based on Bletilla striata polysaccharide with enhanced adhesiveness and antioxidant properties. Colloids Surf. B Biointerfaces 2021, 208, 112066. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Wu, J.; Pang, M.; Hu, D.; Li, Z.; Wang, X.; Sun, L.; Chen, X.; Yao, J. Novel carboxymethyl chitosan/N-acetylneuraminic acid hydrogel for the protection of Pediococcus pentosaceus. Food Res. Int. 2022, 156, 111355. [Google Scholar] [CrossRef] [PubMed]
- Lv, D.; Chen, F.; Yang, X.; Yin, L.; Rashid, M.T.; Li, Y. Spontaneous gelation behaviors and mechanism of Ficus awkeotsang Makino pectin. Int. J. Biol. Macromol. 2023, 247, 125712. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Chen, D.; Patel, B.; Campanella, O.H. Pectin as a natural agent for reinforcement of pea protein gel. Carbohydr. Polym. 2022, 298, 120038. [Google Scholar] [CrossRef]
- Milan, E.P.; Martins, V.C.A.; Horn, M.M.; Plepis, A.M.G. Influence of blend ratio and mangosteen extract in chitosan/collagen gels and scaffolds: Rheological and release studies. Carbohydr. Polym. 2022, 292, 119647. [Google Scholar] [CrossRef]
- Trębacz, H.; Szczęsna, A.; Arczewska, M. Thermal stability of collagen in naturally ageing and in vitro glycated rabbit tissues. J. Therm. Anal. Calorim. 2018, 134, 1903–1911. [Google Scholar] [CrossRef]
- Zou, J.; Wang, L.; Sun, G. Sustainable and Reusable Gelatin-Based Hydrogel “Jelly Ice Cubes” as Food Coolant. II: Ideal Freeze–Thaw Conditions. ACS Sustain. Chem. Eng. 2021, 9, 15365–15374. [Google Scholar] [CrossRef]
- Zhang, B.; Bai, B.; Pan, Y.; Li, X.-M.; Cheng, J.-S.; Chen, H.-Q. Effects of pectin with different molecular weight on gelatinization behavior, textural properties, retrogradation and in vitro digestibility of corn starch. Food Chem. 2018, 264, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Xu, D.; Cui, B.; Wang, Y. Gelation of κ-carrageenan/Konjac glucommanan compound gel: Effect of cyclodextrins. Food Hydrocoll. 2019, 87, 158–164. [Google Scholar] [CrossRef]
- Gadkari, P.V.; Tu, S.; Chiyarda, K.; Reaney, M.J.T.; Ghosh, S. Rheological characterization of fenugreek gum and comparison with other galactomannans. Int. J. Biol. Macromol. 2018, 119, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lei, F.; He, L.; Xu, W.; Jiang, J. Comparative study on the monosaccharides of three typical galactomannans hydrolyzed by different methods. Ind. Crops Prod. 2020, 157, 112895. [Google Scholar] [CrossRef]
- Zavareh, H.S.; Pourmadadi, M.; Moradi, A.; Yazdian, F.; Omidi, M. Chitosan/carbon quantum dot/aptamer complex as a potential anticancer drug delivery system towards the release of 5-fluorouracil. Int. J. Biol. Macromol. 2020, 165, 1422–1430. [Google Scholar] [CrossRef]



| Sample | Hardness (N) | Chewiness (N) | Cohesiveness | Springiness (mm) | Resilience |
|---|---|---|---|---|---|
| LMPH1 | 0.17 ± 0.02 d | 8.59 ± 0.62 d | 0.92 ± 0.06 b | 0.55 ± 0.04 a | 0.26 ± 0.04 b |
| LMPH2 | 0.26 ± 0.01 d | 15.53 ± 1.89 d | 1.05 ± 0.06 a | 0.56 ± 0.06 a | 0.27 ± 0.02 b |
| LMPH3 | 0.61 ± 0.05 c | 39.03 ± 6.28 c | 1.07 ± 0.04 a | 0.60 ± 0.11 a | 0.31 ± 0.03 ab |
| LMPH4 | 0.90 ± 0.06 b | 62.29 ± 4.39 b | 1.09 ± 0.03 a | 0.63 ± 0.03 a | 0.35 ± 0.04 a |
| LMPH5 | 1.13 ± 0.18 a | 82.55 ± 8.39 a | 1.13 ± 0.09 a | 0.66 ± 0.06 a | 0.27 ± 0.05 b |
| Concentration | Water Separation Rate (%) | ||||
|---|---|---|---|---|---|
| Cycle 1 | Cycle 2 | Cycle 3 | Cycle 4 | Cycle 5 | |
| LMPH1 | 1.26 ± 0.09 | 12.0 ± 1.89 | 34.65 ± 2.33 a | 21.73 ± 1.42 a | 12.25 ± 1.26 |
| LMPH2 | ND | 1.61 ± 0.14 | 21.64 ± 2.18 b | 4.57 ± 0.72 b | ND |
| LMPH3 | ND | ND | 10.97 ± 0.11 c | 1.21 ± 0.21 c | ND |
| LMPH4 | ND | ND | 4.28 ± 0.12 d | 0.28 ± 0.03 c | ND |
| LMPH5 | ND | ND | 0.57 ± 0.19 d | ND | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L.; Li, J.; Ding, Y.; Sun, L. Preparation and Characterization of a Novel Longzhua mushroom Polysaccharide Hydrogel and Slow-Release Behavior of Encapsulated Rambutan Peel Polyphenols. Foods 2024, 13, 1711. https://doi.org/10.3390/foods13111711
Zhao L, Li J, Ding Y, Sun L. Preparation and Characterization of a Novel Longzhua mushroom Polysaccharide Hydrogel and Slow-Release Behavior of Encapsulated Rambutan Peel Polyphenols. Foods. 2024; 13(11):1711. https://doi.org/10.3390/foods13111711
Chicago/Turabian StyleZhao, Lingxin, Jiapeng Li, Yangyue Ding, and Liping Sun. 2024. "Preparation and Characterization of a Novel Longzhua mushroom Polysaccharide Hydrogel and Slow-Release Behavior of Encapsulated Rambutan Peel Polyphenols" Foods 13, no. 11: 1711. https://doi.org/10.3390/foods13111711





