Exploring the Nutritional Impact of Sourdough Fermentation: Its Mechanisms and Functional Potential
Abstract
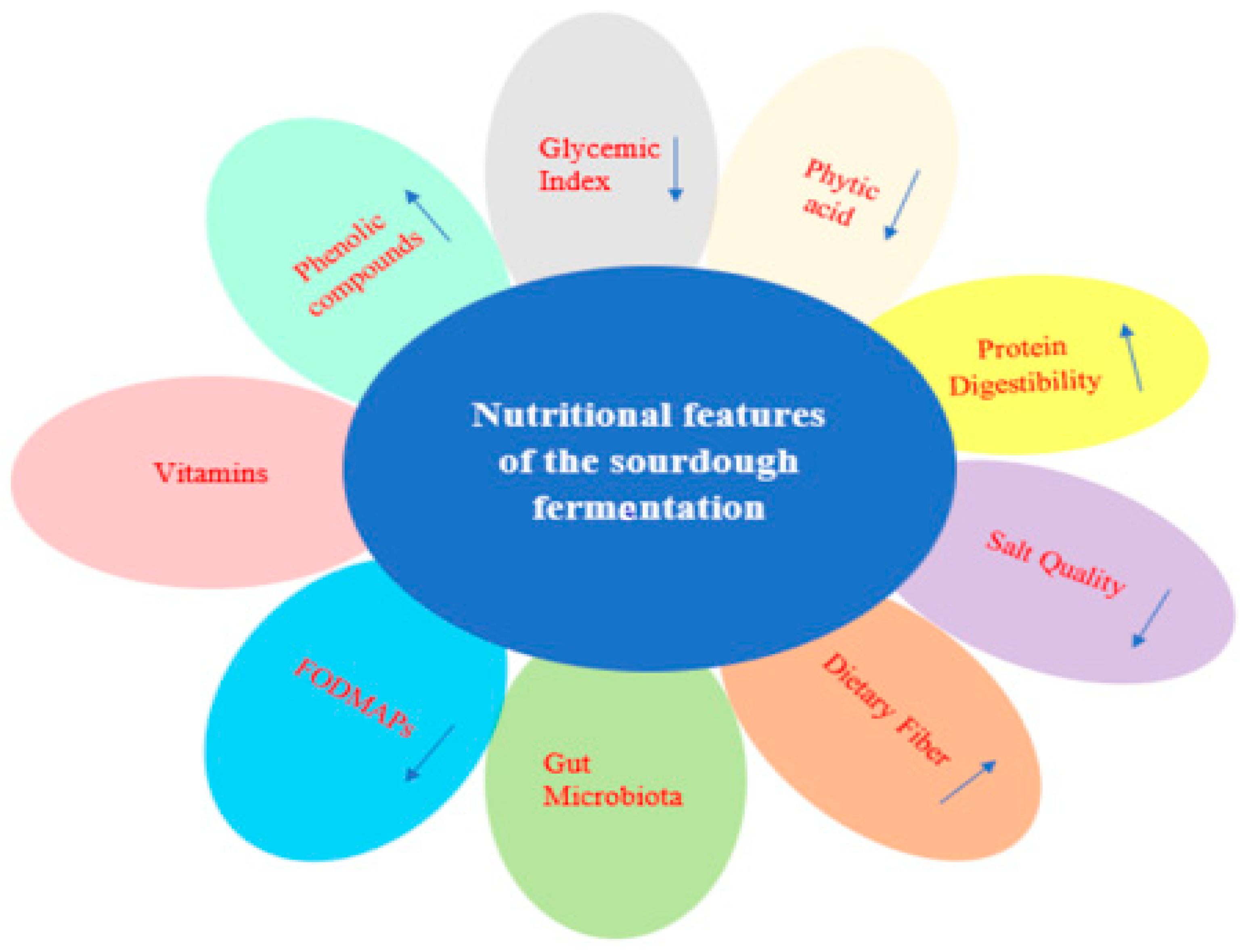
:1. Introduction
2. Nutritional Functionality of Sourdough
2.1. Mineral Bioavailability and Phytic Acid
2.2. Effect on Starch Digestibility and Glycemic Index of Sourdough Fermentation
- ✓
- Its metabolic effects are generally related to the ratio of glucose that is absorbed from the small bowel.
- ✓
- The ratio of glucose decreases after consumption of low-GI carbohydrate foods. For example, intestinal hormones such as incretins and insulin contribute to the reduced rate of absorption of increased postprandial glucose. Prolonged absorption of carbohydrates over time maintains the repression of free fatty acids (FFAs) and counter-regulatory reactions, resulting in lower blood glucose concentrations.
- ✓
- A decrease in FFA concentrations over time and an increase in tissue insulinization and respiratory coefficients lead to faster withdrawal of glucose from the circulation. As a result, glucose absorption from the small intestine continues, but blood glucose concentrations return to baseline. Thus, the increase in postprandial blood glucose decreases with increasing blood glucose area above baseline [49].
2.3. Effect on Protein Digestibility
2.4. Salt Reduction Feature
2.5. Sourdough Fermentation and Dietary Fiber
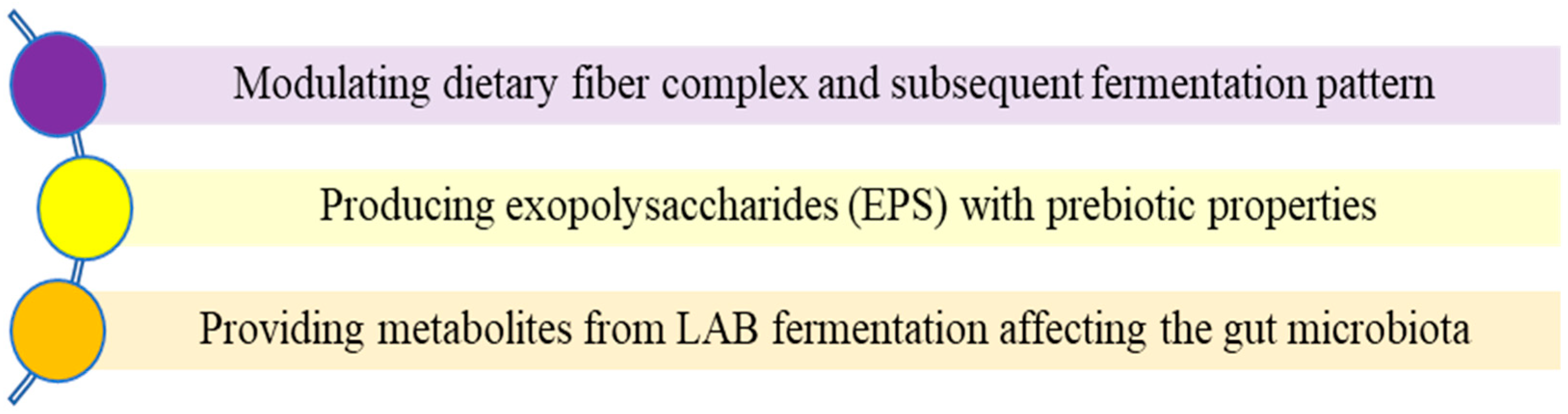
2.6. Sourdough Fermentation and Gut Microbiota
2.7. Sourdough Fermentation and FODMAPs
2.8. The Impact of Sourdough Fermentation on Vitamins
2.9. Sourdough Fermentation and Phenolic Compounds
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mohammadi-Kouchesfahani, M.; Hamidi-Esfahani, Z.; Azizi, M.H. Isolation and identification of lactic acid bacteria with phytase activity from sourdough. Food Sci. Nutr. 2019, 7, 3700–3708. [Google Scholar] [CrossRef]
- Lau, S.W.; Chong, A.Q.; Chin, N.L.; Talib, R.A.; Basha, R.K. Sourdough microbiome comparison and benefits. Microorganisms 2021, 9, 1355. [Google Scholar] [CrossRef] [PubMed]
- Cappelle, S.; Guylaine, L.; Gänzle, M.; Gobbetti, M. History and social aspects of sourdough. In Handbook on Sourdough Biotechnology; Springer International Publishing: Cham, Switzerland, 2023; pp. 1–13. [Google Scholar]
- De Vuyst, L.; Comasio, A.; Kerrebroeck, S.V. Sourdough production: Fermentation strategies, microbial ecology, and use of non-flour ingredients. Crit. Rev. Food Sci. Nutr. 2023, 63, 2447–2479. [Google Scholar] [CrossRef] [PubMed]
- Montemurro, M.; Coda, R.; Rizzello, C.G. Recent advances in the use of sourdough biotechnology in pasta making. Foods 2019, 8, 129. [Google Scholar] [CrossRef]
- Ma, S.; Wang, Z.; Guo, X.; Wang, F.; Huang, J.; Sun, B.; Wang, X. Sourdough improves the quality of whole-wheat flour products: Mechanisms and challenges—A review. Food Chem. 2020, 360, 130038. [Google Scholar] [CrossRef]
- Hulmé, D. The Use of White Bean Flour in Sourdough Bread: Effect on Nutritional Quality. Bachelor’s Thesis, Linnaeus University, Vaxjo, Sweden, 2022. [Google Scholar]
- Gobbetti, M.; Rizzello, C.G.; Di Cagno, R.; De Angelis, M. How the sourdough may affect the functional features of leavened baked goods. Food Microbiol. 2014, 37, 30–40. [Google Scholar] [CrossRef]
- Ameur, H.; Arora, K.; Polo, A.; Gobbetti, M. The sourdough microbiota and its sensory and nutritional performances. In Good Microbes in Medicine, Food Production, Biotechnology, Bioremediation, and Agriculture; Wiley & Sons: Hoboken, NJ, USA, 2022; pp. 169–184. [Google Scholar]
- Sakandar, H.A.; Hussain, R.; Kubow, S.; Sadiq, F.A.; Huang, W.; Imran, M. Sourdough bread: A contemporary cereal fermented product. J. Food Process. Preserv. 2019, 43, e13883. [Google Scholar] [CrossRef]
- Zahra, A.; Farooq, U.; Saeed, M.T.; Quddoos, M.Y.; Hameed, A.; Iftikhar, M.; Noreen, A.; Zahra, S.M.; Hussain, A.; Bukhari, S.R.; et al. Enhancement of sensory attributes and mineral content of Sourdough bread by means of microbial culture and yeast (Saccharomyces cerevisiae). Food Chem. Adv. 2022, 1, 100094. [Google Scholar] [CrossRef]
- Poutanen, K.; Flander, L.; Katina, K. Sourdough and cereal fermentation in a nutritional perspective. Food Microbiol. 2009, 26, 693–699. [Google Scholar] [CrossRef]
- Wang, R.; Guo, S. Phytic acid and its interactions: Contributions to protein functionality, food processing, and safety. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2081–2105. [Google Scholar] [CrossRef]
- Fernández-Peláez, J.; Paesani, C.; Gómez, M. Sourdough technology as a tool for the development of healthier grain-based products: An update. Agronomy 2020, 10, 1962. [Google Scholar] [CrossRef]
- Arora, K.; Ameur, H.; Polo, A.; Di Cagno, R.; Rizzello, C.G.; Gobbetti, M. Thirty years of knowledge on sourdough fermentation: A systematic review. Trends Food Sci. Technol. 2021, 108, 71–83. [Google Scholar] [CrossRef]
- Anastasio, M.; Pepe, O.; Cirillo, T.; Palomba, S.; Blaiotta, G.; Villani, F. Selection and use of phytate-degrading LAB to improve cereal-based products by mineral solubilization during dough fermentation. J. Food Sci. 2010, 75, M28–M35. [Google Scholar] [CrossRef] [PubMed]
- García-Estepa, R.M.; Guerra-Hernández, E.; García-Villanova, B. Phytic acid content in milled cereal products and breads. Food Res. Int. 1999, 32, 217–221. [Google Scholar] [CrossRef]
- Iqbal, T.H.; Lewis, K.O.; Cooper, B.T. Phytase activity in the human and rat small intestine. Gut 1994, 35, 1233–1236. [Google Scholar] [CrossRef]
- Schlemmer, U.; Frølich, W.; Prieto, R.M.; Grases, F. Phytate in foods and significance for humans: Food sources, intake, processing, bioavailability, protective role and analysis. Mol. Nutr. Food Res. 2009, 53, S330–S375. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, M.; Gallo, G.; Corbo, M.R.; McSweeney, P.L.; Faccia, M.; Giovine, M.; Gobbetti, M. Phytase activity in sourdough lactic acid bacteria: Purification and characterization of a phytase from Lactobacillus sanfranciscensis CB1. Int. J. Food Microbiol. 2003, 87, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Zamudio, M.; Gonzalez, A.; Medina, J.A. Lactobacillus plantarum phytase activity is due to non-specific acid phosphatase. Lett. Appl. Microbiol. 2001, 32, 181–184. [Google Scholar] [CrossRef]
- Nuobariene, L.; Cizeikiene, D.; Gradzeviciute, E.; Hansen, Å.S.; Rasmussen, S.K.; Juodeikiene, G.; Vogensen, F.K. Phytase-active lactic acid bacteria from sourdoughs: Isolation and identification. LWT-Food Sci. Technol. 2015, 63, 766–772. [Google Scholar] [CrossRef]
- Fekri, A.; Torbati, M.; Khosrowshahi, A.Y.; Shamloo, H.B.; Azadmard-Damirchi, S. Functional effects of phytate-degrading, probiotic lactic acid bacteria and yeast strains isolated from Iranian traditional sourdough on the technological and nutritional properties of whole wheat bread. Food Chem. 2020, 306, 125620. [Google Scholar] [CrossRef]
- Yildirim, R.M.; Arici, M. Effect of the fermentation temperature on the degradation of phytic acid in whole-wheat sourdough bread. LWT 2019, 112, 108224. [Google Scholar] [CrossRef]
- Karaman, K.; Sagdic, O.; Durak, M.Z. Use of phytase active yeasts and lactic acid bacteria isolated from sourdough in the production of whole wheat bread. LWT 2018, 91, 557–567. [Google Scholar] [CrossRef]
- Shirai, K.; Revah-Moiseev, S.; García-Garibay, M.; Marshall, V.M. Ability of some strains of lactic acid bacteria to degrade phytic acid. Lett. Appl. Microbiol. 1994, 19, 366–369. [Google Scholar] [CrossRef]
- Lopez, H.W.; Ouvry, A.; Bervas, E.; Guy, C.; Messager, A.; Demigne, C.; Remesy, C. Strains of lactic acid bacteria isolated from sour doughs degrade phytic acid and improve calcium and magnesium solubility from whole wheat flour. J. Agric. Food Chem. 2000, 48, 2281–2285. [Google Scholar] [CrossRef] [PubMed]
- Preedy, V.R.; Watson, R.R. (Eds.) Flour and Breads and Their Fortification in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Türk, M.; Carlsson, N.G.; Sandberg, A.S. Reduction in the levels of phytate during wholemeal bread making; effect of yeast and wheat phytases. J. Cereal Sci. 1996, 23, 257–264. [Google Scholar] [CrossRef]
- Haros, M.; Rosell, C.M.; Benedito, C. Use of fungal phytase to improve breadmaking performance of whole wheat bread. J. Agric. Food Chem. 2001, 49, 5450–5454. [Google Scholar] [CrossRef]
- Rickard, S.E.; Thompson, L.U. Interactions and Biological Effects of Phytic Acid; Shaidi, F., Ed.; Antinutrients and Phytochemicals in Food; American Chemical Society: Washington, DC, USA, 1997; pp. 294–312. [Google Scholar]
- Reddy, N.R. Occurrence, distribution, content, and dietary intake of phytate. In Food Phytates; CRC Press: Boca Raton, FL, USA, 2001; pp. 41–68. [Google Scholar]
- Houssni, I.E.L.; Zahidi, A.; Khedid, K.; Hassikou, R. A Review of Spontaneous Sourdough as a Functional Ingredient for Improving the Sensory and Nutritional Quality of Wheat Bread. J. Mater. Environ. Sci. 2022, 13, 9–28. [Google Scholar]
- Aller, E.E.; Abete, I.; Astrup, A.; Martinez, J.A.; van Baak, M.A. Starches, sugars and obesity. Nutrients 2011, 3, 341–369. [Google Scholar] [CrossRef]
- Barclay, A.W.; Petocz, P.; McMillan-Price, J.; Flood, V.M.; Prvan, T.; Mitchell, P.; Brand-Miller, J.C. Glycemic index, glycemic load, and chronic disease risk—A meta-analysis of observational studies. Am. J. Clin. Nutr. 2008, 87, 627–637. [Google Scholar] [CrossRef]
- Björck, I.; Elmståhl, H.L. The glycaemic index: Importance of dietary fibre and other food properties. Proc. Nutr. Soc. 2003, 62, 201–206. [Google Scholar] [CrossRef]
- Fardet, A.; Leenhardt, F.; Lioger, D.; Scalbert, A.; Rémésy, C. Parameters controlling the glycaemic response to breads. Nutr. Res. Rev. 2006, 19, 18–25. [Google Scholar] [CrossRef]
- Maioli, M.; Pes, G.M.; Sanna, M.; Cherchi, S.; Dettori, M.; Manca, E.; Farris, G.A. Sourdough-leavened bread improves postprandial glucose and insulin plasma levels in subjects with impaired glucose tolerance. Acta Diabetol. 2008, 45, 91–96. [Google Scholar] [CrossRef]
- Katina, K.; Arendt, E.; Liukkonen, K.H.; Autio, K.; Flander, L.; Poutanen, K. Potential of sourdough for healthier cereal products. Trends Food Sci. Technol. 2005, 16, 104–112. [Google Scholar] [CrossRef]
- Demirkesen-Bicak, H.; Arici, M.; Yaman, M.; Karasu, S.; Sagdic, O. Effect of different fermentation condition on estimated glycemic index, in vitro starch digestibility, and textural and sensory properties of sourdough bread. Foods 2021, 10, 514. [Google Scholar] [CrossRef]
- Björck, I.; Granfeldt, Y.; Liljeberg, H.; Tovar, J.; Asp, N.G. Food properties affecting the digestion and absorption of carbohydrates. Am. J. Clin. Nutr. 1994, 59, 699S–705S. [Google Scholar] [CrossRef]
- Lauro, M.; Poutanen, K.; Forssell, P. Effect of partial gelatinization and lipid addition on α-amylolysis of barley starch granules. Cereal Chem. 2000, 77, 595–601. [Google Scholar] [CrossRef]
- Östman, E. Fermentation as a Means of Optimizing the Glycaemic Index-Food Mechanisms and Metabolic Merits with Emphasis on Lactic Acid in Cereal Products; Lund University: Lund, Sweden, 2003. [Google Scholar]
- Tsafrakidou, P.; Michaelidou, A.M.; Biliaderis, C.G. Fermented cereal-based products: Nutritional aspects, possible impact on gut microbiota and health implications. Foods 2020, 9, 734. [Google Scholar] [CrossRef]
- Șerban, L.R.; Păucean, A.; Man, S.M.; Chiş, M.S.; Mureşan, V. Ancient Wheat Species: Biochemical Profile and Impact on Sourdough Bread Characteristics—A Review. Processes 2021, 9, 2008. [Google Scholar] [CrossRef]
- Available online: www.glycemicindex.com.tr (accessed on 1 January 2024).
- Coulston, A.M.; Hollenbeck, C.B.; Swislocki, A.L.; Reaven, G.M. Effect of source of dietary carbohydrate on plasma glucose and insulin responses to mixed meals in subjects with NIDDM. Diabetes Care 1987, 10, 395–400. [Google Scholar] [CrossRef]
- Wolever, T.M.; Nuttall, F.Q.; Lee, R.; Wong, G.S.; Josse, R.G.; Csima, A.; Jenkins, D.J. Prediction of the relative blood glucose response of mixed meals using the white bread glycemic index. Diabetes Care 1985, 8, 418–428. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Kendall, C.W.; Augustin, L.S.; Franceschi, S.; Hamidi, M.; Marchie, A.; Jenkins, A.L.; Axelsen, M. Glycemic index: Overview of implications in health and disease. Am. J. Clin. Nutr. 2002, 76, 266S–273S. [Google Scholar] [CrossRef]
- Food and Agriculture Organization; World Health Organization. Carbohydrates in Human Nutrition, Report of a Joint FAO/WHO Expert Consultation, Rome, 14–18 April 1997; FAO/WHO: Rome, Italy, 1997. [Google Scholar]
- Bo, S.; Seletto, M.; Choc, A.; Ponzo, V.; Lezo, A.; Demagistris, A.; Evangelista, A.; Ciccone, G.; Bertolino, M.; Cassader, M.; et al. The acute impact of the intake of four types of bread on satiety and blood concentrations of glucose, insulin, free fatty acids, triglyceride and acylated ghrelin. A randomized controlled cross-over trial. Food Res. Int. 2017, 92, 40–47. [Google Scholar] [CrossRef]
- Gobbetti, M.; De Angelis, M.; Di Cagno, R.; Calasso, M.; Archetti, G.; Rizzello, C.G. Novel insights on the functional/nutritional features of the sourdough fermentation. Int. J. Food Microbiol. 2019, 302, 103–113. [Google Scholar] [CrossRef]
- Canesin, M.R.; Cazarin, C.B.B. Nutritional quality and nutrient bioaccessibility in sourdough bread. Curr. Opin. Food Sci. 2021, 40, 81–86. [Google Scholar] [CrossRef]
- Champ, M. Definition, analysis, physical and chemical characterization and intake of RS. In Proceedings of the Concluding Plenary Meeting of EURESTA: Including the Final Reports of the Working Groups; April 1994. European Flair-Concerted Action no. 11 (COST 911); Asp, N.-G., van Amelsvoort, J.M.M., Hautvast, J.G.A.J., Eds.; EURESTA: Wageningen, The Netherlands, 1994; pp. 1–11. [Google Scholar]
- Östman, E.M.; Nilsson, M.; Elmståhl, H.L.; Molin, G.; Björck, I.M.E. On the effect of lactic acid on blood glucose and insulin responses to cereal products: Mechanistic studies in healthy subjects and in vitro. J. Cereal Sci. 2002, 36, 339–346. [Google Scholar] [CrossRef]
- Brighenti, F.; Casiraghi, M.C.; Baggio, C. Resistant starch in the Italian diet. Br. J. Nutr. 1998, 80, 333–341. [Google Scholar]
- Liljeberg, H.; Åkerberg, A.; Björck, I. Resistant starch formation in bread as influenced by choice of ingredients or baking conditions. Food Chem. 1996, 56, 389–394. [Google Scholar] [CrossRef]
- Rolim, M.E.; Fortes, M.I.; Von Frankenberg, A.; Duarte, C.K. Consumption of sourdough bread and changes in the glycemic control and satiety: A systematic review. Crit. Rev. Food Sci. Nutr. 2024, 64, 801–806. [Google Scholar] [CrossRef]
- Najjar, A.M.; Parsons, P.M.; Duncan, A.M.; Robinson, L.E.; Yada, R.Y.; Graham, T.E. The acute impact of ingestion of breads of varying composition on blood glucose, insulin and incretins following first and second meals. Br. J. Nutr. 2008, 101, 391–398. [Google Scholar] [CrossRef]
- Novotni, D.; Ćurić, D.; Bituh, M.; Colić Barić, I.; Škevin, D.; Čukelj, N. Glycemic index and phenolics of partially-baked frozen bread with sourdough. Int. J. Food Sci. Nutr. 2011, 62, 26–33. [Google Scholar] [CrossRef]
- Wolter, A.; Hager, A.S.; Zannini, E.; Arendt, E.K. In vitro starch digestibility and predicted glycaemic indexes of buckwheat, oat, quinoa, sorghum, teff and commercial gluten-free bread. J. Cereal Sci. 2013, 58, 431–436. [Google Scholar] [CrossRef]
- Wolter, A.; Hager, A.S.; Zannini, E.; Arendt, E.K. Influence of sourdough on in vitro starch digestibility and predicted glycemic indices of gluten-free breads. Food Funct. 2014, 5, 564–572. [Google Scholar] [CrossRef]
- Johansson, D.P.; Gutiérrez, J.L.V.; Landberg, R.; Alminger, M.; Langton, M. Impact of food processing on rye product properties and their in vitro digestion. Eur. J. Nutr. 2018, 57, 1651–1666. [Google Scholar] [CrossRef]
- Liljeberg, H.G.; Björck, I.M. Delayed gastric emptying rate as a potential mechanism for lowered glycemia after eating sourdough bread: Studies in humans and rats using test products with added organic acids or an organic salt. Am. J. Clin. Nutr. 1996, 64, 886–893. [Google Scholar] [CrossRef]
- Shumoy, H.; Van Bockstaele, F.; Devecioglu, D.; Raes, K. Effect of sourdough addition and storage time on in vitro starch digestibility and estimated glycemic index of tef bread. Food Chem. 2018, 264, 34–40. [Google Scholar] [CrossRef]
- Stamataki, N.S.; Yanni, A.E.; Karathanos, V.T. Bread making technology influences postprandial glucose response: A review of the clinical evidence. Br. J. Nutr. 2017, 117, 1001–1012. [Google Scholar] [CrossRef]
- Scazzina, F.; Siebenhandl-Ehn, S.; Pellegrini, N. The effect of dietary fibre on reducing the glycaemic index of bread. Br. J. Nutr. 2013, 109, 1163–1174. [Google Scholar] [CrossRef]
- Gil-Cardoso, K.; Saldana, G.; Luengo, E.; Pastor, J.; Virto, R.; Alcaide-Hidalgo, J.M.; del Bas, J.M.; Arola, L.; Caimari, A. Consumption of sourdough breads improves postprandial glucose response and produces sourdough-specific effects on biochemical and inflammatory parameters and mineral absorption. J. Agric. Food Chem. 2021, 69, 3044–3059. [Google Scholar] [CrossRef]
- Di Cagno, R.; De Angelis, M.; Lavermicocca, P.; De Vincenzi, M.; Giovannini, C.; Faccia, M.; Gobbetti, M. Proteolysis by sourdough lactic acid bacteria: Effects on wheat flour protein fractions and gliadin peptides involved in human cereal intolerance. Appl. Environ. Microbiol. 2002, 68, 623–633. [Google Scholar] [CrossRef]
- Pessione, E.; Cirrincione, S. Bioactive molecules released in food by lactic acid bacteria: Encrypted peptides and biogenic amines. Front. Microbiol. 2016, 7, 876. [Google Scholar] [CrossRef]
- Kopeć, A.; Pysz, M.; Borczak, B.; Sikora, E.; Rosell, C.M.; Collar, C.; Sikora, M. Effects of sourdough and dietary fibers on the nutritional quality of breads produced by bake-off technology. J. Cereal Sci. 2011, 54, 499–505. [Google Scholar] [CrossRef]
- Curiel, J.A.; Coda, R.; Centomani, I.; Summo, C.; Gobbetti, M.; Rizzello, C.G. Exploitation of the nutritional and functional characteristics of traditional Italian legumes: The potential of sourdough fermentation. Int. J. Food Microbiol. 2015, 196, 51–61. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Portincasa, P.; Montemurro, M.; Di Palo, D.M.; Lorusso, M.P.; De Angelis, M.; Bonfrate, L.; Genot, B.; Gobbetti, M. Sourdough fermented breads are more digestible than those started with baker’s yeast alone: An in vivo challenge dissecting distinct gastrointestinal responses. Nutrients 2019, 11, 2954. [Google Scholar] [CrossRef]
- Polo, A.; Arora, K.; Ameur, H.; Di Cagno, R.; De Angelis, M.; Gobbetti, M. Gluten-free diet and gut microbiome. J. Cereal Sci. 2020, 95, 103058. [Google Scholar] [CrossRef]
- Gaglio, R.; Alfonzo, A.; Barbera, M.; Franciosi, E.; Francesca, N.; Moschetti, G.; Settanni, L. Persistence of a mixed lactic acid bacterial starter culture during lysine fortification of sourdough breads by addition of pistachio powder. Food Microbiol. 2020, 86, 103349. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, L.; Qian, H.; Zhang, H.; Qi, X. Contribution of spontaneously-fermented sourdoughs with pear and navel orange for the bread-making. LWT 2018, 89, 336–343. [Google Scholar] [CrossRef]
- Siragusa, S.; De Angelis, M.; Di Cagno, R.; Rizzello, C.G.; Coda, R.; Gobbetti, M. Synthesis of γ-aminobutyric acid by lactic acid bacteria isolated from a variety of Italian cheeses. Appl. Environ. Microbiol. 2007, 73, 7283–7290. [Google Scholar] [CrossRef]
- Lorusso, A.; Coda, R.; Montemurro, M.; Rizzello, C.G. Use of selected lactic acid bacteria and quinoa flour for manufacturing novel yogurt-like beverages. Foods 2018, 7, 51. [Google Scholar] [CrossRef]
- Silow, C.; Axel, C.; Zannini, E.; Arendt, E.K. Current status of salt reduction in bread and bakery products–a review. J. Cereal Sci. 2016, 72, 135–145. [Google Scholar] [CrossRef]
- Zhao, C.J.; Kinner, M.; Wismer, W.; Gänzle, M.G. Effect of glutamate accumulation during sourdough fermentation with Lactobacillus reuteri on the taste of bread and sodium-reduced bread. Cereal Chem. 2015, 92, 224–230. [Google Scholar] [CrossRef]
- Ferreyra, L.S.; Verdini, R.A.; Soazo, M.; Piccirilli, G.N. Impact of whey protein addition on wheat bread fermented with a spontaneous sourdough. Int. J. Food Sci. Technol. 2021, 56, 4738–4745. [Google Scholar] [CrossRef]
- Hamaker, B.R.; Tuncil, Y.E. A perspective on the complexity of dietary fiber structures and their potential effect on the gut microbiota. J. Mol. Biol. 2014, 426, 3838–3850. [Google Scholar] [CrossRef] [PubMed]
- Raninen, K.; Lappi, J.; Mykkänen, H.; Poutanen, K. Dietary fiber type reflects physiological functionality: Comparison of grain fiber, inulin, and polydextrose. Nutr. Rev. 2011, 69, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Pontonio, E.; Lorusso, A.; Gobbetti, M.; Rizzello, C.G. Use of fermented milling by-products as functional ingredient to develop a low-glycaemic index bread. J. Cereal Sci. 2017, 77, 235–242. [Google Scholar] [CrossRef]
- Saa, D.T.; Di Silvestro, R.; Dinelli, G.; Gianotti, A. Effect of sourdough fermentation and baking process severity on dietary fibre and phenolic compounds of immature wheat flour bread. LWT-Food Sci. Technol. 2017, 83, 26–32. [Google Scholar] [CrossRef]
- Katina, K. Sourdough: A Tool for the Improved Flavour, Texture and Shelf-Life of Wheat Bread. Ph.D. Thesis, University of Helsinki, Helsinki, Finland, 2005. [Google Scholar]
- Elleuch, M.; Bedigian, D.; Roiseux, O.; Besbes, S.; Blecker, C.; Attia, H. Dietary fibre and fibre-rich by-products of food processing: Characterisation, technological functionality and commercial applications: A review. Food Chem. 2011, 124, 411–421. [Google Scholar] [CrossRef]
- Boskov Hansen, H.; Andreasen, M.; Nielsen, M.; Larsen, L.; Knudsen, B.K.; Meyer, A.; Christensen, L.; Hansen, Å. Changes in dietary fibre, phenolic acids and activity of endogenous enzymes during rye bread-making. Eur. Food Res. Technol. 2002, 214, 33–42. [Google Scholar] [CrossRef]
- Mihhalevski, A.; Nisamedtinov, I.; Hälvin, K.; Ošeka, A.; Paalme, T. Stability of B-complex vitamins and dietary fiber during rye sourdough bread production. J. Cereal Sci. 2013, 57, 30–38. [Google Scholar] [CrossRef]
- Pejcz, E.; Czaja, A.; Wojciechowicz-Budzisz, A.; Gil, Z.; Spychaj, R. The potential of naked barley sourdough to improve the quality and dietary fibre content of barley enriched wheat bread. J. Cereal Sci. 2017, 77, 97–101. [Google Scholar] [CrossRef]
- Tuukkanen, K.; Loponen, J.; Mikola, M.; Sontag-Strohm, T.; Salovaara, H. Degradation of secalins during rye sourdough fermentation. Cereal Chem. 2005, 82, 677–682. [Google Scholar] [CrossRef]
- Olojede, A.O.; Sanni, A.I.; Banwo, K. Effect of legume addition on the physiochemical and sensorial attributes of sorghum-based sourdough bread. LWT 2020, 118, 108769. [Google Scholar] [CrossRef]
- Çetin-Babaoğlu, H.; Arslan-Tontul, S.; Akın, N. Effect of immature wheat flour on nutritional and technological quality of sourdough bread. J. Cereal Sci. 2020, 94, 103000. [Google Scholar] [CrossRef]
- Das, S.; Pegu, K.; Arya, S.S. Functional sourdough millet bread rich in dietary fibre—An optimization study using fuzzy logic analysis. Bioact. Carbohydr. Diet. Fibre 2021, 26, 100279. [Google Scholar] [CrossRef]
- Olojede, A.O.; Sanni, A.I.; Banwo, K.; Michael, T. Improvement of Texture, Nutritional Qualities, and Consumers’ Perceptions of Sorghum-Based Sourdough Bread Made with Pediococcus pentosaceus and Weissella confusa Strains. Fermentation 2022, 8, 32. [Google Scholar] [CrossRef]
- Subaşı, A.S.; Ercan, R. The effects of wheat variety, sourdough treatment and sourdough level on nutritional characteristics of whole wheat bread. J. Cereal Sci. 2023, 110, 103637. [Google Scholar] [CrossRef]
- Parker, A.; Lawson, M.A.; Vaux, L.; Pin, C. Host-microbe interaction in the gastrointestinal tract. Environ. Microbiol. 2018, 20, 2337–2353. [Google Scholar] [CrossRef]
- Da Ros, A.; Polo, A.; Rizzello, C.G.; Acin-Albiac, M.; Montemurro, M.; Di Cagno, R.; Gobbetti, M. Feeding with sustainably sourdough bread has the potential to promote the healthy microbiota metabolism at the colon level. Microbiol. Spectr. 2021, 9, e00494-21. [Google Scholar] [CrossRef]
- Portune, K.J.; Benítez-Páez, A.; Del Pulgar, E.M.G.; Cerrudo, V.; Sanz, Y. Gut microbiota, diet, and obesity-related disorders—The good, the bad, and the future challenges. Mol. Nutr. Food Res. 2017, 61, 1600252. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef]
- Dahiya, D.; Nigam, P.S. The gut microbiota influenced by the intake of probiotics and functional foods with prebiotics can sustain wellness and alleviate certain ailments like gut-inflammation and colon-cancer. Microorganisms 2022, 10, 665. [Google Scholar] [CrossRef]
- Scazzina, F.; Del Rio, D.; Pellegrini, N.; Brighenti, F. Sourdough bread: Starch digestibility and postprandial glycemic response. J. Cereal Sci. 2009, 49, 419–421. [Google Scholar] [CrossRef]
- Korakli, M.; Gänzle, M.G.; Vogel, R.F. Metabolism by bifidobacteria and lactic acid bacteria of polysaccharides from wheat and rye, and exopolysaccharides produced by Lactobacillus sanfranciscensis. J. Appl. Microbiol. 2002, 92, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Schwab, C.; Mastrangelo, M.; Corsetti, A.; Gänzle, M. Formation of oligosaccharides and polysaccharides by Lactobacillus reuteri LTH5448 and Weissella cibaria 10M in sorghum sourdoughs. Cereal Chem. 2008, 85, 679–684. [Google Scholar] [CrossRef]
- Jann, A.; Arrigoni, E.; Rochat, F.; Schmid, D.; Bauche, A.U. S. Patent No. 7,091,194; U.S. Patent and Trademark Office: Washington, DC, USA, 2006. [Google Scholar]
- Abbondio, M.; Palomba, A.; Tanca, A.; Fraumene, C.; Pagnozzi, D.; Serra, M.; Marongiu, F.; Laconi, E.; Uzzau, S. Fecal metaproteomic analysis reveals unique changes of the gut microbiome functions after consumption of sourdough Carasau bread. Front. Microbiol. 2019, 10, 1733. [Google Scholar] [CrossRef]
- Van Baarlen, P.; Troost, F.J.; van Hemert, S.; van der Meer, C.; de Vos, W.M.; de Groot, P.J.; Hooiveld, G.J.; Brummer, R.J.M.; Kleerebezem, M. Differential NF-κB pathways induction by Lactobacillus plantarum in the duodenum of healthy humans correlating with immune tolerance. Proc. Natl. Acad. Sci. USA 2009, 106, 2371–2376. [Google Scholar] [CrossRef] [PubMed]
- Korem, T.; Zeevi, D.; Zmora, N.; Weissbrod, O.; Bar, N.; Lotan-Pompan, M.; Avnit-Sagi, T.; Kosower, N.; Malka, G.; Rein, M.; et al. Bread affects clinical parameters and induces gut microbiome-associated personal glycemic responses. Cell Metab. 2017, 25, 1243–1253. [Google Scholar] [CrossRef]
- Lopez, H.W.; Duclos, V.; Coudray, C.; Krespine, V.; Feillet-Coudray, C.; Messager, A.; Demigné, C.; Rémésy, C. Making bread with sourdough improves mineral bioavailability from reconstituted whole wheat flour in rats. Nutrition 2003, 19, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Arias, M.; Cobo, M.; Jaime-Sánchez, P.; Pastor, J.; Marijuan, P.; Pardo, J.; Rezusta, A.; Del Campo, R. Gut microbiota and systemic inflammation changes after bread consumption: The ingredients and the processing influence. J. Funct. Foods 2017, 32, 98–105. [Google Scholar] [CrossRef]
- Koistinen, V.M. Effects of Food Processing and Gut Microbial Metabolism on Whole Grain Phytochemicals: A Metabolomics Approach. Ph.D. Thesis, Itä-Suomen yliopisto, Kuopio, Finland, 2019. [Google Scholar]
- Kwon, J.G.; Park, S.H.; Kwak, J.E.; Cho, J.H.; Kim, G.; Lee, D.; Kim, D.H.; Kim, H.B.; Lee, J.H. Mouse feeding study and microbiome analysis of sourdough bread for evaluation of its health effects. Front. Microbiol. 2022, 13, 989421. [Google Scholar] [CrossRef]
- Whelan, K.; Abrahmsohn, O.; David, G.J.; Staudacher, H.; Irving, P.; Lomer, M.C.; Ellis, P.R. Fructan content of commonly consumed wheat, rye and gluten-free breads. Int. J. Food Sci. Nutr. 2011, 62, 498–503. [Google Scholar] [CrossRef]
- Yan, Y.L.; Hu, Y.; Gänzle, M.G. Prebiotics, FODMAPs and dietary fiber—Conflicting concepts in development of functional food products? Curr. Opin. Food Sci. 2018, 20, 30–37. [Google Scholar] [CrossRef]
- Gibson, P.R.; Shepherd, S.J. Evidence-based dietary management of functional gastrointestinal symptoms: The FODMAP approach. J. Gastroenterol. Hepatol. 2010, 25, 252–258. [Google Scholar] [CrossRef]
- Menezes, L.A.; Minervini, F.; Filannino, P.; Sardaro, M.L.; Gatti, M.; Lindner, J.D.D. Effects of sourdough on FODMAPs in bread and potential outcomes on irritable bowel syndrome patients and healthy subjects. Front. Microbiol. 2018, 9, 1972. [Google Scholar] [CrossRef] [PubMed]
- El-Salhy, M.; Gundersen, D.; Hatlebakk, J.G.; Hausken, T.; Watson, R.R. Diet and irritable bowel syndrome, with a focus on appetite-regulating hormones. In Nutrition in the Prevention and Treatment of Abdominal Obesity; Elsevier: San Diego, CA, USA, 2014; pp. 5–16. [Google Scholar]
- Shah, S.L.; Lacy, B.E. Dietary interventions and irritable bowel syndrome: A review of the evidence. Curr. Gastroenterol. Rep. 2016, 18, 41. [Google Scholar] [CrossRef]
- Biesiekierski, J.R.; Rosella, O.; Rose, R.; Liels, K.; Barrett, J.S.; Shepherd, S.J.; Gibson, P.R.; Muir, J.G. Quantification of fructans, galacto-oligosacharides and other short-chain carbohydrates in processed grains and cereals. J. Hum. Nutr. Diet. 2011, 24, 154–176. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, H.; Porter, J.; Gibson, P.R.; Barrett, J.; Garg, M. implementation of a diet low in FODMAPs for patients with irritable bowel syndrome—Directions for future research. Aliment. Pharmacol. Ther. 2019, 49, 124–139. [Google Scholar] [CrossRef]
- Pejcz, E.; Lachowicz-Wiśniewska, S.; Nowicka, P.; Wojciechowicz-Budzisz, A.; Spychaj, R.; Gil, Z. Effect of Inoculated Lactic Acid Fermentation on the Fermentable Saccharides and Polyols, Polyphenols and Antioxidant Activity Changes in Wheat Sourdough. Molecules 2021, 26, 4193. [Google Scholar] [CrossRef] [PubMed]
- Muir, J.G.; Varney, J.E.; Ajamian, M.; Gibson, P.R. Gluten-free and low-FODMAP sourdoughs for patients with coeliac disease and irritable bowel syndrome: A clinical perspective. Int. J. Food Microbiol. 2019, 290, 237–246. [Google Scholar] [CrossRef]
- Loponen, J.; Gänzle, M.G. Use of sourdough in low FODMAP baking. Foods 2018, 7, 96. [Google Scholar] [CrossRef]
- Struyf, N.; Laurent, J.; Verspreet, J.; Verstrepen, K.J.; Courtin, C.M. Saccharomyces cerevisiae and Kluyveromyces marxianus cocultures allow reduction of fermentable oligo-, di-, and monosaccharides and polyols levels in whole wheat bread. J. Agric. Food Chem. 2017, 65, 8704–8713. [Google Scholar]
- Laurent, J.; Struyf, N.; Bautil, A.; Bakeeva, A.; Chmielarz, M.; Lyly, M.; Herrera-Malaver, B.; Passoth, V.; Verstrepen, K.J.; Courtin, C.M. The Potential of Kluyveromyces marxianus to Produce Low-FODMAP Straight-Dough and Sourdough Bread: A Pilot-Scale Study. Food Bioprocess Technol. 2021, 14, 1920–1935. [Google Scholar] [CrossRef]
- Menezes, L.A.A.; Molognoni, L.; de Sá Ploêncio, L.A.; Costa, F.B.M.; Daguer, H.; Dea Lindner, J.D. Use of sourdough fermentation to reducing FODMAPs in breads. Eur. Food Res. Technol. 2019, 245, 1183–1195. [Google Scholar] [CrossRef]
- Schmidt, M.; Sciurba, E. Determination of FODMAP contents of common wheat and rye breads and the effects of processing on the final contents. Eur. Food Res. Technol. 2021, 247, 395–410. [Google Scholar] [CrossRef]
- Boakye, P.G.; Kougblenou, I.; Murai, T.; Okyere, A.Y.; Anderson, J.; Bajgain, P.; Philipp, B.; LaPlante, B.; Schlecht, S.; Vogel, C.; et al. Impact of sourdough fermentation on FODMAPs and amylase-trypsin inhibitor levels in wheat dough. J. Cereal Sci. 2022, 108, 103574. [Google Scholar] [CrossRef]
- Axel, C.; Röcker, B.; Brosnan, B.; Zannini, E.; Furey, A.; Coffey, A.; Arendt, E.K. Application of Lactobacillus amylovorus DSM19280 in gluten-free sourdough bread to improve the microbial shelf life. Food Microbiol. 2015, 47, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Amr, A.S.; Alkhamaiseh, A.M. Sourdough use in Bread Production. Jordan J. Agric. Sci. 2022, 18, 81–98. [Google Scholar] [CrossRef]
- Liukkonen, K.H.; Katina, K.; Wilhelmsson, A.; Myllymaki, O.; Lampi, A.M.; Kariluoto, S.; Piironen, V.; Heinonen, S.M.; Nurmi, T.; Adlercreutz, H.; et al. Process-induced changes on bioactive compounds in whole grain rye. Proc. Nutr. Soc. 2003, 62, 117–122. [Google Scholar] [CrossRef]
- Chawla, S.; Nagal, S. Sourdough in bread-making: An ancient technology to solve modern issues. Int. J. Ind. Biotechnol. Biomater. 2015, 1, 1–10. [Google Scholar]
- Sieuwerts, S.; Bron, P.A.; Smid, E.J. Mutually stimulating interactions between lactic acid bacteria and Saccharomyces cerevisiae in sourdough fermentation. LWT 2018, 90, 201–206. [Google Scholar] [CrossRef]
- Mutukumira, A.N.; Tian, H.; Rutherfurd-Markwick, K. Reducing FODMAPs in bread-the case for sourdough fermentation. Food N. Z. 2021, 21, 41–44. [Google Scholar]
- Verni, M.; Verardo, V.; Rizzello, C.G. How fermentation affects the antioxidant properties of cereals and legumes. Foods 2019, 8, 362. [Google Scholar] [CrossRef] [PubMed]
- Gänzle, M.G. Enzymatic and bacterial conversions during sourdough fermentation. Food Microbiol. 2014, 37, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Poojary, M.M.; Nguyen, T.D.; Dekiwadia, C.; Dias, D.A.; Huynh, T. Phenolic compounds-containing fruit peel extracts of Garcinia humilis exhibit anti-melanoma activity. Food Biosci. 2023, 52, 102428. [Google Scholar] [CrossRef]
- De Souza Silva, A.P.; de Camargo, A.C.; Lazarini, J.G.; Franchin, M.; Sardi, J.D.C.O.; Rosalen, P.L.; de Alencar, S.M. Phenolic Profile and the Antioxidant, Anti-Inflammatory, and Antimicrobial Properties of Açaí (Euterpe oleracea) Meal: A Prospective Study. Foods 2023, 12, 86. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, J.G.; de Ávila, P.M.; Schimitberger, R.; da Cunha, L.R.; Gomes, R.A.B.; Vieira, M.C.; Monteiro, R.D.S.; Vieira, S.M.; Pereira, P.A.P. Evaluation of the effect of substrates and types of wheat flour on microbiological characteristics, pH values, levels of total phenolic compounds, antioxidant capacity and fermentative capacity of sourdough. Res. Soc. Dev. 2022, 11, e13211932401. [Google Scholar] [CrossRef]
- Zamora, R.; Hidalgo, F.J. Carbonyl-trapping abilities of 5-alkylresorcinols. Food Chem. 2022, 393, 133372. [Google Scholar] [CrossRef] [PubMed]
- Dapčević-Hadnađev, T.; Stupar, A.; Stevanović, D.; Škrobot, D.; Maravić, N.; Tomić, J.; Hadnađev, M. Ancient Wheat Varieties and Sourdough Fermentation as a Tool to Increase Bioaccessibility of Phenolics and Antioxidant Capacity of Bread. Foods 2022, 11, 3985. [Google Scholar] [CrossRef] [PubMed]
- Rizzello, C.G.; Coda, R.; Mazzacane, F.; Minervini, D.; Gobbetti, M. Micronized by-products from debranned durum wheat and sourdough fermentation enhanced the nutritional, textural and sensory features of bread. Food Res. Int. 2012, 46, 304–313. [Google Scholar] [CrossRef]
- Bei, Q.; Chen, G.; Liu, Y.; Zhang, Y.; Wu, Z. Improving phenolic compositions and bioactivity of oats by enzymatic hydrolysis and microbial fermentation. J. Funct. Foods 2018, 47, 512–520. [Google Scholar] [CrossRef]
- Melini, F.; Melini, V. Impact of fermentation on phenolic compounds and antioxidant capacity of quinoa. Fermentation 2021, 7, 20. [Google Scholar] [CrossRef]


| Reference | Flour Type | Microorganism | Total Dietary Fiber Ratio Determined as a Result |
|---|---|---|---|
| Saa et al. [85] | Kamut khorasan and durum wheat-grain flour (milky and entirely mature) | Lpb. plantarum, Fructilactobacillus sanfranciscensis, Levl. Brevis, and S. cerevisiae | Khorasan flour at immature phase using sour yeast fermentation at high temperatures resulted in (10.26 mg/100 g). Khorasan flour at entirely mature phase using sour yeast fermentation at high temperature resulted in (19.25 mg/100 g). Breads acquired with durum wheat flour at entirely mature phase using industrial fermentation at high temperatures resulted in (15.48 mg/100 g). |
| Pejcz et al. [90] | Wheat flour and a wheat–barley blen | Saccharomyces chevalieri, Lacticaseibacillus casei, and Levl. brevis | Barley sour yeast ended in a higher condensation of both dietary fiber and the arabinoxylans and β-glucan fractions compared to barley whole wheat. Total dietary fiber was 10%. |
| Olojede et al. [92] | Sorghum flour | Pediococcus pentosaceus SA8 and S. cerevisiae YC1 | The highest total dietary fiber amount (17.2%) was found in sour yeast bread made with P. pentosaceus SA8 and S. cerevisiae YC1 strains. |
| Çetin-Babaoğlu et al. [93] | Sourdough breads are prepared from immature wheat flour (26 and 36 days) | Liml. reuteri, Levl. brevis, Lbp. plantarum, Liml. Fermentum, and Lacticaseibacillus rhamnosus | Immature wheat sourdough bread (26 day) resulted inm2.18%. Immature wheat sourdough bread (36 day) resulted in 2.10% |
| Das et al. [94] | Millet flours such as kodo, barn, small, and foxtail were used for sourdough bread production | Sourdough starter culture mix | Foxtail 20–50%, refined flour 65–35%, chickpea flour 10%, and tapioca flour 5%. |
| Olojede et al. [95] | Sorghum flour and corn starch were used | P. pentosaceus and Weissella confusa | The highest total dietary fiber amount (15.9%) was found in sourdough bread with P. pentosaceus, while the lowest total dietary fiber amount was observed in the control bread without sour yeast (13.25%). |
| Subaşı and Ercan [96] | Whole wheat flour (Tosunbey, Kenanbey, İkizce-96, Bezostaja-1) | Lpb. Plantarum and Fruc. sanfranciscensis | Whole wheat bread of Bezostaja-1 had the highest total dietary fiber amount (15.94%). |
| Human Studies | ||
|---|---|---|
| Reference | Type of Flour, Type of Sourdough Bread Made with LAB, and Number of Volunteers | Results for Gut Microbiota |
| Da Ros et al. [98] | Wheat flour, Lpb. plantarum CR1, Furl. rossiae CR5, and S. cerevisiae E10, 40 healthy volunteers | The amount of SCFAs and isovaleric and 2-methylbutyric acids increased. |
| Animal Studies | ||
| Reference | Type of Sourdough Bread and Type of Animal Used | Results for Gut Microbiota |
| Arias et al. [110] | Celta bread, S. cerevisiae, S. pastorianus, C. sakei, Lpb. paralimentarius, P. parvulus, Levl. Brevis, and Leu. citreum, 10 female 8-week-old C57BL/6 J mice. | In general, after bread ingestion, there was a significant reduction in the F. phylum relative to the baseline. There was a substantial rise in Bacteroidetes bacteria in the intestinal microbiota of the mice fed with the commercial bread. In the group fed with sour yeast bread in the Celta class, the main change was related to Verrucomicrobiaphylum. |
| Koistinen et al. [111] | Whole-grain wheat and whole-grain rye, C. milleri, Levl. Brevis, and Lpb. plantarum, C57BL/6 J male mice (n = 74) | It has been noted that diets enriched with bran produce a rise in the relative amount of a few bacterial taxa, like Akkermansia, Bifidobacterium, Coriobacteriaceae, Lactobacillus, Parasutterella, and Ruminococcus. |
| Kwon et al. [112] | Yeast-leavened white bread and sourdough bread, male C57BL/6 mice | Mice fed with sourdough bread showed a diabetes-lowering effect by decreasing the GI, owing to the existence of dietary fiber and SCFAs. Some useful bowel bacteria like Akkermansia, Bifidobacterium, and Lactobacillus were increased in mice in the sourdough bread-fed group. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkay, Z.; Falah, F.; Cankurt, H.; Dertli, E. Exploring the Nutritional Impact of Sourdough Fermentation: Its Mechanisms and Functional Potential. Foods 2024, 13, 1732. https://doi.org/10.3390/foods13111732
Alkay Z, Falah F, Cankurt H, Dertli E. Exploring the Nutritional Impact of Sourdough Fermentation: Its Mechanisms and Functional Potential. Foods. 2024; 13(11):1732. https://doi.org/10.3390/foods13111732
Chicago/Turabian StyleAlkay, Zuhal, Fereshteh Falah, Hasan Cankurt, and Enes Dertli. 2024. "Exploring the Nutritional Impact of Sourdough Fermentation: Its Mechanisms and Functional Potential" Foods 13, no. 11: 1732. https://doi.org/10.3390/foods13111732





