Optimizing Screw Speed and Barrel Temperature for Textural and Nutritional Improvement of Soy-Based High-Moisture Extrudates
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Proximate Composition of the Protein Ingredients
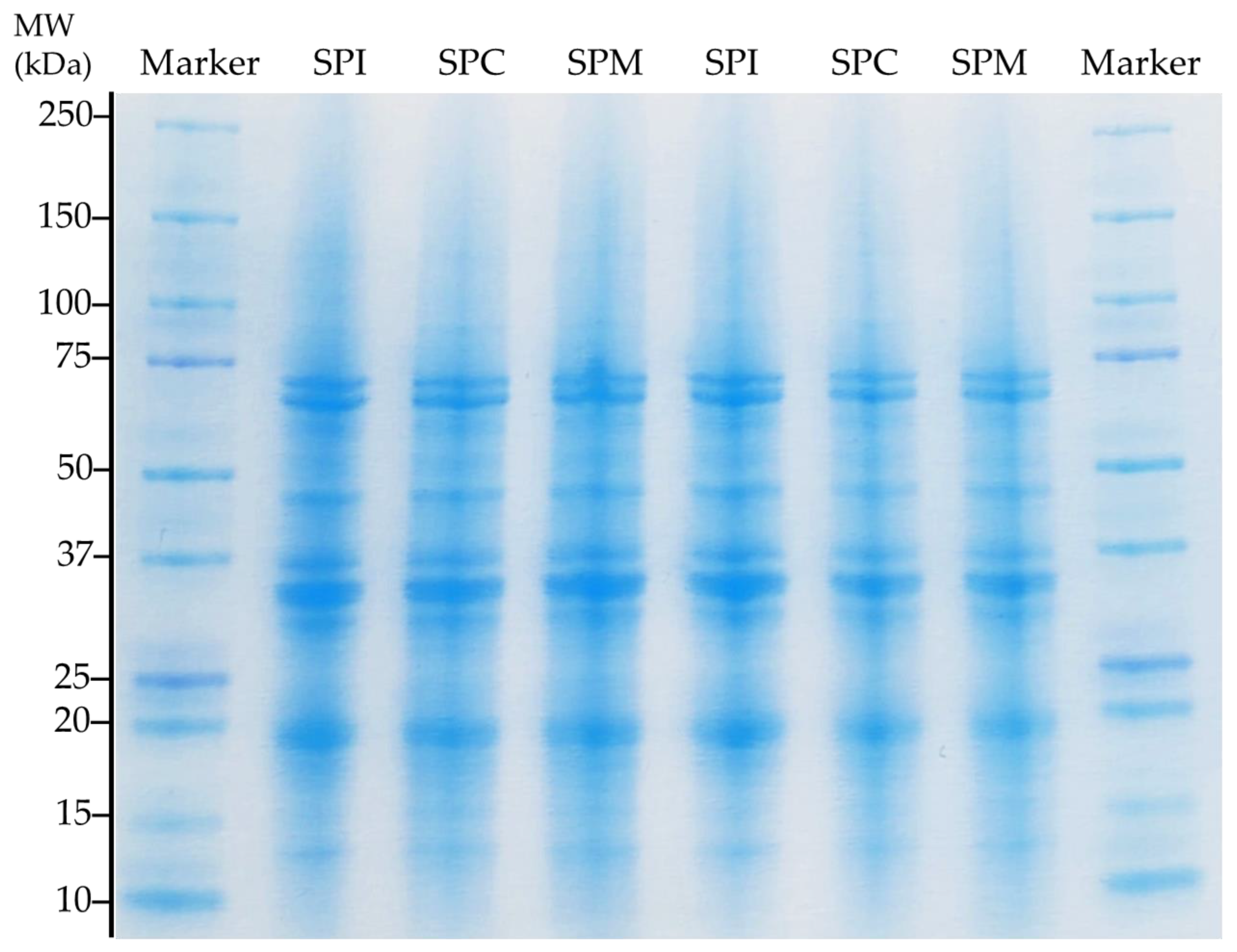
2.2.2. Protein Molecular Profile (SDS-PAGE)
2.2.3. Amino Acid Composition of the Protein Ingredients
2.2.4. Thermal Properties
2.2.5. Physicochemical Properties
Water-Binding and Oil-Binding Capacity
Least Gelation Concentration
Foaming and Emulsifying Properties
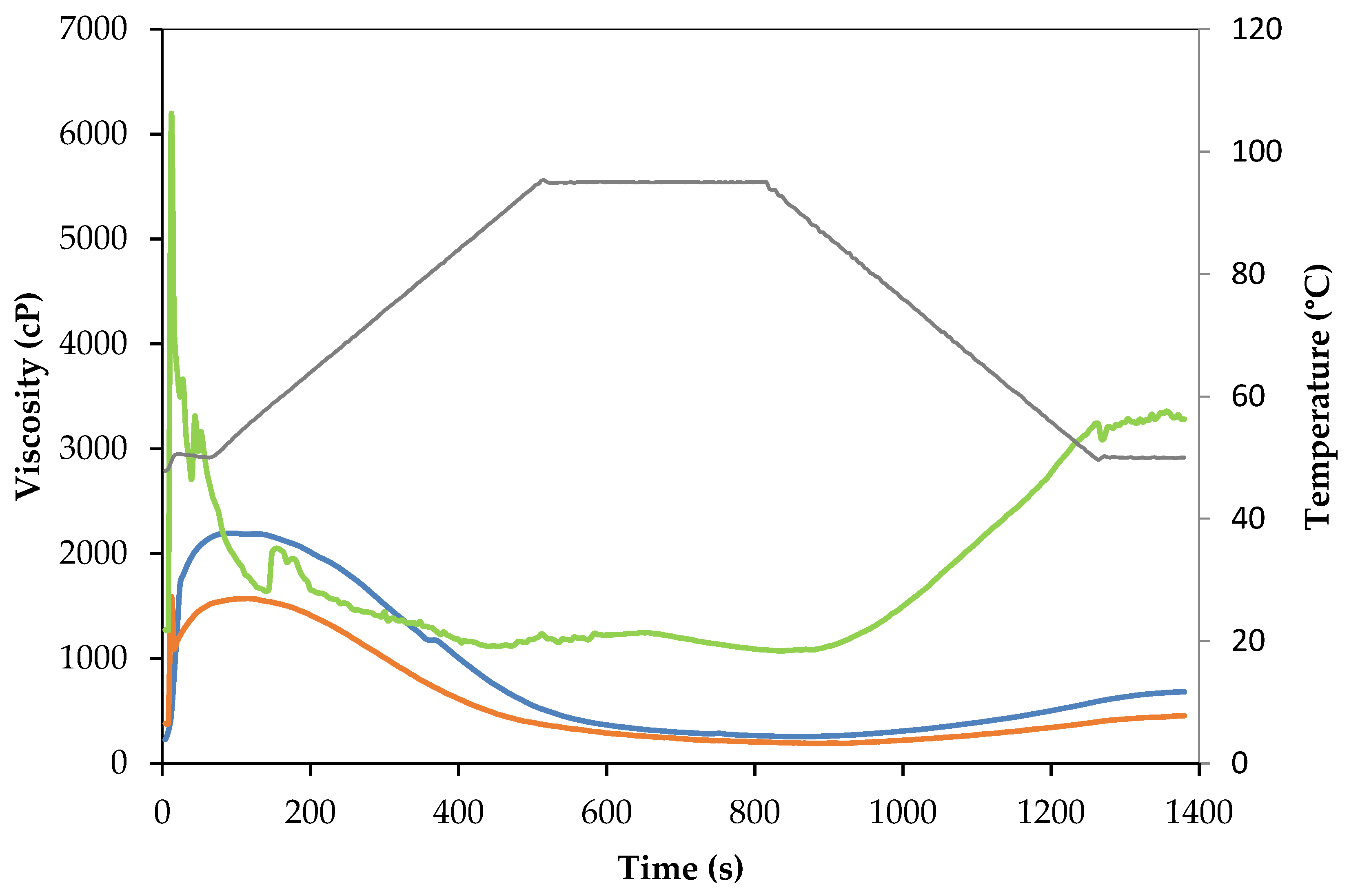
Pasting Properties
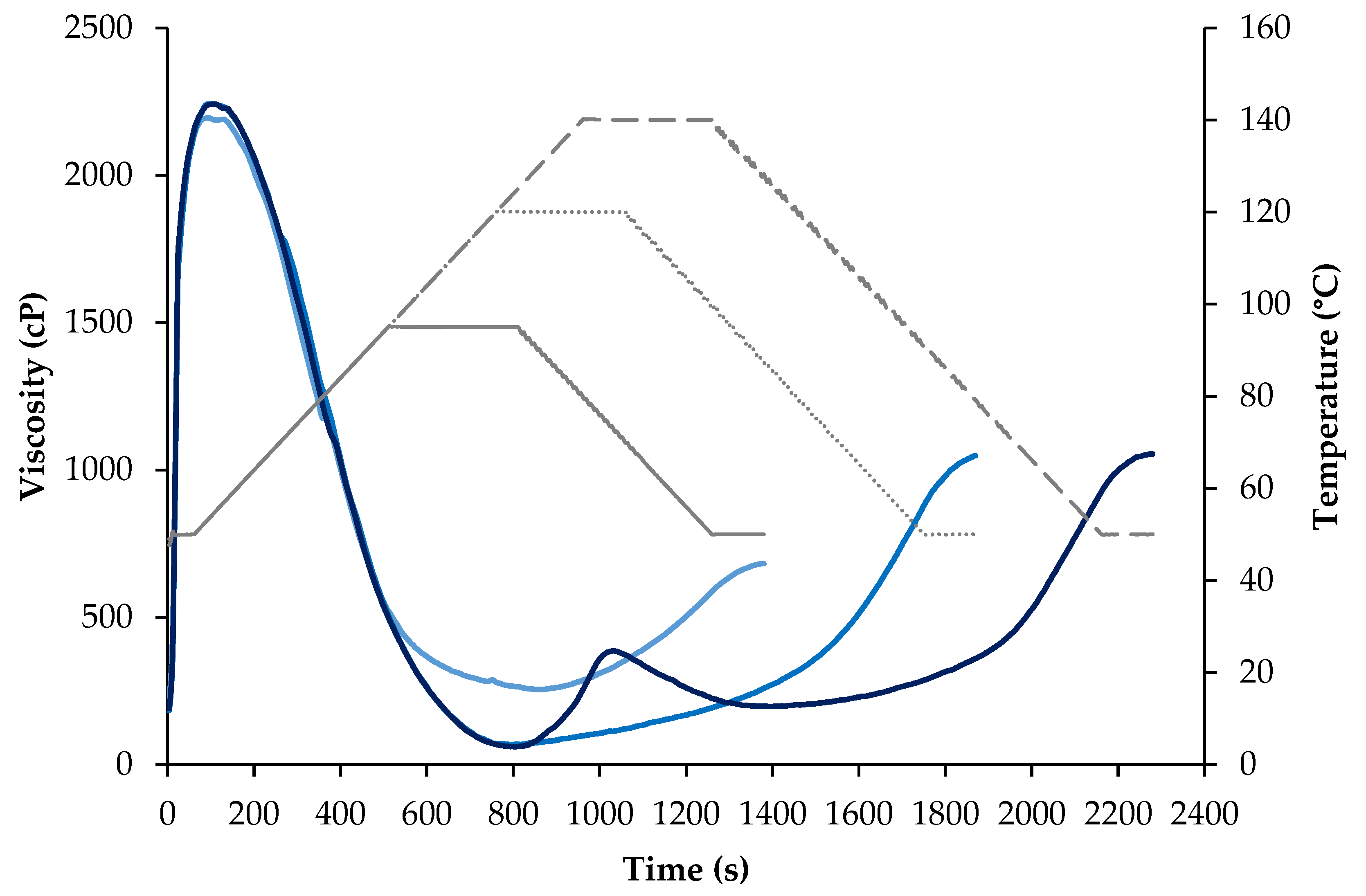
2.2.6. Extrusion Processing
2.2.7. Evaluation of the Visual, Textural, Nutritional, and Protein Structural Changes upon Extrusion
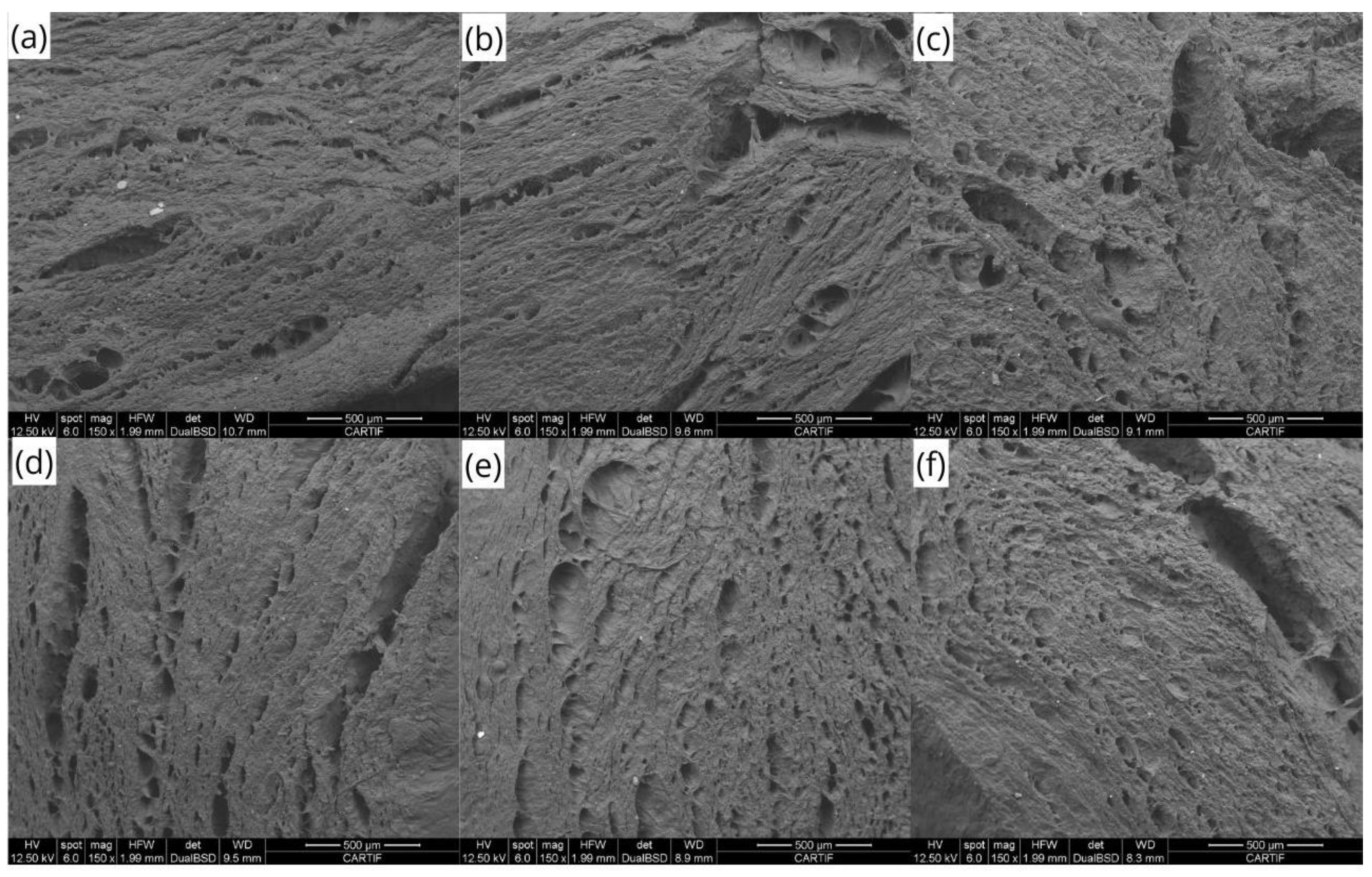
Macro- and Microscopical Evaluation
Color Evaluation
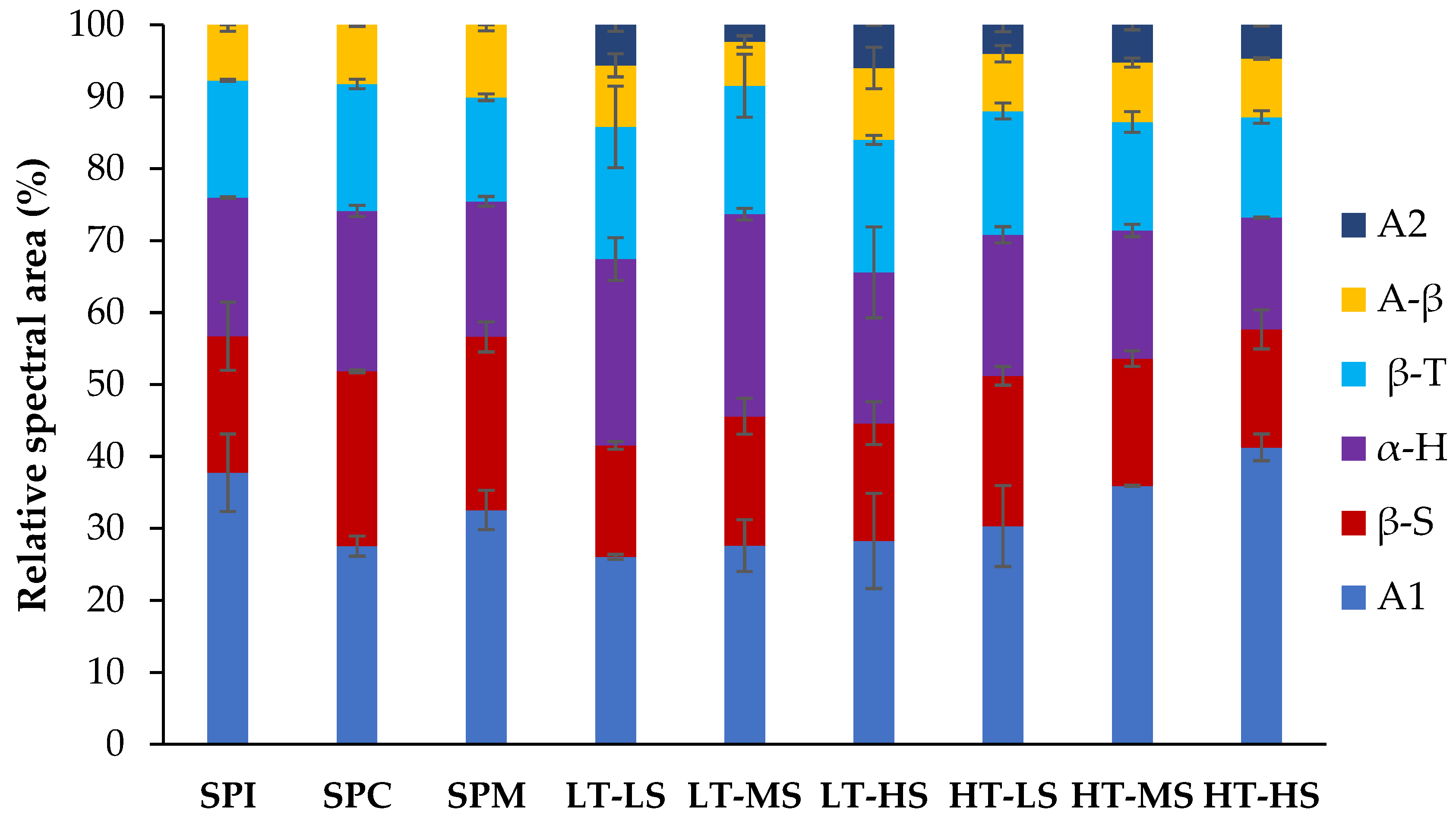
Protein Secondary Structure
Textural Properties
Antinutritional Factors (Trypsin Inhibitor Activity)
2.3. Statistical Analysis
3. Results and Discussion
3.1. Proximate Composition of Protein Powders
3.2. Protein Molecular Properties
| Amino Acid | SPI | SPC | SPM | Daily Requirement ** |
|---|---|---|---|---|
| Histidine * | 2.83 ± 0.28 a | 3.02 ± 0.25 a | 2.91 ± 0.25 a | 1.50 |
| Threonine * | 2.97 ± 0.23 a | 3.24 ± 0.25 b | 3.11 ± 0.25 a | 2.30 |
| Valine * | 3.12 ± 0.42 a | 3.26 ± 0.46 a | 3.19 ± 0.47 a | 3.90 |
| Methionine * | 1.33 ± 0.21 a | 1.36 ± 0.22 a | 1.38 ± 0.22 a | 2.20 *** |
| Phenylalanine * | 4.18 ± 0.24 a | 4.14 ± 0.25 a | 4.05 ± 0.25 a | 4.00 |
| Isoleucine * | 2.94 ± 0.30 a | 2.97 ± 0.33 a | 2.89 ± 0.33 a | 3.00 |
| Leucine * | 6.60 ± 1.02 a | 6.75 ± 1.75 a | 6.57± 1.26 a | 5.90 |
| Lysine * | 6.22 ± 0.14 a | 6.51 ± 0.11 b | 6.37 ± 0.11 ab | 4.50 |
| Tryptophan * | 0.15 ±0.70 a | 0.12 ±0.86 a | 0.11 ±0.94 a | 0.60 |
| Glycine | 3.69 ± 0.35 a | 3.89 ± 0.38 a | 3.77 ± 0.38 a | |
| Arginine | 5.87 ±1.27 a | 5.49 ± 0.92 a | 5.49 ± 0.95 a | |
| Tyrosine | 3.15 ± 0.8 a | 2.99 ± 0.7 a | 2.91 ± 0.8 a | |
| Cysteine | 0.88 ± 0.73 a | 1.00 ± 0.67 a | 0.98 ± 0.70 a | |
| Proline | 4.87 ± 0.56 a | 3.56 ± 0.51 b | 4.00 ± 0.25 ab | |
| Aspartic acid | 10.57 ± 1.69 a | 10.59 ± 1.81 a | 10.31 ± 1.76 a | |
| Glutamic acid | 16.93 ± 1.81 a | 16.46 ± 1.92 a | 16.16 ± 1.94 a | |
| Serine | 4.56 ± 0.43 a | 4.69 ± 0.75 a | 4.56 ± 0.55 a | |
| Alanine | 3.83 ± 0.40 a | 4.22 ± 0.42 a | 4.04 ± 0.43 a | |
| E/T (%) | 30.32 | 31.35 | 30.58 | |
| E/NE (%) | 57.44 | 59.93 | 58.54 |
3.3. Physicochemical Properties of Protein Powders
3.4. Extrusion Processing of Soy Protein Powders
3.4.1. Macro- and Microscopic Evaluation of Extruded Meat Analogs
3.4.2. Changes in Protein Secondary Structures upon Extrusion
3.4.3. Textural Properties of Extruded Meat Analogs
3.4.4. Reduction of Trypsin Inhibitors upon Extrusion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sha, L.; Xiong, Y.L. Plant Protein-Based Alternatives of Reconstructed Meat: Science, Technology, and Challenges. Trends Food Sci. Technol. 2020, 102, 51–61. [Google Scholar] [CrossRef]
- Springmann, M.; Clark, M.; Mason-D’Croz, D.; Wiebe, K.; Bodirsky, B.L.; Lassaletta, L.; de Vries, W.; Vermeulen, S.J.; Herrero, M.; Carlson, K.M.; et al. Options for Keeping the Food System within Environmental Limits. Nature 2018, 562, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Wang, Z.; Adhikari, B.; He, Z.; Zeng, M.; Qin, F.; Chen, J. Soluble and Insoluble Fractions of Soy Protein Isolate Affect the Properties of Its High-Moisture Extrudates. Food Biosci. 2024, 59, 103850. [Google Scholar] [CrossRef]
- Wittek, P.; Zeiler, N.; Karbstein, H.P.; Emin, M.A. High Moisture Extrusion of Soy Protein: Investigations on the Formation of Anisotropic Product Structure. Foods 2021, 10, 102. [Google Scholar] [CrossRef]
- Mateen, A.; Mathpati, M.; Singh, G. A Study on High Moisture Extrusion for Making Whole Cut Meat Analogue: Characterization of System, Process and Product Parameters. Innov. Food Sci. Emerg. Technol. 2023, 85, 103315. [Google Scholar] [CrossRef]
- Ryu, G.-H. Extrusion cooking of high-moisture meat analogues. In Extrusion Cooking; Woodhead Publishing: Cambridge, UK, 2020; pp. 205–224. [Google Scholar] [CrossRef]
- Nasrollahzadeh, F.; Alexi, N.; Skov, K.B.; Roman, L.; Sfyra, K.; Martinez, M.M. Texture Profiling of Muscle Meat Benchmarks and Plant-Based Analogues: An Instrumental and Sensory Design Approach with Focus on Correlations. Food Hydrocoll. 2024, 151, 109829. [Google Scholar] [CrossRef]
- Day, L.; Swanson, B.G. Functionality of Protein-Fortified Extrudates. Compr. Rev. Food Sci. Food Saf. 2013, 12, 546–564. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahzadeh, F.; Roman, L.; Skov, K.; Jakobsen, L.M.A.; Trinh, B.M.; Tsochatzis, E.D.; Mekonnen, T.; Corredig, M.; Dutcher, J.R.; Martinez, M.M. A Comparative Investigation of Seed Storage Protein Fractions: The Synergistic Impact of Molecular Properties and Composition on Anisotropic Structuring. Food Hydrocoll. 2023, 137, 108400. [Google Scholar] [CrossRef]
- Guerrero, P.; Beatty, E.; Kerry, J.P.; De La Caba, K. Extrusion of Soy Protein with Gelatin and Sugars at Low Moisture Content. J. Food Eng. 2012, 110, 53–59. [Google Scholar] [CrossRef]
- Wei, Y.; Kang, L.; Zhang, C. Food Extrusion Theory and Technology, 2nd ed.; China Light Industry Press: Beijing, China, 2009. [Google Scholar]
- Nasrollahzadeh, F.; Roman, L.; Swaraj, V.J.S.; Ragavan, K.V.; Vidal, N.P.; Dutcher, J.R.; Martinez, M.M. Hemp (Cannabis sativa L.) Protein Concentrates from Wet and Dry Industrial Fractionation: Molecular Properties, Nutritional Composition, and Anisotropic Structuring. Food Hydrocoll. 2022, 131, 107755. [Google Scholar] [CrossRef]
- Kyriakopoulou, K.; Dekkers, B.; van der Goot, A.J. Plant-Based Meat Analogues; Elsevier Inc.: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- McClements, I.F.; McClements, D.J. Designing Healthier Plant-Based Foods: Fortification, Digestion, and Bioavailability. Food Res. Int. 2023, 169, 112853. [Google Scholar] [CrossRef]
- Webb, D.; Dogan, H.; Li, Y.; Alavi, S. Physico-Chemical Properties and Texturization of Pea, Wheat and Soy Proteins Using Extrusion and Their Application in Plant-Based Meat. Foods 2023, 12, 1586. [Google Scholar] [CrossRef] [PubMed]
- Bakhsh, A.; Lee, E.Y.; Ncho, C.M.; Kim, C.J.; Son, Y.M.; Hwang, Y.H.; Joo, S.T. Quality Characteristics of Meat Analogs through the Incorporation of Textured Vegetable Protein: A Systematic Review. Foods 2022, 11, 1242. [Google Scholar] [CrossRef]
- Pietsch, V.L.; Bühler, J.M.; Karbstein, H.P.; Emin, M.A. High Moisture Extrusion of Soy Protein Concentrate: Influence of Thermomechanical Treatment on Protein-Protein Interactions and Rheological Properties. J. Food Eng. 2019, 251, 11–18. [Google Scholar] [CrossRef]
- Cheftel, J.C.; Kitagawa, M.; Queguiner, C. New Protein Texturization Processes by Extrusion Cooking at High Moisture Levels. Food Rev. Int. 1992, 8, 235–275. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, B.; Wei, Y. Effects of the Specific Mechanical Energy on the Physicochemical Properties of Texturized Soy Protein during High-Moisture Extrusion Cooking. J. Food Eng. 2014, 121, 32–38. [Google Scholar] [CrossRef]
- Grabowska, K.J.; Zhu, S.; Dekkers, B.L.; De Ruijter, N.C.A.; Gieteling, J.; Van Der Goot, A.J. Shear-Induced Structuring as a Tool to Make Anisotropic Materials Using Soy Protein Concentrate. J. Food Eng. 2016, 188, 77–86. [Google Scholar] [CrossRef]
- Sandoval Murillo, J.L.; Osen, R.; Hiermaier, S.; Ganzenmüller, G. Towards Understanding the Mechanism of Fibrous Texture Formation during High-Moisture Extrusion of Meat Substitutes. J. Food Eng. 2019, 242, 8–20. [Google Scholar] [CrossRef]
- Coscueta, E.R.; Pellegrini Malpiedi, L.; Pintado, M.M.; Nerli, B.B. Production of Soy Protein Concentrate with the Recovery of Bioactive Compounds: From Destruction to Valorization. Food Hydrocoll. 2023, 137, 108314. [Google Scholar] [CrossRef]
- Waggle, D.H.; Steinke, F.H.; Shen, J.L. Isolated soy proteins. Ch. 4. In Legumes; Matthews, R.H., Ed.; Marcel Dekker: New York, NY, USA, 1989; pp. 99–138. [Google Scholar]
- Clarke, E.; Wiseman, J. Effects of Extrusion Conditions on Trypsin Inhibitor Activity of Full Fat Soybeans and Subsequent Effects on Their Nutritional Value for Young Broilers. Br. Poult. Sci. 2007, 48, 703–712. [Google Scholar] [CrossRef]
- Nwabueze, T.U. Effect of Process Variables on Trypsin Inhibitor Activity (TIA), Phytic Acid and Tannin Content of Extruded African Breadfruit-Corn-Soy Mixtures: A Response Surface Analysis. LWT 2007, 40, 21–29. [Google Scholar] [CrossRef]
- Petres, J.; Czukor, B. Investigation of the Effects of Extrusion Cooking on Antinutritional Factors in Soybeans Employing Response Surface Analysis Part 1. Effect of Extrusion Cooking on Trypsin-inhibitor Activity. Food/Nahrung. 1989, 33, 275–281. [Google Scholar] [CrossRef]
- Chen, F.L.; Wei, Y.M.; Zhang, B.; Ojokoh, A.O. System Parameters and Product Properties Response of Soybean Protein Extruded at Wide Moisture Range. J. Food Eng. 2010, 96, 208–213. [Google Scholar] [CrossRef]
- Lin, S.; Huff, H.E.; Hsieh, F. Texture and Chemical Characteristics of Soy Protein Meat Analog Extruded at High Moisture. J. Food Sci. 2000, 65, 264–269. [Google Scholar] [CrossRef]
- Dekkers, B.L.; Nikiforidis, C.V.; van der Goot, A.J. Shear-induced fibrous structure formation from a pectin/SPI blend. Innov. Food Sci. Emerg. Technol. 2016, 36, 193–200. [Google Scholar] [CrossRef]
- AOAC. AOAC Official Method 934.01: Proximate analysis and calculations moisture. In Official Methods of Analysis of AOAC International; AOAC International: Washington, DC, USA, 2002. [Google Scholar]
- AOAC. AOAC Official Method 923.03: Ash of Flour. Gravimetric. In Official Methods of Analysis of AOAC International; AOAC International: Washington, DC, USA, 2002. [Google Scholar]
- AOAC. AOAC Official Method 991.43: Total, soluble and insoluble dietary fiber in foods. Gravimetric. In Official Methods of Analysis of AOAC International; AOAC International: Washington, DC, USA, 2002. [Google Scholar]
- AOAC. AOAC Official Method 920.39: Fat (crude) or ether extract in animal feed. Gravimetric. In Official Methods of Analysis of AOAC International; AOAC International: Washington, DC, USA, 2002. [Google Scholar]
- AACC International. Approved Methods of the American Association of Cereal Chemists, Methods 46–30.01 (Protein), 11th ed.; American Association of Cereal Chemists: St. Paul, MN, USA, 2015. [Google Scholar]
- Laemmli. Cleavage of Structural Protein during the Assembly of the Head of Bacteriophage T4; Nature Publishing Group: London, UK, 1970; Volume 227, pp. 680–685. [Google Scholar] [CrossRef]
- Guideline 98/64/EG (1998) of the European Union: Commission Directive 98/64/EC of 3 September 1998 Establishing Community Methods of Analysis for the Determination of Amino-Acids, Crude Oils and Fats, and Olaquindox in Feedingstuffs and Amending Directive 71/393/EEC (Text with EEA Relevance) (OJ L 257 19.09.1998, p. 14, ELI). Available online: http://data.europa.eu/eli/dir/1998/64/oj (accessed on 5 April 2024).
- AACC International. Approved Methods of the American Association of Cereal Chemists International, 11th ed.; Methods: 56-30.01 (WBC); American Association of Cereal Chemists: St. Paul, MN, USA, 2012. [Google Scholar]
- Lin, M.J.Y.; Humbert, E.S.; Sosulski, F.W. Functional Properties Sunflower Meal Products of. J. Food Sci. 1974, 39, 5–7. [Google Scholar] [CrossRef]
- Adebiyi, A.P.; Aluko, R.E. Functional Properties of Protein Fractions Obtained from Commercial Yellow Field Pea (Pisum sativum L.) Seed Protein Isolate. Food Chem. 2011, 128, 902–908. [Google Scholar] [CrossRef]
- Pearce, K.N.; Kinsella, J.E. Emulsifying Properties of Proteins: Evaluation of a Turbidimetric Technique. J. Agric. Food Chem. 1978, 26, 716–723. [Google Scholar] [CrossRef]
- Karaca, A.C.; Low, N.; Nickerson, M. Emulsifying Properties of Chickpea, Faba Bean, Lentil and Pea Proteins Produced by Isoelectric Precipitation and Salt Extraction. Food Res. Int. 2011, 44, 2742–2750. [Google Scholar] [CrossRef]
- Moore, E.; Jennifer, M.C.; Hallin, M. Physical Testing—Food RVA Method 50.01 Pulse Flour and Pulse Starch Method. Perkin Elmer, Inc.: Waltham, MA, USA, 2022. Available online: www.perkinelmer.com (accessed on 30 May 2024).
- Carbonaro, M.; Maselli, P.; Nucara, A. Relationship between Digestibility and Secondary Structure of Raw and Thermally Treated Legume Proteins: A Fourier Transform Infrared (FT-IR) Spectroscopic Study. Amino Acids 2012, 43, 911–921. [Google Scholar] [CrossRef]
- Vidal, N.P.; Roman, L.; Swaraj, V.J.S.; Ragavan, K.V.; Simsek, S.; Rahimi, J.; Kroetsch, B.; Martinez, M.M. Enhancing the Nutritional Value of Cold-Pressed Oilseed Cakes through Extrusion Cooking. Innov. Food Sci. Emerg. Technol. 2022, 77, 102956. [Google Scholar] [CrossRef]
- Wang, C.; Jiang, L.; Wei, D.; Li, Y.; Sui, X.; Wang, Z.; Li, D. Effect of Secondary Structure Determined by FTIR Spectra on Surface Hydrophobicity of Soybean Protein Isolate. Procedia Eng. 2011, 15, 4819–4827. [Google Scholar] [CrossRef]
- Fevzioglu, M.; Ozturk, O.K.; Hamaker, B.R.; Campanella, O.H. Quantitative Approach to Study Secondary Structure of Proteins by FT-IR Spectroscopy, Using a Model Wheat Gluten System. Int. J. Biol. Macromol. 2020, 164, 2753–2760. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.R. Basic Aspects of the Technique and Applications of Infrared Spectroscopy of Peptides and Proteins. ACS Symp. Ser. 2000, 750, 2–37. [Google Scholar] [CrossRef]
- Osen, R.; Toelstede, S.; Wild, F.; Eisner, P.; Schweiggert-Weisz, U. High Moisture Extrusion Cooking of Pea Protein Isolates: Raw Material Characteristics, Extruder Responses, and Texture Properties. J. Food Eng. 2014, 127, 67–74. [Google Scholar] [CrossRef]
- ISO 14902:2001; ISO Method 14902—Animal Feeding Stuffs—Determination of Trypsin Inhibitor Activity of Soya Products, n.d. ISO: Geneva, Switzerland, 2001.
- Bainy, E.M.; Tosh, S.M.; Corredig, M.; Poysa, V.; Woodrow, L. Varietal Differences of Carbohydrates in Defatted Soybean Flour and Soy Protein Isolate By-Products. Carbohydr. Polym. 2008, 72, 664–672. [Google Scholar] [CrossRef]
- Ning, L.; Villota, R. Influence of 7S and 11S globulins on the extrusion performance of soy protein concentrates. J. Food Process. Preserv. 1994, 18, 421–436. [Google Scholar] [CrossRef]
- Liu, Q.; Geng, R.; Zhao, J.; Chen, Q.; Kong, B. Structural and Gel Textural Properties of Soy Protein Isolate When Subjected to Extreme Acid PH-Shifting and Mild Heating Processes. J. Agric. Food Chem. 2015, 63, 4853–4861. [Google Scholar] [CrossRef] [PubMed]
- Flory, J.; Alavi, S. Use of Hydration Properties of Proteins to Understand Their Functionality and Tailor Texture of Extruded Plant-Based Meat Analogues. J. Food Sci. 2024, 89, 245–258. [Google Scholar] [CrossRef]
- Xia, W.; Pan, S.; Cheng, Z.; Tian, Y.; Huang, X. High-Intensity Ultrasound Treatment on Soy Protein after Selectively Proteolyzing Glycinin Component: Physical, Structural, and Aggregation Properties. Foods 2020, 9, 839. [Google Scholar] [CrossRef]
- Fernández-Quintela, A.; Macarulla, M.T.; Del Barrio, A.S.; Martínez, J.A. Composition and Functional Properties of Protein Isolates Obtained from Commercial Legumes Grown in Northern Spain. Plant Foods Hum. Nutr. 1997, 51, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Mateen, A.; Singh, G. Evaluating the Potential of Millets as Blend Components with Soy Protein Isolate in a High Moisture Extrusion System for Improved Texture, Structure, and Colour Properties of Meat Analogues. Food Res. Int. 2023, 173, 113395. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, L.A.; Mes, J.J.; Mensink, M.; Wanders, A.J. Protein Quality of Soy and the Effect of Processing: A Quantitative Review. Front. Nutr. 2022, 9, 1004754. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.Y. Soybean: Soy Concentrates and Isolates. In Encyclopedia of Food Grains, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2015; Volumes 3–4, pp. 482–488. [Google Scholar] [CrossRef]
- FAO. Dietary Protein Quality Evaluation in Human Nutrition. Report of an FAO Expert Consultation; FAO: Rome, Italy, 2013; Volume 92. [Google Scholar]
- Tang, C.H.; Choi, S.M.; Ma, C.Y. Study of Thermal Properties and Heat-Induced Denaturation and Aggregation of Soy Proteins by Modulated Differential Scanning Calorimetry. Int. J. Biol. Macromol. 2007, 40, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Ryu, H.S.; Rhee, K.C. Protein Solubility Characteristics of Commercial Soy Protein Products. JAOCS J. Am. Oil Chem. Soc. 2003, 80, 85–90. [Google Scholar] [CrossRef]
- Li, S.; Wei, Y.; Fang, Y.; Zhang, W.; Zhang, B. DSC Study on the Thermal Properties of Soybean Protein Isolates/Corn Starch Mixture. J. Therm. Anal. Calorim. 2014, 115, 1633–1638. [Google Scholar] [CrossRef]
- Damodaran, S. Refolding of Thermally Unfolded Soy Proteins during the Cooling Regime of the Gelation Process: Effect on Gelation. J. Agric. Food Chem. 1988, 36, 262–269. [Google Scholar] [CrossRef]
- Tang, X.; Shen, Y.; Zhang, Y.; Schilling, M.W.; Li, Y. Parallel Comparison of Functional and Physicochemical Properties of Common Pulse Proteins. LWT 2021, 146, 111594. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Q.; Zhang, W.; Bakalis, S.; Luo, Y.; Lametsch, R. Different Source of Commercial Soy Protein Isolates: Structural, Compositional, and Physicochemical Characteristics in Relation to Protein Functionalities. Food Chem. 2024, 433, 137315. [Google Scholar] [CrossRef]
- Farooq, Z.; Boye, J.I. Novel Food and Industrial Applications of Pulse Flours and Fractions. In Pulse Foods; Academic Press: Cambridge, MA, USA, 2011; pp. 283–323. [Google Scholar] [CrossRef]
- Day, L. Proteins from Land Plants—Potential Resources for Human Nutrition and Food Security. Trends Food Sci. Technol. 2013, 32, 25–42. [Google Scholar] [CrossRef]
- Du, S.-K.; Jiang, H.; Yu, X.; Jane, J.-L. Physicochemical and Functional Properties of Whole Legume Flour. LWT 2014, 55, 308–313. [Google Scholar] [CrossRef]
- Keerati-U-Rai, M.; Corredig, M. Heat-Induced Changes Occurring in Oil/Water Emulsions Stabilized by Soy Glycinin and β-Conglycinin. J. Agric. Food Chem. 2010, 58, 9171–9180. [Google Scholar] [CrossRef]
- Kinsella, J.E. Functional Properties of Soy Proteins. J. Am. Oil Chem. Soc. 1979, 56, 242–258. [Google Scholar] [CrossRef]
- Wang, C.; Johnson, L.A. Functional Properties of Hydrothermally Cooked Soy Protein Products. JAOCS J. Am. Oil Chem. Soc. 2001, 78, 189–195. [Google Scholar] [CrossRef]
- Zahari, I.; Ferawati, F.; Helstad, A.; Ahlström, C.; Östbring, K.; Rayner, M.; Purhagen, J.K. Development of High-Moisture Meat Analogues with Hemp and Soy Protein Using Extrusion Cooking. Foods 2020, 9, 772. [Google Scholar] [CrossRef]
- McClements, D.J.; Weiss, J.; Kinchla, A.J.; Nolden, A.A.; Grossmann, L. Methods for Testing the Quality Attributes of Plant-Based Foods: Meat-and Processed-Meat Analogs. Foods 2021, 10, 260. [Google Scholar] [CrossRef]
- Dekkers, B.L.; Boom, R.M.; van der Goot, A.J. Structuring Processes for Meat Analogues. Trends Food Sci. Technol. 2018, 81, 25–36. [Google Scholar] [CrossRef]
- Chiang, A. Protein-Protein Interaction of Soybean Protein from Extrusion Processing. Master’s Thesis, University of Missouri-Columbia, Columbia, MO, USA, 2007. [Google Scholar]
- Wang, H.; van den Berg, F.W.J.; Zhang, W.; Czaja, T.P.; Zhang, L.; Jespersen, B.M.; Lametsch, R. Differences in Physicochemical Properties of High-Moisture Extrudates Prepared from Soy and Pea Protein Isolates. Food Hydrocoll. 2022, 128, 107540. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, B.; Wei, Y.; Li, S. Effects of Specific Mechanical Energy on Soy Protein Aggregation during Extrusion Process Studied by Size Exclusion Chromatography Coupled with Multi-Angle Laser Light Scattering. J. Food Eng. 2013, 115, 220–225. [Google Scholar] [CrossRef]
- Beck, S.M.; Knoerzer, K.; Sellahewa, J.; Emin, M.A.; Arcot, J. Effect of Different Heat-Treatment Times and Applied Shear on Secondary Structure, Molecular Weight Distribution, Solubility and Rheological Properties of Pea Protein Isolate as Investigated by Capillary Rheometry. J. Food Eng. 2017, 208, 66–76. [Google Scholar] [CrossRef]
- Osen, R.J.J. Texturization of Pea Protein Isolates Using High Moisture Extrusion Cooking. Ph.D. Thesis, Technische Universität München, Munchen, Germany, 2017. [Google Scholar]
- Bouvier, J.; Campanella, O.H. Extrusion Processing Technology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014. [Google Scholar] [CrossRef]
- Peace, R.W.; Sarwar, G.; Touchburn, S.P. Trypsin Inhibitor Levels in Soy-Based Infant Formulas and Commercial Soy Protein Isolates and Concentrates. Food Res. Int. 1992, 25, 137–141. [Google Scholar] [CrossRef]
- Avilés-Gaxiola, S.; Chuck-Hernández, C.; Serna Saldívar, S.O. Inactivation Methods of Trypsin Inhibitor in Legumes: A Review. J. Food Sci. 2018, 83, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Vanga, S.K.; Wang, J.; Raghavan, V. Effect of Ultrasound and Microwave Processing on the Structure, in-Vitro Digestibility and Trypsin Inhibitor Activity of Soymilk Proteins. LWT 2020, 131, 109708. [Google Scholar] [CrossRef]
- Nikbakht Nasrabadi, M.; Sedaghat Doost, A.; Mezzenga, R. Modification Approaches of Plant-Based Proteins to Improve Their Techno-Functionality and Use in Food Products. Food Hydrocoll. 2021, 118, 106789. [Google Scholar] [CrossRef]
- Rackis, J.; Honig, D.H.; Sessa, D.J.; Moser, H.A. Lipoxygenase and Peroxidase Activities of Soybeans as Related to the Flavor Profile During Maturation. Cereal Chem. 1972, 49, 586–597. [Google Scholar]


| Parameter | Value | Time (min:s) | ||
|---|---|---|---|---|
| 95 °C | 120 °C | 140 °C | ||
| Initial Temperature (°C) | 50 | 00:00 | 00:00 | 00:00 |
| Speed (rpm) | 960 | 00:00 | 00:00 | 00:00 |
| Speed (rpm) | 160 | 00:10 | 00:10 | 00:10 |
| Temperature (°C) | 50 | 01:00 | 01:00 | 01:00 |
| Temperature (°C) | Hold * | 08:30 | 12:40 | 16:00 |
| Temperature (°C) | Hold * | 13:30 | 17:40 | 21:00 |
| Temperature (°C) | 50 | 21:00 | 29:10 | 36:00 |
| End Temperature (°C) | 50 | 23:00 | 31:10 | 38:00 |
| Barrel Segment Temperatures (°C) | Screw Speed (rpm) | Motor Torque (%) | Melt Temperature (°C) | Melt Pressure (bar) | SME (Wh/Kg) | Extrusion Conditions | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Z1 | Z2 | Z3 | Z4 | Z5 | Z6 | ||||||
| 30 | 50 | 75 | 105 | 115 | 120 | 300 | 11.2 | 115 | 18.0 | 41.1 | LT-LS |
| 350 | 12.3 | 117 | 20.4 | 52.6 | LT-MS | ||||||
| 400 | 9.2 | 115 | 14.3 | 44.9 | LT-HS | ||||||
| 30 | 70 | 95 | 115 | 125 | 140 | 300 | 10.4 | 131 | 16.5 | 38.1 | HT-LS |
| 350 | 9.8 | 130 | 15.5 | 41.9 | HT-MS | ||||||
| 400 | 7.9 | 130 | 11.6 | 38.7 | HT-HS | ||||||
| SPI | SPC | SPM | |
|---|---|---|---|
| Proximate composition | |||
| Moisture (g/100 g, w.b.) | 7.6 ± 0.2 b | 6.1 ± 0.1 a | 6.3 ± 0.2 a |
| Protein (g/100 g d.b.) | 95.4 ± 1.1 c | 73.9 ± 0.1 a | 76.5 ± 0.1 b |
| Crude Fiber (g/100 g d.b.) | 0.8 ± 0.0 a | 3.5 ± 0.1 c | 3.1 ± 0.1 b |
| Carbohydrates (g/100 g d.b.) | 0.2 ± 0.0 a | 11.7 ± 0.1 c | 10.3 ± 0.1 b |
| Lipids (g/100 g d.b.) | 0.4 ± 0.1 a | 0.6 ± 0.1 a | 0.6 ± 0.1 a |
| Ash (g/100 g, d.b.) | 4.0 ± 0.1 a | 4.4 ± 0.2 a | 4.3 ± 0.2 a |
| Physicochemical properties | |||
| Td1 (°C) | nd | 86.4 ± 0.7 a | 86.1 ± 1.9 a |
| Td2 (°C) | nd | 116.6 ± 0.1 a | 117.4 ± 0.6 a |
| ΔH1 (J/g protein) | nd | 1.8 ± 0.6 a | 1.4 ± 0.1 a |
| ΔH2 (J/g protein) | nd | 3.4 ± 0.1 b | 3.2 ± 0.1 a |
| WBC (g/g) | 4.7 ± 0.1 a | 5.0 ± 0.1 b | 5.0 ± 0.0 b |
| OBC (mL/g) | 3.4 ± 0.1 a | 3.0 ± 0.2 a | 3.0 ± 0.1 a |
| LGC (%) | 14 ± 0 c | 12 ± 0 a | 13 ± 0 b |
| FC (%) | 43.1 ± 2.0 a | 50.0 ± 0.0 b | 49.2 ± 0.2 b |
| FS (%) | 79.2 ± 5.9 b | 62.7 ± 0.2 a | 64.7 ± 0.9 a |
| EAI (m2/g) | 99.5 ± 0.0 b | 94.5 ± 0.3 a | 95.1 ± 0.2 a |
| ESI (min) | 1350 ± 0.0 c | 80.2 ± 0.2 a | 555.0 ± 0.2 b |
| L* | a* | b* | ΔE | |
|---|---|---|---|---|
| SPI | 86.07 ± 0.06 e | −0.27 ± 0.01 a | 16.33 ± 0.09 b | - |
| SPC | 88.05 ± 0.00 g | −0.55 ± 0.01 a | 14.58 ± 0.02 a | - |
| SPM | 87.38 ± 0.05 f | 0.10 ± 0.01 a | 14.68 ± 0.08 a | - |
| LT-LS | 64.25 ± 0.23 c | 3.22 ± 0.03 b | 18.85 ± 0.19 de | 23.71 ± 0.25 b |
| LT-MS | 64.55 ± 0.22 c | 3.29 ± 0.07 b | 18.59 ± 0.05 d | 23.38 ± 0.28 b |
| LT-HS | 66.08 ± 0.17 d | 2.87 ± 0.01 b | 17.42 ± 0.25 c | 21.65 ± 0.19 a |
| HT-LS | 60.84 ± 0.25 a | 4.31 ± 0.25 c | 19.66 ± 0.24 ef | 27.33 ± 0.22 d |
| HT-MS | 61.83 ± 0.32 b | 4.69 ± 0.47 c | 20.15 ± 0.93 f | 26.53 ± 0.51 cd |
| HT-HS | 61.74 ± 0.4 b | 4.24 ± 0.88 c | 18.95 ± 0.79 de | 26.32 ± 0.72 c |
| Conditions | Hardness | Resilience | Cohesiveness | Springiness | Cutting Force Longitudinal (FL) | Cutting ForceTransverse (FT) | FL/FT |
|---|---|---|---|---|---|---|---|
| N | % | % | % | N | N | ||
| LT-LS | 127.62 ± 8.37 c | 0.41 ± 0.03 bc | 0.81 ± 0.02 ab | 0.78 ± 0.02 a | 3.18 ± 0.15 b | 3.73 ± 0.25 b | 0.85 ± 0.05 c |
| LT-MS | 113.60 ± 3.52 b | 0.40 ± 0.03 ab | 0.80 ± 0.02 ab | 0.78 ± 0.04 a | 2.95 ± 0.31 b | 3.65 ± 0.22 b | 0.81 ± 0.07 c |
| LT-HS | 111.48 ± 1.35 b | 0.35 ± 0.04 a | 0.78 ± 0.04 a | 0.74 ± 0.04 a | 2.02 ± 0.08 a | 2.46 ± 0.09 a | 0.82 ± 0.05 c |
| HT-LS | 109.06 ± 6.97 b | 0.47 ± 0.07 c | 0.83 ± 0.04 b | 0.78 ± 0.04 a | 5.23 ± 0.27 d | 7.23 ± 0.44 e | 0.73 ± 0.05 b |
| HT-MS | 89.25 ± 4.41 a | 0.39 ± 0.06 ab | 0.76 ± 0.05 a | 0.78 ± 0.07 a | 4.05 ± 0.16 c | 6.31 ± 0.27 d | 0.64 ± 0.04 a |
| HT-HS | 86.16 ± 4.90 a | 0.37 ± 0.06 ab | 0.76 ± 0.05 a | 0.78 ± 0.09 a | 4.01 ± 0.15 c | 5.62 ± 0.24 c | 0.72 ± 0.03 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, G.; Piñero, M.-Y.; Parle, F.; Blanco, B.; Roman, L. Optimizing Screw Speed and Barrel Temperature for Textural and Nutritional Improvement of Soy-Based High-Moisture Extrudates. Foods 2024, 13, 1748. https://doi.org/10.3390/foods13111748
Ribeiro G, Piñero M-Y, Parle F, Blanco B, Roman L. Optimizing Screw Speed and Barrel Temperature for Textural and Nutritional Improvement of Soy-Based High-Moisture Extrudates. Foods. 2024; 13(11):1748. https://doi.org/10.3390/foods13111748
Chicago/Turabian StyleRibeiro, Gabriela, María-Ysabel Piñero, Florencia Parle, Belén Blanco, and Laura Roman. 2024. "Optimizing Screw Speed and Barrel Temperature for Textural and Nutritional Improvement of Soy-Based High-Moisture Extrudates" Foods 13, no. 11: 1748. https://doi.org/10.3390/foods13111748
APA StyleRibeiro, G., Piñero, M.-Y., Parle, F., Blanco, B., & Roman, L. (2024). Optimizing Screw Speed and Barrel Temperature for Textural and Nutritional Improvement of Soy-Based High-Moisture Extrudates. Foods, 13(11), 1748. https://doi.org/10.3390/foods13111748






