Storage Stability of Plant-Based Drinks Related to Proteolysis and Generation of Free Amino Acids
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples of Plant-Based Drinks and Experimental Set-Up
2.2. Color and pH Measurement
2.3. Protein Content and Protein Stability during Storage
2.4. Particle Size Distribution
2.5. Free N-Terminals via o-Phthalaldehyde Assay
2.6. Quantification of Free Amino Acids on Days 0, 175 and 364 of Storage Period Using Triple-Quadrupole Mass Spectrometry
2.7. Statistical Analysis
3. Results
3.1. Color, pH and Protein Solubility
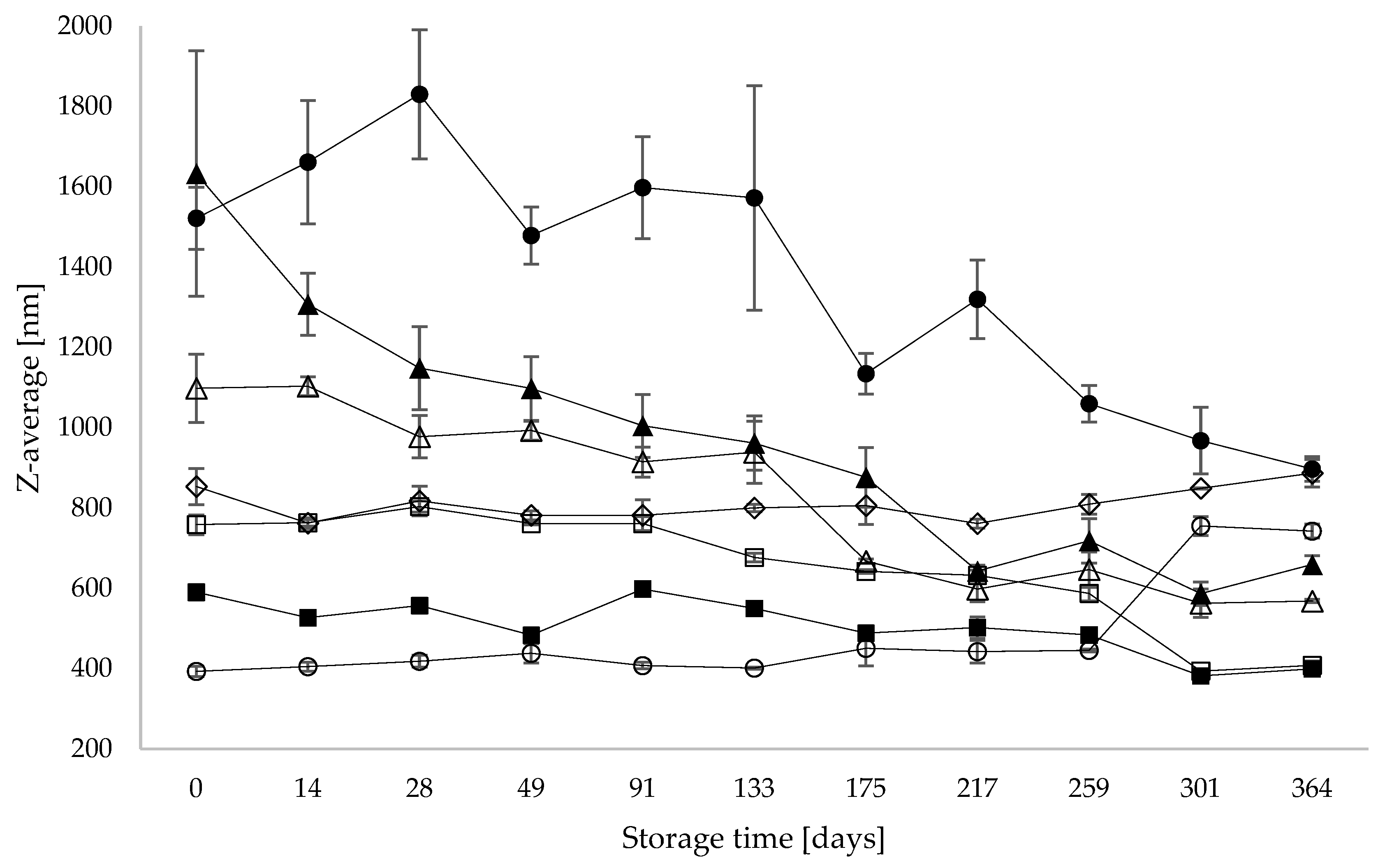
3.2. Particle Size
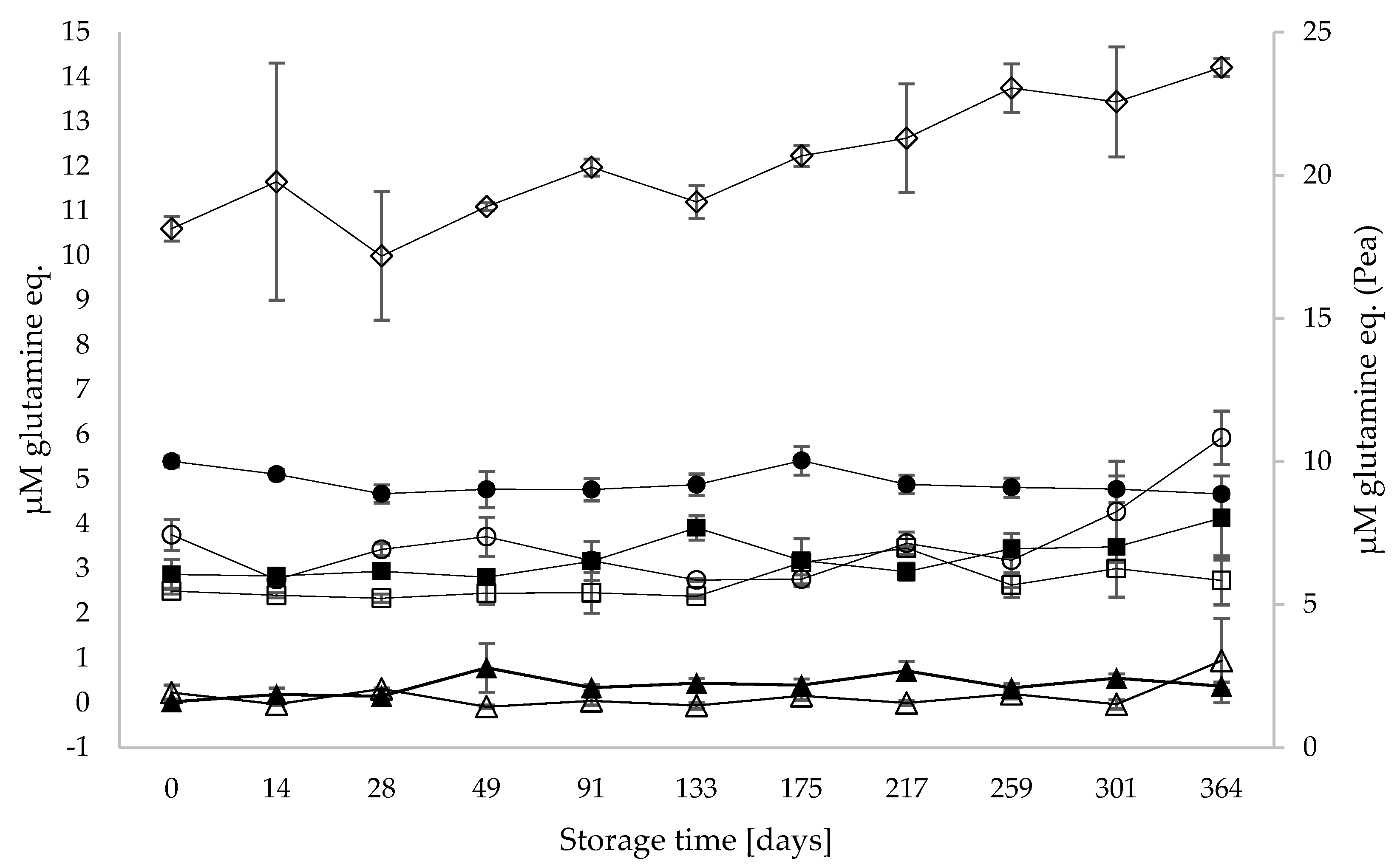
3.3. Proteolysis and Generation of Free Amino Acids during Storage
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rosenfeld, D.L. The psychology of vegetarianism: Recent advances and future directions. Appetite 2018, 131, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Tyagi, S.K.; Anurag, R.K. Plant-based milk alternatives an emerging segment of functional beverages: A review. J. Food Sci. Technol. 2016, 53, 3408–3423. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, C.; Fuentes, M. Making a market for alternatives: Marketing devices and the qualification of a vegan milk substitute. J. Mark. Manag. 2017, 33, 529–555. [Google Scholar] [CrossRef]
- Ingram, C.J.E.; Mulcare, C.A.; Itan, Y.; Thomas, M.G.; Swallow, D.M. Lactose digestion and the evolutionary genetics of lactase persistence. Hum. Genet. 2009, 124, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Patra, T.; Rinnan, Å.; Olsen, K. The physical stability of plant-based drinks and the analysis methods thereof. Food Hydrocoll. 2021, 118, 106770. [Google Scholar] [CrossRef]
- Thorning, T.K.; Raben, A.; Tholstrup, T.; Soedamah-Muthu, S.S.; Givens, I.; Astrup, A. Milk and dairy products: Good or bad for human health? An assessment of the totality of scientific evidence. Food Nutr. Res. 2016, 60, 32527. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Domínguez, R.; Budaraju, S.; Roselló-Soto, E.; Barba, F.J.; Mallikarjunan, K.; Roohinejad, S.; Lorenzo, J.M. Effect of Innovative Food Processing Technologies on the Physicochemical and Nutritional Properties and Quality of Non-Dairy Plant-Based Beverages. Foods 2020, 4, 288. [Google Scholar] [CrossRef]
- Roland, I.S.; Aguilera-Toro, M.; Nielsen, S.D.-H.; Poulsen, N.A.; Larsen, L.B. Processing-Induced Markers in Proteins of Commercial Plant-Based Drinks in Relation to Compositional Aspects. Foods 2023, 12, 3282. [Google Scholar] [CrossRef] [PubMed]
- Schilling, W.; Lesak, D. The making of a plant-based beverage. Food Technol. Mag. 2022, 76, 73–75. [Google Scholar]
- Mäkinen, O.E.; Sozer, N.; Ercili-Cura, D.; Poutanen, K. Protein from Oat: Structure, process, functionality, and nutrition. In Sustainable Protein Sources; Academic Press: Cambridge, MA, USA, 2016; pp. 105–119. [Google Scholar]
- Nielsen, S.D.; Knudsen, L.J.; Baekgaard, L.T.; Rauh, V.; Larsen, L.B. Influence of Lactose on the Maillard Reaction and Dehydroalanine-Mediated Protein Cross-Linking in Casein and Whey. Foods 2022, 11, 897. [Google Scholar] [CrossRef]
- Aguilera-Toro, M.; Poulsen, N.A.; Akkerman, M.; Rauh, V.; Larsen, L.B.; Nielsen, S.D. Development in Maillard Reaction and Dehydroalanine Pathway Markers during Storage of UHT Milk Representing Differences in Casein Micelle Size and Sedimentation. Foods 2022, 11, 1525. [Google Scholar] [CrossRef] [PubMed]
- Anema, S.G. Age gelation, sedimentation, and creaming in UHT milk: A review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 140–166. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, E.Y.; Son, H.J.; Lee, J.J.; Choi, Y.H.; Rhyu, M.R. Identification of a key, umami -active fraction in modernized Korean soy sauce and the impact thereof on bitter-masking. Food Chem. 2017, 233, 256–262. [Google Scholar] [CrossRef]
- McGuire, R.G. Reporting of Objective Color Measurements. HortScience 1992, 27, 1254–1255. [Google Scholar] [CrossRef]
- Jones, D.B. Factors for Converting Percentages of Nitrogen in Foods and Feeds into Percentages of Protein; US Department of Agriculture: Washington, DC, USA, 1931; Volume 183, pp. 1–22.
- Salazar, C.; Armenta, J.M.; Shulaev, V. An UPLC-ESI-MS/MS Assay Using 6-Aminoquinolyl-N-Hydroxysuccinimidyl Carbamate Derivatization for Targeted Amino Acid Analysis: Application to Screening of Arabidopsis thaliana Mutants. Metabolites 2012, 2, 398–428. [Google Scholar] [CrossRef] [PubMed]
- Diep, T.T.; Pook, C.; Yoo, M.J.Y. Physicochemical properties and proximate composition of tamarillo (Solanum betaceum Cav.) fruits from New Zealand. J. Food Compos. Anal. 2020, 92, 103563. [Google Scholar] [CrossRef]
- Giacalone, D.; Clausen, M.P.; Jaeger, S.R. Understanding barriers to consumption of plant-based foods and beverages: Insights from sensory and consumer science. Curr. Opin. Food Sci. 2022, 48, 100919. [Google Scholar] [CrossRef]
- Durand, A.; Franks, G.V.; Hosken, R.W. Particle sizes and stability of UHT bovine, cereal and grain milks. Food Hydrocoll. 2003, 17, 671–678. [Google Scholar] [CrossRef]
- Jensen, S.; Jansson, T.; Eggers, N. Storage-induced changes in the sensory characteristics and volatiles of conventional and lactose-hydrolyzed UHT processed milk. Eur. Food Res. Technol. 2015, 240, 1247–1257. [Google Scholar] [CrossRef]
- Deswal, A.; Deora, N.S.; Mishra, H.N. Optimization of Enzymatic Production Process of Oat Milk Using Response Surface Methodology. Food Bioprocess Technol. 2013, 7, 610–618. [Google Scholar] [CrossRef]
- Ryan, B.J.; Henehan, G.T. Avoiding Proteolysis During Protein Purification. Methods Mol. Biol. 2017, 1485, 53–69. [Google Scholar] [CrossRef]
- Nielsen, S.D.; Jansson, T.; Le, T.T.; Jensen, S.; Eggers, N.; Rauh, V.; Sundekilde, U.K.; Sørensen, J.; Andersen, H.J.; Bertram, H.C. Correlation between sensory properties and peptides derived from hydrolysed-lactose uht milk during storage. Int. Dairy. J. 2017, 68, 23–31. [Google Scholar] [CrossRef]
- Chen, D.-W.; Zhang, M. Non-volatile taste active compounds in the meat of Chinese mitten crab (Eriocheir sinensis). Food Chem. 2007, 104, 1200–1205. [Google Scholar] [CrossRef]
- Gorissen, S.H.M.; Crombag, J.J.R.; Senden, J.M.G.; Waterval, W.A.H.; Bierau, J.; Verdijk, L.B.; van Loon, L.J.C. Protein content and amino acid composition of commercially available plant-based protein isolates. Amino Acids 2018, 50, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
| Oat | Oat/Hemp | Almond | Roasted Almond | Pea | Soy | Soy/Rice | |
|---|---|---|---|---|---|---|---|
| Energy (kJ/kcal) | 219/52 | 205/48 | 87/21 | 89/21 | 142/34 | 147/35 | 231/55 |
| Protein (g/100 g) | 0.4 | 1.1 | 0.5 | 0.4 | 2 | 3.7 | 3.5 |
| Fat (g/100 g) | 1.9 | 1.0 | 1.2 | 0.9 | 2.1 | 2.1 | 2.0 |
| Saturated fat (g/100 g) | 0.3 | 0 | 0.1 | 0.1 | 0.2 | 0.4 | 0.3 |
| Carbohydrates (g/100 g) | 8 | 8.7 | 2 | 2.7 | 2 | 0.6 | 5.6 |
| Sugars (g/100 g) | 3.2 | 3.3 | 1.9 | 2.4 | 2 | 0.1 | 4.0 |
| Fibre (g/100 g) | 1 | N/A | 0.1 | 0.3 | N/A | 0.6 | 0.5 |
| Salt (g/100 g) | 0.08 | 0.1 | 0.1 | 0.1 | 0.2 | 0.04 | 0.19 |
| Calcium (mg/100 g) | N/A | N/A | 120 | 120 | 120 | N/A | 120 |
| Shelf life 1 | 9 mo., RT | 1–2 mo., 5 °C | 9 mo., RT | 9 mo., RT | 12 mo., RT | 9 mo., RT | 9 mo., RT |
| Heat treatment | UHT | ESL | UHT | UHT | UHT | UHT | UHT |
| Sample | Day | pH | L* | a* | b* | Protein PBD (%) | Protein Supernatant (%) |
|---|---|---|---|---|---|---|---|
| Oat | 0 | 6.77 | 58.89 | −0.33 | 10.02 | 0.456 ± 0.007 b | 0.164 ± 0.018 a |
| 175 | 6.45 | 54.41 | 0.20 | 9.93 | |||
| 364 | 6.54 | 53.97 | 0.44 | 9.92 | 0.226 ± 0.001 a | ||
| Oat/hemp | 0 | 6.80 | 54.42 | −1.51 | 8.46 | 0.930 ± 0.003 b | 0.328 ± 0.000 a |
| 175 | 6.32 | 49.55 | −0.67 | 7.60 | |||
| 364 | 6.39 | 48.55 | −0.40 | 7.47 | 0.334 ± 0.002 a | ||
| Roasted Almond | 0 | 7.60 | 55.84 | 0.48 | 7.87 | 0.441 ± 0.005 b | 0.265 ± 0.000 a |
| 175 | 7.08 | 52.32 | 0.65 | 7.18 | |||
| 364 | 7.37 | 52.43 | 0.44 | 6.93 | 0.368 ± 0.002 ab | ||
| Almond | 0 | 8.08 | 59.32 | −0.19 | 2.84 | 0.449 ± 0.019 b | 0.310 ± 0.003 a |
| 175 | 7.42 | 55.61 | −0.01 | 2.26 | |||
| 364 | 7.50 | 55.60 | −0.19 | 2.25 | 0.312 ± 0.125 a | ||
| Pea | 0 | 7.62 | 63.75 | −1.40 | 8.41 | 1.702 ± 0.035 c | 1.144 ± 0.003 a |
| 175 | 7.45 | 59.74 | −1.02 | 8.28 | |||
| 364 | 7.61 | 59.57 | −1.03 | 8.01 | 1.173 ± 0.000 b | ||
| Soy | 0 | 7.18 | 62.48 | −3.00 | 11.57 | 4.223 ± 0.182 b | 3.201 ± 0.033 a |
| 175 | 7.05 | 58.71 | −2.49 | 10.27 | |||
| 364 | 7.24 | 58.40 | −2.16 | 9.42 | 3.222 ± 0.001 ab | ||
| Soy/rice | 0 | 7.94 | 58.70 | −1.10 | 10.73 | 2.617 ± 0.584 a | 2.884 ± 0.065 a |
| 175 | 7.56 | 53.37 | 0.16 | 9.80 | |||
| 364 | 7.54 | 52.85 | 0.52 | 9.68 | 2.984 ± 0.000 a |
| Free AA | Day | Oat | Oat/Hemp | Almond | Roasted Almond | Pea | Soy | Soy/Rice |
|---|---|---|---|---|---|---|---|---|
| Histidine | 0 | 0.18 ± 0.00 | 0.05 ± 0.00 | ND | ND | 0.22 ± 0.05 a | 0.04 ± 0.00 | 0.17 ± 0.01 |
| 175 | 0.27 ± 0.05 | 0.06 ± 0.00 | ND | ND | 0.37 ± 0.07 ab | 0.07 ± 0.01 | 0.23 ± 0.05 | |
| 364 | 0.15 ± 0.00 | 0.05 ± 0.00 | ND | ND | 0.48 ± 0.11 b | 0.14 ± 0.00 | 0.16 ± 0.03 | |
| Isoleucine | 0 | 0.25 ± 0.01 | 0.13 ± 0.01 | 0.10 ± 0.01 | 0.09 ± 0.00 | 0.64 ± 0.04 a | 0.02 ± 0.00 | 0.05 ± 0.00 |
| 175 | 0.27 ± 0.03 | 0.13 ± 0.01 | 0.09 ± 0.01 | 0.09 ± 0.00 | 1.14 ± 0.20 b | 0.02 ± 0.00 | 0.05 ± 0.00 | |
| 364 | 0.19 ± 0.01 | 0.17 ± 0.02 | 0.10 ± 0.02 | 0.10 ± 0.01 | 1.30 ± 0.09 b | 0.04 ± 0.00 | 0.05 ± 0.00 | |
| Leucine | 0 | 0.21 ± 0.00 | 0.29 ± 0.03 | 0.05 ± 0.01 | 0.03 ± 0.00 | 2.20 ± 0.13 a | 0.01 ± 0.00 | 0.06 ± 0.00 |
| 175 | 0.24 ± 0.03 | 0.32 ± 0.03 | 0.06 ± 0.01 | 0.02 ± 0.00 | 3.60 ± 0.57 b | 0.02 ± 0.00 | 0.06 ± 0.00 | |
| 364 | 0.09 ± 0.00 | 0.38 ± 0.04 | 0.06 ± 0.01 | 0.03 ± 0.00 | 4.00 ± 0.17 b | 0.07 ± 0.00 | 0.06 ± 0.01 | |
| Lysine | 0 | 0.13 ± 0.03 | 0.60 ± 0.09 a | 0.28 ± 0.04 | 0.05 ± 0.00 | 1.42 ± 0.05 a | 0.06 ± 0.01 | 0.05 ± 0.00 |
| 175 | 0.34 ± 0.04 | 0.84 ± 0.10 ab | 0.30 ± 0.06 | 0.12 ± 0.03 | 3.24 ± 0.35 b | 0.08 ± 0.02 | 0.07 ± 0.00 | |
| 364 | 0.61 ± 0.02 | 1.19 ± 0.03 b | 0.31 ± 0.05 | 0.19 ± 0.01 | 3.77 ± 0.35 c | 0.04 ± 0.00 | 0.05 ± 0.01 | |
| Methionine | 0 | 0.02 ± 0.00 | 0.05 ± 0.00 a | 0.02 ± 0.00 | 0.01 ± 0.00 | 0.08 ± 0.01 a | 0.01 ± 0.00 | 0.02 ± 0.00 |
| 175 | 0.02 ± 0.00 | 0.08 ± 0.01 b | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.14 ± 0.01 b | 0.01 ± 0.00 | 0.03 ± 0.00 | |
| 364 | 0.01 ± 0.00 | 0.08 ± 0.01 b | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.18 ± 0.01 c | 0.02 ± 0.00 | 0.02 ± 0.00 | |
| Phenylalanine | 0 | 0.16 ± 0.01 | 0.14 ± 0.01 | 0.07 ± 0.01 | 0.01 ± 0.01 | 0.76 ± 0.07 a | 0.01 ± 0.00 | 0.04 ± 0.00 |
| 175 | 0.24 ± 0.01 | 0.15 ± 0.02 | 0.07 ± 0.02 | 0.01 ± 0.00 | 1.45 ± 0.27 b | 0.01 ± 0.00 | 0.05 ± 0.01 | |
| 364 | 0.24 ± 0.00 | 0.19 ± 0.03 | 0.07 ± 0.01 | 0.02 ± 0.00 | 1.54 ± 0.12 b | 0.05 ± 0.01 | 0.04 ± 0.00 | |
| Threonine | 0 | 0.40 ± 0.03 ab | 0.17 ± 0.01 | 0.09 ± 0.02 | 0.06 ± 0.01 | 0.35 ± 0.07 a | 0.01 ± 0.00 | 0.02 ± 0.00 |
| 175 | 0.50 ± 0.08 b | 0.19 ± 0.03 | 0.09 ± 0.01 | 0.04 ± 0.01 | 0.65 ± 0.09 b | 0.01 ± 0.00 | 0.03 ± 0.00 | |
| 364 | 0.27 ± 0.01 a | 0.25 ± 0.03 | 0.08 ± 0.01 | 0.07 ± 0.01 | 0.82 ± 0.04 c | 0.01 ± 0.00 | 0.02 ± 0.00 | |
| Tryptophan | 0 | 0.95 ± 0.02 b | 0.11 ± 0.02 | 0.05 ± 0.01 | 0.03 ± 0.00 | 0.02 ± 0.00 | 0.10 ± 0.02 a | 0.24 ± 0.01 |
| 175 | 1.16 ± 0.20 c | 0.11 ± 0.01 | 0.04 ± 0.01 | 0.03 ± 0.01 | 0.02 ± 0.00 | 0.11 ± 0.02 a | 0.24 ± 0.02 | |
| 364 | 0.30 ± 0.04 a | 0.15 ± 0.02 | 0.04 ± 0.01 | 0.02 ± 0.00 | 0.04 ± 0.01 | 0.35 ± 0.02 b | 0.22 ± 0.01 | |
| Valine | 0 | 0.45 ± 0.01 ab | 0.24 ± 0.02 | 0.15 ± 0.01 | 0.08 ± 0.00 | 0.61 ± 0.05 a | 0.02 ± 0.00 | 0.05 ± 0.00 |
| 175 | 0.59 ± 0.12 b | 0.26 ± 0.03 | 0.13 ± 0.02 | 0.08 ± 0.01 | 1.10 ± 0.02 b | 0.02 ± 0.00 | 0.06 ± 0.00 | |
| 364 | 0.33 ± 0.01 a | 0.36 ± 0.03 | 0.15 ± 0.04 | 0.11 ± 0.03 | 1.43 ± 0.08 c | 0.04 ± 0.00 | 0.05 ± 0.01 | |
| L-Alanine | 0 | 1.46 ± 0.03 a | 0.90 ± 0.06 | 0.70 ± 0.09 | 0.72 ± 0.10 | 0.79 ± 0.04 a | 0.11 ± 0.01 | 0.22 ± 0.01 |
| 175 | 1.88 ± 0.33 a | 1.07 ± 0.09 | 0.79 ± 0.09 | 0.64 ± 0.06 | 1.97 ± 0.16 b | 0.15 ± 0.02 | 0.24 ± 0.01 | |
| 364 | 2.45 ± 0.09 b | 1.28 ± 0.08 | 0.67 ± 0.09 | 0.79 ± 0.07 | 2.50 ± 0.12 c | 0.11 ± 0.01 | 0.22 ± 0.02 | |
| Arginine | 0 | ND | ND | 0.24 ± 0.08 | ND | 0.62 ± 0.18 a | 0.23 ± 0.07 | 0.80 ± 0.16 |
| 175 | 1.26 ± 0.22 | 0.42 ± 0.39 | ND | ND | 1.35 ± 0.26 b | 0.27 ± 0.15 | 1.05 ± 0.21 | |
| 364 | ND | 0.08 ± 0.00 | ND | ND | 1.45 ± 0.06 b | 0.63 ± 0.03 | 0.88 ± 0.05 | |
| Asparagine | 0 | 12.30 ± 0.45 a | 1.97 ± 0.17 | 1.22 ± 0.13 | 0.72 ± 0.16 | 0.84 ± 0.06 | 0.07 ± 0.00 | 0.43 ± 0.01 |
| 175 | 15.39 ± 2.30 b | 2.07 ± 0.13 | 1.30 ± 0.30 | 0.72 ± 0.04 | 1.79 ± 0.22 | 0.07 ± 0.02 | 0.45 ± 0.03 | |
| 364 | 9.49 ± 1.35 a | 3.02 ± 0.09 | 1.19 ± 0.19 | 0.82 ± 0.14 | 1.68 ± 0.20 | 0.12 ± 0.01 | 0.41 ± 0.03 | |
| Aspartic acid | 0 | 4.14 ± 0.01 a | 0.73 ± 0.11 | 0.59 ± 0.07 | 0.55 ± 0.09 | 0.15 ± 0.03 | 0.15 ± 0.03 | 0.32 ± 0.01 |
| 175 | 5.37 ± 0.86 b | 0.82 ± 0.05 | 0.66 ± 0.15 | 0.50 ± 0.03 | 0.35 ± 0.04 | 0.18 ± 0.04 | 0.35 ± 0.02 | |
| 364 | 3.35 ± 0.16 a | 1.01 ± 0.15 | 0.59 ± 0.08 | 0.68 ± 0.09 | 0.49 ± 0.02 | 0.26 ± 0.01 | 0.33 ± 0.02 | |
| Cystine | 0 | 0.52 ± 0.02 a | 0.53 ± 0.07 | 0.32 ± 0.04 | 0.30 ± 0.07 | 0.33 ± 0.02 a | 0.03 ± 0.01 | 0.06 ± 0.01 |
| 175 | 0.7 ± 0.16 a | 0.64 ± 0.07 | 0.37 ± 0.10 | 0.31 ± 0.03 | 1.14 ± 0.17 b | 0.04 ± 0.02 | 0.06 ± 0.00 | |
| 364 | 1.62 ± 0.29 b | 0.86 ± 0.11 | 0.33 ± 0.06 | 0.40 ± 0.07 | 1.44 ± 0.13 b | 0.02 ± 0.00 | 0.05 ± 0.01 | |
| Glutamic acid | 0 | 7.00 ± 0.01 b | 0.89 ± 0.07 | 0.61 ± 0.07 | 0.14 ± 0.01 | 0.29 ± 0.01 | 0.16 ± 0.03 | 0.53 ± 0.02 |
| 175 | 8.96 ± 1.43 c | 1.09 ± 0.09 | 0.66 ± 0.16 | 0.10 ± 0.01 | 0.50 ± 0.03 | 0.21 ± 0.04 | 0.57 ± 0.04 | |
| 364 | 3.85 ± 0.25 a | 1.35 ± 0.14 | 0.59 ± 0.06 | 0.08 ± 0.01 | 0.55 ± 0.00 | 0.39 ± 0.02 | 0.52 ± 0.06 | |
| Glutamine | 0 | ND | 0.19 ± 0.03 b | ND | ND | 0.01 ± 0.00 | ND | ND |
| 175 | ND | 0.12 ± 0.02 ab | ND | ND | 0.01 ± 0.00 | ND | ND | |
| 364 | ND | 0.10 ± 0.03 a | ND | ND | ND | ND | ND | |
| Glycine | 0 | 0.52 ± 0.02 a | 0.53 ± 0.07 | 0.32 ± 0.04 | 0.30 ± 0.07 | 0.33 ± 0.02 a | 0.03 ± 0.01 | 0.06 ± 0.01 |
| 175 | 0.70 ± 0.16 a | 0.64 ± 0.07 | 0.37 ± 0.10 | 0.31 ± 0.03 | 1.14 ± 0.17 b | 0.04 ± 0.02 | 0.06 ± 0.00 | |
| 364 | 1.62 ± 0.29 b | 0.86 ± 0.11 | 0.33 ± 0.06 | 0.40 ± 0.07 | 1.44 ± 0.13 b | 0.02 ± 0.00 | 0.05 ± 0.01 | |
| Proline | 0 | 5.15 ± 0.15 b | 0.67 ± 0.02 | 1.05 ± 0.14 | 0.68 ± 0.06 | 0.05 ± 0.01 | 0.03 ± 0.00 | 0.07 ± 0.00 |
| 175 | 6.46 ± 1.01 c | 0.75 ± 0.05 | 1.07 ± 0.19 | 0.61 ± 0.03 | 0.09 ± 0.01 | 0.03 ± 0.01 | 0.08 ± 0.00 | |
| 364 | 3.99 ± 0.17 a | 0.94 ± 0.03 | 1.02 ± 0.13 | 0.72 ± 0.04 | 0.15 ± 0.01 | 0.04 ± 0.00 | 0.07 ± 0.01 | |
| Serine | 0 | 0.60 ± 0.04 | 0.34 ± 0.03 | 0.29 ± 0.00 | 0.26 ± 0.06 | 1.03 ± 0.05 a | 0.02 ± 0.00 | 0.06 ± 0.01 |
| 175 | 0.77 ± 0.09 | 0.41 ± 0.04 | 0.32 ± 0.08 | 0.23 ± 0.01 | 2.89 ± 0.36 b | 0.03 ± 0.01 | 0.06 ± 0.01 | |
| 364 | 0.95 ± 0.07 | 0.58 ± 0.05 | 0.28 ± 0.06 | 0.31 ± 0.04 | 3.37 ± 0.38 c | 0.04 ± 0.01 | 0.06 ± 0.01 | |
| Tyrosine | 0 | 0.44 ± 0.02 b | 0.09 ± 0.01 | 0.04 ± 0.01 | 0.03 ± 0.01 | 0.18 ± 0.03 a | 0.01 ± 0.00 | 0.05 ± 0.01 |
| 175 | 0.55 ± 0.09 b | 0.10 ± 0.02 | 0.05 ± 0.00 | 0.03 ± 0.00 | 0.47 ± 0.09 b | 0.01 ± 0.00 | 0.06 ± 0.00 | |
| 364 | 0.29 ± 0.00 a | 0.14 ± 0.02 | 0.03 ± 0.00 | 0.04 ± 0.00 | 0.52 ± 0.02 b | 0.02 ± 0.00 | 0.05 ± 0.00 | |
| Total free AA | 0 | 35.53 ± 0.87 a | 8.72 ± 0.82 | 6.67 ± 0.83 | 4.46 ± 0.68 | 10.67 ± 0.91 a | 1.22 ± 0.19 | 3.69 ± 0.29 |
| 175 | 46.31 ± 7.26 b | 10.30 ± 1.24 | 6.83 ± 1.37 | 4.21 ± 0.29 | 22.40 ± 2.94 b | 1.52 ± 0.39 | 4.19 ± 0.44 | |
| 364 | 29.44 ± 2.61 a | 13.10 ± 1.00 | 6.27 ± 0.89 | 5.19 ± 0.57 | 25.86 ± 1.92 b | 2.54 ± 0.14 | 3.64 ± 0.33 | |
| mg Free AA/100 g PBD | 0 | 16.20 ± 0.40 | 8.11 ± 0.76 | 2.94 ± 0.36 | 2.00 ± 0.30 | 18.16 ± 1.55 | 5.20 ± 0.80 | 9.66 ± 0.76 |
| 175 | 21.12 ± 3.31 | 9.58 ± 1.15 | 3.01 ± 0.60 | 1.89 ± 0.13 | 38.13 ± 5.00 | 6.41 ± 1.63 | 10.96 ± 1.16 | |
| 364 | 13.43 ± 1.19 | 12.18 ± 0.93 | 2.77 ± 0.39 | 2.33 ± 0.26 | 44.01 ± 3.27 | 10.70 ± 0.60 | 9.51 ± 0.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roland, I.S.; Le, T.T.; Chen, T.; Aguilera-Toro, M.; Nielsen, S.D.-H.; Larsen, L.B.; Poulsen, N.A. Storage Stability of Plant-Based Drinks Related to Proteolysis and Generation of Free Amino Acids. Foods 2024, 13, 367. https://doi.org/10.3390/foods13030367
Roland IS, Le TT, Chen T, Aguilera-Toro M, Nielsen SD-H, Larsen LB, Poulsen NA. Storage Stability of Plant-Based Drinks Related to Proteolysis and Generation of Free Amino Acids. Foods. 2024; 13(3):367. https://doi.org/10.3390/foods13030367
Chicago/Turabian StyleRoland, Ida Schwartz, Thao T. Le, Tony Chen, Miguel Aguilera-Toro, Søren Drud-Heydary Nielsen, Lotte Bach Larsen, and Nina Aagaard Poulsen. 2024. "Storage Stability of Plant-Based Drinks Related to Proteolysis and Generation of Free Amino Acids" Foods 13, no. 3: 367. https://doi.org/10.3390/foods13030367
APA StyleRoland, I. S., Le, T. T., Chen, T., Aguilera-Toro, M., Nielsen, S. D.-H., Larsen, L. B., & Poulsen, N. A. (2024). Storage Stability of Plant-Based Drinks Related to Proteolysis and Generation of Free Amino Acids. Foods, 13(3), 367. https://doi.org/10.3390/foods13030367








