Improvement of Theaflavins on Glucose and Lipid Metabolism in Diabetes Mellitus
Abstract
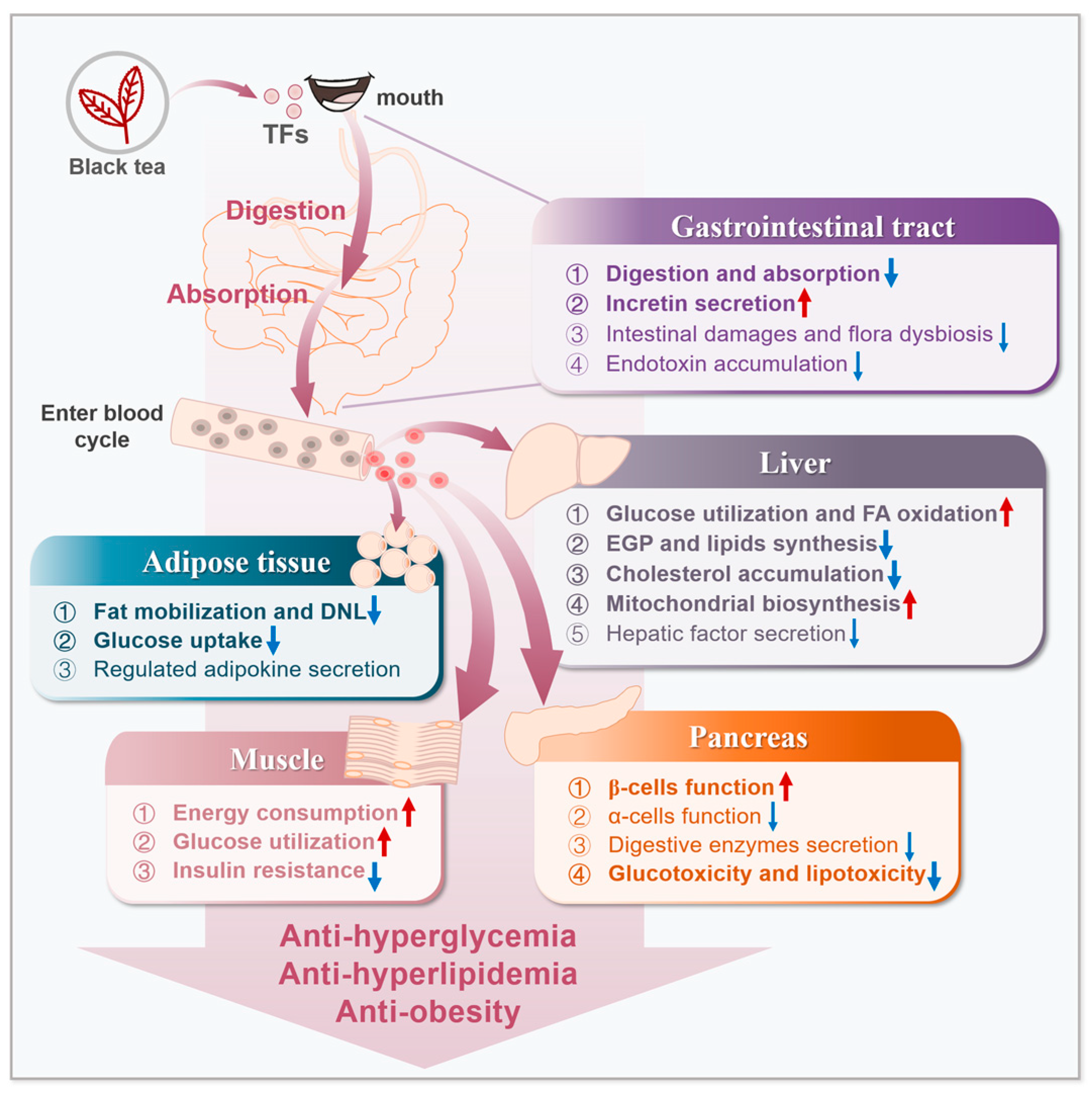
:1. Introduction
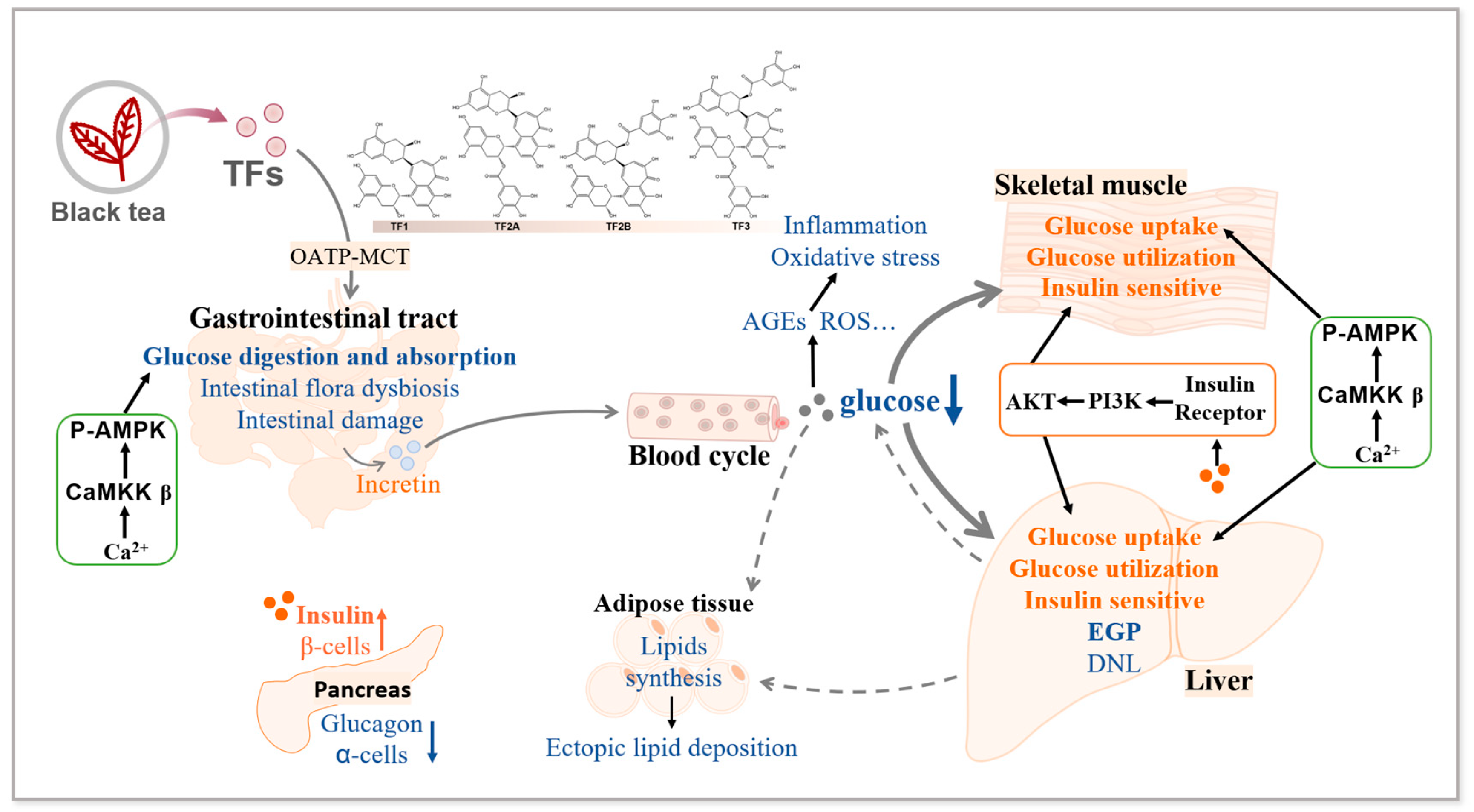
2. Effects of TFs on Glucose Metabolism
2.1. Glucose Metabolism Disorders in Diabetes
2.2. TFs Ameliorate Diabetic Glucose Metabolism Disorders
2.3. Mechanisms Associated with the Improvement of Diabetic Glucose Metabolism Disorders by TFs
2.4. Summary
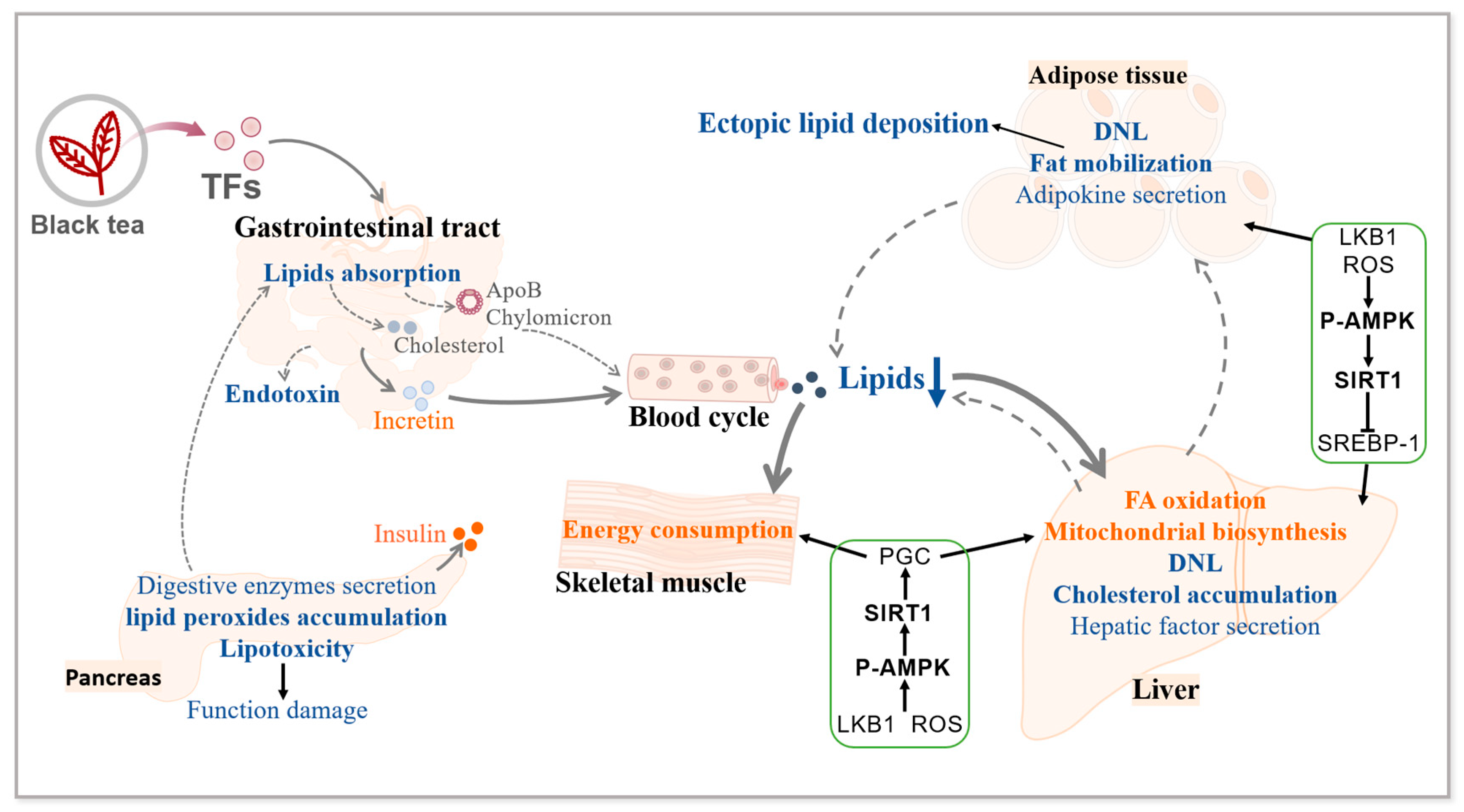
3. Effects of TFs on Lipid Metabolism
3.1. Lipid Metabolism Disorders in Diabetes
3.2. TFs Ameliorate Diabetic Lipid Metabolism Disorders
3.3. Mechanisms Associated with the Improvement of Diabetic Lipid Metabolism Disorders by TFs
3.4. Summary
4. Conclusions
5. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Theaflavin Derivatives | Precursors | Structural Formulas | |
|---|---|---|---|
| 1 | Theaflavin (TF) | EC + EGC | 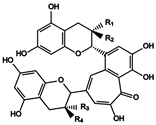 (1) R1 = R4 = OH, R2 = R3 = H (2) R1 = R3 = H, R2 = R4 = OH (3) R1 = R3 = OH, R2 = R4 = H (4) R1 = OH, R2 = R3 = H, R4 = gallate (5) R1 = R3 = H, R2 = OH, R4 = gallate (6) R1 = gallate, R2 = R3 = H, R4 = OH (7) R1 = gallate, R2 = R4 = H, R3 = OH (8) R1 = R4 = gallate, R2 = R3 = H |
| 2 | Isotheaflavin B (TF1b) | EC + (+)-GC | |
| 3 | Neotheaflavin C (TF1c) | (+)-C + EGC | |
| 4 | Theaflavin-3-gallate (TF-3-G) | EC + EGCG | |
| 5 | Neotheaflavin-3-gallate | (+)-C + EGCG | |
| 6 | Theaflavin-3′-gallate (TF-3′-G) | ECG + EGC | |
| 7 | Isotheaflavin-3′-gallate | ECG + (+)-GC | |
| 8 | Theaflavin-3,3′-gallate (TF-D-G) | ECG + EGCG | |
| 9 | Theaflavic acid | (+)-EC + GA |  (9) R1 = OH, R2 = H (10) R1 = H, R2 = OH (11) R1 = H, R2 = gallate |
| 10 | Epitheaflavic acid | (-)-EC + GA | |
| 11 | Epitheaflavic acid-3-gallate | (-)-ECG + GA | |
| 12 | Theaflagallin | (±)-GC + GA (Pyrogallol) | 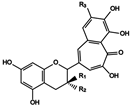 (12) R1 = OH, R2 = H, R3 = OH (13) R1 = H, R2 = OH, R3 = OH (14) R1 = H, R2 = gallate, R3 = OH (15) R1 = OH, R2 = H, R3 = H (16) R1 = gallate, R2 = R3 = H |
| 13 | Epitheaflgallin | ||
| 14 | Epitheaflagallin-3- gallate | EGCG + GA (Pyrogallol) | |
| 15 | Undecided | EGC + Catechol | |
| 16 | Undecided | EGCG + Catechol | |
| 17 | Purpurogallin | Pyrogallol | 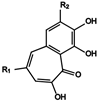 (17) R1 = H, R2 = OH (18) R1 = COOH, R2 = OH (19) R1 = COOH, R2 = H |
| 18 | Purpurogallin carboxylic acid | GA + GA (Pyrogallol) | |
| 19 | Undecided | GA + Catechol | |
| 20 | Theaflavate A | ECG |  (20) R1 = gallate, R2 = H (21) R1 = OH, R2 = H (22) R1 = H, R2 = OH |
| 21 | Theaflavate B | ECG + EC | |
| 22 | Neotheaflavate B | C + EGCG | |
| 23 | Theadibenzotropolone A | Theaflavin-3-gallate + EC |  (23) R1 = H, R2 = OH, R3 = H, R4 = OH (24) R1 = OH, R2 = H, R3 = H, R4 = OH (25) R1 = H, R2 = OH, R3 = OH, R4 = H |
| 24 | Theadibenzotropolone B | Theaflavin-3-gallate + C | |
| 25 | Theadibenzotropolone C | Neotheaflavin-3-gallate + EC | |
| 26 | Theatribenzotropolone A | Theaflavin-3,3′-gallate + 2EC | 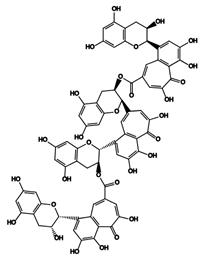 |
| 27 | Bistheaflavin A | Theaflavin + Theaflavin | 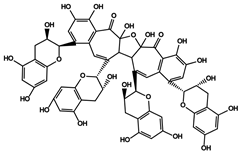 |
| 28 | Bistheaflavin B | Theaflavin + Theaflavin | 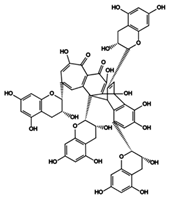 |
References
- IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021.
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 4, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Umpierrez, G.; Korytkowski, M. Diabetic emergencies—Ketoacidosis, hyperglycaemic hyperosmolar state and hypoglycaemia. Nat. Rev. Endocrinol. 2016, 12, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Kharroubi, A.T.; Darwish, H.M. Diabetes mellitus: The epidemic of the century. World J. Diabetes 2015, 6, 850–867. [Google Scholar] [CrossRef] [PubMed]
- Stefan, N.; Cusi, K. A global view of the interplay between non-alcoholic fatty liver disease and diabetes. Lancet Diabetes Endocrinol. 2022, 10, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Infante, B.; Conserva, F.; Pontrelli, P.; Leo, S.; Stasi, A.; Fiorentino, M.; Troise, D.; Dello Strologo, A.; Alfieri, C.; Gesualdo, L.; et al. Recent advances in molecular mechanisms of acute kidney injury in patients with diabetes mellitus. Front. Endocrinol. 2022, 5, 903970. [Google Scholar] [CrossRef] [PubMed]
- Haas, A.V.; McDonnell, M.E. Pathogenesis of Cardiovascular Disease in Diabetes. Endocrinol. Metab. Clin. N. Am. 2018, 47, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Batista, T.M.; Haider, N.; Kahn, C.R. Defining the underlying defect in insulin action in type 2 diabetes. Diabetologia 2021, 64, 994–1006. [Google Scholar] [CrossRef] [PubMed]
- Weir, G.C.; Bonner-Weir, S. Five Stages of Evolving Beta-Cell Dysfunction During Progression to Diabetes. Diabetes 2004, 53 (Suppl. S3), S16–S21. [Google Scholar] [CrossRef] [PubMed]
- Cinti, F.; Bouchi, R.; Kim-Muller, J.Y.; Ohmura, Y.; Sandoval, P.R.; Masini, M.; Marselli, L.; Suleiman, M.; Ratner, L.E.; Marchetti, P.; et al. Evidence of β-Cell Dedifferentiation in Human Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2016, 101, 1044–1054. [Google Scholar] [CrossRef]
- Talchai, C.; Xuan, S.; Lin, H.V.; Sussel, L.; Accili, D. Pancreatic β cell dedifferentiation as a mechanism of diabetic β cell failure. Cell 2012, 150, 1223–1234. [Google Scholar] [CrossRef]
- Hunter, R.W.; Hughey, C.C.; Lantier, L.; Sundelin, E.I.; Peggie, M.; Zeqiraj, E.; Sicheri, F.; Jessen, N.; Wasserman, D.H.; Sakamoto, K. Metformin reduces liver glucose production by inhibition of fructose-1-6-bisphosphatase. Nat. Med. 2018, 24, 1395–1406. [Google Scholar] [CrossRef] [PubMed]
- Coll, A.P.; Chen, M.; Taskar, P.; Rimmington, D.; Patel, S.; Tadross, J.A.; Cimino, I.; Yang, M.; Welsh, P.; Virtue, S.; et al. GDF15 mediates the effects of metformin on body weight and energy balance. Nature 2020, 578, 444–448. [Google Scholar] [CrossRef]
- Zabielski, P.; Hady, H.R.; Chacinska, M.; Roszczyc, K.; Gorski, J.; Blachnio-Zabielska, A.U. The effect of high fat diet and metformin treatment on liver lipids accumulation and their impact on insulin action. Sci. Rep. 2018, 8, 7249. [Google Scholar] [CrossRef]
- Foretz, M.; Guigas, B.; Viollet, B. Metformin: Update on mechanisms of action and repurposing potential. Nat. Rev. Endocrinol. 2023, 19, 460–476. [Google Scholar] [CrossRef]
- LaMoia, T.E.; Shulman, G.I. Cellular and Molecular Mechanisms of Metformin Action. Endocr. Rev. 2021, 42, 77–96. [Google Scholar] [CrossRef] [PubMed]
- Bauer, P.V.; Duca, F.A.; Waise, T.M.Z.; Rasmussen, B.A.; Abraham, M.A.; Dranse, H.J.; Puri, A.; O’Brien, C.A.; Lam, T.K.T. Metformin Alters Upper Small Intestinal Microbiota that Impact a Glucose-SGLT1-Sensing Glucoregulatory Pathway. Cell Metab. 2018, 27, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Westcott, G.P.; Segal, A.R.; Mitri, J.; Brown, F.M. Prolonged glucosuria and relapse of diabetic ketoacidosis related to SGLT2-inhibitor therapy. Endocrinol. Diabetes Metab. 2020, 3, e00117. [Google Scholar] [CrossRef]
- Neumiller, J.J.; White, J.R., Jr.; Campbell, R.K. Sodium-glucose co-transport inhibitors: Progress and therapeutic potential in type 2 diabetes mellitus. Drugs 2010, 70, 377–385. [Google Scholar] [CrossRef]
- Cubeddu, L.X.; Bönisch, H.; Göthert, M.; Molderings, G.; Racké, K.; Ramadori, G.; Miller, K.J.; Schwörer, H. Effects of metformin on intestinal 5-hydroxytryptamine (5-HT) release and on 5-HT3 receptors. Naunyn Schmiedebergs Arch. Pharmacol. 2000, 361, 85–91. [Google Scholar] [CrossRef]
- McCreight, L.J.; Bailey, C.J.; Pearson, E.R. Metformin and the gastrointestinal tract. Diabetologia 2016, 59, 426–435. [Google Scholar] [CrossRef]
- Quaile, M.P.; Melich, D.H.; Jordan, H.L.; Nold, J.B.; Chism, J.P.; Polli, J.W.; Smith, G.A.; Rhodes, M.C. Toxicity and toxicokinetics of metformin in rats. Toxicol. Appl. Pharmacol. 2010, 243, 340–347. [Google Scholar] [CrossRef]
- Li, J.-X.; Hung, Y.-T.; Bair, H.; Hsu, S.-B.; Hsu, C.-Y.; Lin, C.-J. Sodium-glucose co-transporter 2 inhibitor add-on therapy for metformin delays diabetic retinopathy progression in diabetes patients: A population-based cohort study. Sci. Rep. 2023, 13, 17049. [Google Scholar] [CrossRef]
- Huang, D.D.; Shi, G.; Jiang, Y.; Yao, C.; Zhu, C. A review on the potential of Resveratrol in prevention and therapy of diabetes and diabetic complications. Biomed. Pharmacother. 2020, 125, 109767. [Google Scholar] [CrossRef]
- Hameed, A.; Galli, M.; Adamska-Patruno, E.; Krętowski, A.; Ciborowski, M. Select Polyphenol-Rich Berry Consumption to Defer or Deter Diabetes and Diabetes-Related Complications. Nutrients 2020, 12, 2538. [Google Scholar] [CrossRef]
- Cao, H.; Ou, J.; Chen, L.; Zhang, Y.; Szkudelski, T.; Delmas, D.; Daglia, M.; Xiao, J. Dietary polyphenols and type 2 diabetes: Human Study and Clinical Trial. Crit. Rev. Food Sci. Nutr. 2019, 59, 3371–3379. [Google Scholar] [CrossRef]
- Cai, Q.; Ji, S.; Li, M.; Zheng, S.; Zhou, X.; Guo, H.; Deng, S.; Zhu, J.; Li, D.; Xie, Z. Theaflavin-regulated Imd condensates control Drosophila intestinal homeostasis and aging. iScience 2021, 24, 102150. [Google Scholar] [CrossRef]
- Wang, J.; Qin, Y.; Jiang, J.; Shan, H.; Zhao, C.; Li, S. The Effect of Theaflavins on the Gut Microbiome and Metabolites in Diabetic Mice. Foods 2023, 12, 3865. [Google Scholar] [CrossRef]
- Łuczaj, W.; Skrzydlewska, E. Antioxidative properties of black tea. Prev. Med. 2005, 40, 910–918. [Google Scholar] [CrossRef]
- O’Neill, E.J.; Termini, D.; Albano, A.; Tsiani, E. Anti-Cancer Properties of Theaflavins. Molecules 2021, 26, 987. [Google Scholar] [CrossRef]
- Gothandam, K.; Ganesan, V.S.; Ayyasamy, T.; Ramalingam, S. Antioxidant potential of theaflavin ameliorates the activities of key enzymes of glucose metabolism in high fat diet and streptozotocin—Induced diabetic rats. Redox Rep. 2019, 24, 41–50. [Google Scholar] [CrossRef]
- Jin, D.; Xu, Y.; Mei, X.; Meng, Q.; Gao, Y.; Li, B.; Tu, Y. Antiobesity and lipid lowering effects of theaflavins on high-fat diet induced obese rats. Food Funct. 2013, 5, 1142–1150. [Google Scholar] [CrossRef]
- Wang, K.; Wu, J.; Chen, S.; Zhao, H.; He, P.; Tu, Y.; Li, B. Transcriptome analysis provides insight into the anti-diabetic mechanism of theaflavins in high-fat diet and streptozotocin-induced mice. Food Funct. 2022, 13, 2033–2043. [Google Scholar] [CrossRef]
- Fang, Y.; Wang, J.; Cao, Y.; Liu, W.; Duan, L.; Hu, J.; Peng, J. The Antiobesity Effects and Potential Mechanisms of Theaflavins. J. Med. Food 2023, 27, 1–11. [Google Scholar] [CrossRef]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Gaglia, J.L.; Hilliard, M.E.; Isaacs, D.; et al. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes—2023. Diabetes Care 2022, 46 (Suppl. S1), S19–S40. [Google Scholar] [CrossRef]
- Xing, J.; Chen, C. Hyperinsulinemia: Beneficial or harmful or both on glucose homeostasis. Am. J. Physiol. Endocrinol. Metab. 2022, 323, E2–E7. [Google Scholar] [CrossRef]
- Gothandam, K.; Ganesan, V.S.; Ayyasamy, T.; Ramalingam, S. Protective effect of theaflavin on glycoprotein components and TCA cycle enzymes in high-fat diet and streptozotocin-induced diabetic rats. J. Basic Appl. Zool. 2019, 80, 43. [Google Scholar] [CrossRef]
- Haythorne, E.; Rohm, M.; van de Bunt, M.; Brereton, M.F.; Tarasov, A.I.; Blacker, T.S.; Sachse, G.; Silva Dos Santos, M.; Terron Exposito, R.; Davis, S.; et al. Diabetes causes marked inhibition of mitochondrial metabolism in pancreatic β-cells. Nat. Commun. 2019, 10, 2474. [Google Scholar] [CrossRef]
- Krssak, M.; Brehm, A.; Bernroider, E.; Anderwald, C.; Nowotny, P.; Dalla Man, C.; Cobelli, C.; Cline, G.W.; Shulman, G.I.; Waldhäusl, W.; et al. Alterations in postprandial hepatic glycogen metabolism in type 2 diabetes. Diabetes 2004, 53, 3048–3056. [Google Scholar] [CrossRef]
- Dyer, J.; Wood, I.S.; Palejwala, A.; Ellis, A.; Shirazi-Beechey, S.P. Expression of monosaccharide transporters in intestine of diabetic humans. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 282, G241–G248. [Google Scholar] [CrossRef]
- Koepsell, H. Glucose transporters in the small intestine in health and disease. Pflugers Arch. 2020, 472, 1207–1248. [Google Scholar] [CrossRef]
- Maianu, L.; Keller, S.R.; Garvey, W.T. Adipocytes exhibit abnormal subcellular distribution and translocation of vesicles containing glucose transporter 4 and insulin-regulated aminopeptidase in type 2 diabetes mellitus: Implications regarding defects in vesicle trafficking. J. Clin. Endocrinol. Metab. 2001, 86, 5450–5456. [Google Scholar] [CrossRef]
- Tong, T.; Ren, N.; Soomi, P.; Wu, J.; Guo, N.; Kang, H.; Kim, E.; Wu, Y.; He, P.; Tu, Y.; et al. Theaflavins Improve Insulin Sensitivity through Regulating Mitochondrial Biosynthesis in Palmitic Acid-Induced HepG2 Cells. Molecules 2018, 23, 3382. [Google Scholar] [CrossRef]
- Jiang, S.; Young, J.L.; Wang, K.; Qian, Y.; Cai, L. Diabetic-induced alterations in hepatic glucose and lipid metabolism: The role of type 1 and type 2 diabetes mellitus (Review). Mol. Med. Rep. 2020, 22, 603–611. [Google Scholar] [CrossRef]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Laffin, M.; Fedorak, R.; Zalasky, A.; Park, H.; Gill, A.; Agrawal, A.; Keshteli, A.; Hotte, N.; Madsen, K.L. A high-sugar diet rapidly enhances susceptibility to colitis via depletion of luminal short-chain fatty acids in mice. Sci. Rep. 2019, 9, 12294. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Levy, M.; Grosheva, I.; Zheng, D.; Soffer, E.; Blacher, E.; Braverman, S.; Tengeler, A.C.; Barak, O.; Elazar, M.; et al. Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science 2018, 359, 1376–1383. [Google Scholar] [CrossRef]
- Im, S.S.; Kang, S.Y.; Kim, S.Y.; Kim, H.I.; Kim, J.W.; Kim, K.S.; Ahn, Y.H. Glucose-stimulated upregulation of GLUT2 gene is mediated by sterol response element-binding protein-1c in the hepatocytes. Diabetes 2005, 54, 1684–1691. [Google Scholar] [CrossRef]
- Huebschmann, A.G.; Regensteiner, J.G.; Vlassara, H.; Reusch, J.E. Diabetes and advanced glycoxidation end products. Diabetes Care 2006, 29, 1420–1432. [Google Scholar] [CrossRef]
- Khalid, M.; Petroianu, G.; Adem, A. Advanced Glycation End Products and Diabetes Mellitus: Mechanisms and Perspectives. Biomolecules 2022, 12, 542. [Google Scholar] [CrossRef]
- Fukami, K.; Yamagishi, S.; Okuda, S. Role of AGEs-RAGE system in cardiovascular disease. Curr. Pharm. Des. 2014, 20, 2395–2402. [Google Scholar] [CrossRef]
- Macdonald Ighodaro, O. Molecular pathways associated with oxidative stress in diabetes mellitus. Biomed. Pharmacother. 2018, 108, 656–662. [Google Scholar] [CrossRef]
- Cai, X.; Liu, Z.; Dong, X.; Wang, Y.; Zhu, L.; Li, M.; Xu, Y. Hypoglycemic and lipid lowering effects of theaflavins in high-fat diet-induced obese mice. Food Funct. 2021, 12, 9922–9931. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, M.; Takemoto, H.; Kouno, H.; Soeda, T.; Moriya, T. A Simple, Enzymatic Biotransformation Method Using Fresh Green Tea Leaves Efficiently Generates Theaflavin-Containing Fermentation Water That Has Potent Physiological Functions in Mice and Humans. Biol. Pharm. Bull. 2017, 40, 860–866. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, J.; Zhao, C.; Shan, H.; Shao, Z.; Wang, C.; Guan, J.; Xie, Z.; Li, S. The Protective Effect of Theaflavins on the Kidney of Mice with Type II Diabetes Mellitus. Nutrients 2022, 15, 201. [Google Scholar] [CrossRef]
- Li, B.; Fu, L.; Kojima, R.; Yamamoto, A.; Ueno, T.; Matsui, T. Theaflavins prevent the onset of diabetes through ameliorating glucose tolerance mediated by promoted incretin secretion in spontaneous diabetic Torii rats. J. Funct. Foods 2021, 86, 104702. [Google Scholar] [CrossRef]
- Bahorun, T.; Luximon-Ramma, A.; Neergheen-Bhujun, V.S.; Gunness, T.K.; Googoolye, K.; Auger, C.; Crozier, A.; Aruoma, O.I. The effect of black tea on risk factors of cardiovascular disease in a normal population. Prev. Med. 2012, 54, S98–S102. [Google Scholar] [CrossRef]
- Butacnum, A.; Chongsuwat, R.; Bumrungpert, A. Black tea consumption improves postprandial glycemic control in normal and pre-diabetic subjects: A randomized, double-blind, placebo-controlled crossover study. Asia Pac. J. Clin. Nutr. 2017, 26, 59–64. [Google Scholar] [CrossRef]
- Kondo, Y.; Goto, A.; Noma, H.; Iso, H.; Hayashi, K.; Noda, M. Effects of Coffee and Tea Consumption on Glucose Metabolism: A Systematic Review and Network Meta-Analysis. Nutrients 2018, 11, 48. [Google Scholar] [CrossRef]
- Anderson, R.A.; Polansky, M.M. Tea enhances insulin activity. J. Agric. Food Chem. 2002, 50, 7182–7186. [Google Scholar] [CrossRef]
- Zhou, H.; Wu, Y.; Kim, E.; Pan, H.; He, P.; Li, B.; Chen, Y.C.; Tu, Y. Simultaneous Tests of Theaflavin-3,3′-digallate as an Anti-Diabetic Drug in Human Hepatoma G2 Cells and Zebrafish (Danio rerio). Nutrients 2021, 13, 4379. [Google Scholar] [CrossRef]
- Lu, Y.; Lu, P.; Wang, Y.; Fang, X.; Wu, J.; Wang, X. A Novel Dipeptidyl Peptidase IV Inhibitory Tea Peptide Improves Pancreatic β-Cell Function and Reduces α-Cell Proliferation in Streptozotocin-Induced Diabetic Mice. Int. J. Mol. Sci. 2019, 20, 322. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, R.; Sundaram, R.; Thiagarajan, R.; Sivakumar, M.R.; Meiyalagan, V.; Arumugam, M. Effect of black tea on histological and immunohistochemical changes in pancreatic tissues of normal and streptozotocin-induced diabetic mice (Mus musculus). Microsc. Res. Tech. 2009, 72, 723–726. [Google Scholar] [CrossRef] [PubMed]
- Toshima, A.; Matsui, T.; Noguchi, M.; Qiu, J.; Tamaya, K.; Miyata, Y.; Tanaka, T.; Tanaka, K. Identification of alpha-glucosidase inhibitors from a new fermented tea obtained by tea-rolling processing of loquat (Eriobotrya japonica) and green tea leaves. J. Sci. Food Agric. 2010, 90, 1545–1550. [Google Scholar] [CrossRef] [PubMed]
- Hara, Y.; Honda, M. The Inhibition of α-Amylase by Tea Polyphenols. Agric. Biol. Chem. 1990, 54, 1939–1945. [Google Scholar] [CrossRef]
- Kan, L.; Capuano, E.; Fogliano, V.; Verkerk, R.; Mes, J.J.; Tomassen, M.M.M.; Oliviero, T. Inhibition of α-glucosidases by tea polyphenols in rat intestinal extract and Caco-2 cells grown on Transwell. Food Chem. 2021, 361, 130047. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G. Possible effects of dietary polyphenols on sugar absorption and digestion. Mol. Nutr. Food Res. 2013, 57, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Tanaka, T.; Tamura, S.; Toshima, A.; Tamaya, K.; Miyata, Y.; Tanaka, K.; Matsumoto, K. alpha-Glucosidase inhibitory profile of catechins and theaflavins. J. Agric. Food Chem. 2007, 55, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Tsimihodimos, V.; Filippas-Ntekouan, S.; Elisaf, M. SGLT1 inhibition: Pros and cons. Eur. J. Pharmacol. 2018, 838, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Turk, E.; Zabel, B.; Mundlos, S.; Dyer, J.; Wright, E.M. Glucose/galactose malabsorption caused by a defect in the Na+/glucose cotransporter. Nature 1991, 350, 354–356. [Google Scholar] [CrossRef]
- Li, B.; Fu, L.; Abe, C.; Nectoux, A.M.; Yamamoto, A.; Matsui, T. Theaflavins inhibit glucose transport across Caco-2 cells through the downregulation of the Ca2+/AMP-activated protein kinase-mediated glucose transporter SGLT1. J. Funct. Foods 2020, 75, 104273. [Google Scholar] [CrossRef]
- Pereira-Caro, G.; Moreno-Rojas, J.M.; Brindani, N.; Del Rio, D.; Lean, M.E.J.; Hara, Y.; Crozier, A. Bioavailability of Black Tea Theaflavins: Absorption, Metabolism, and Colonic Catabolism. J. Agric. Food Chem. 2017, 65, 5365–5374. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.-N.; Tanaka, M.; Li, B.; Ueno, T.; Matsuda, H.; Matsui, T. Novel in situ visualisation of rat intestinal absorption of polyphenols via matrix-assisted laser desorption/ionisation mass spectrometry imaging. Sci. Rep. 2019, 9, 3166. [Google Scholar] [CrossRef] [PubMed]
- Nishiumi, S.; Bessyo, H.; Kubo, M.; Aoki, Y.; Tanaka, A.; Yoshida, K.; Ashida, H. Green and black tea suppress hyperglycemia and insulin resistance by retaining the expression of glucose transporter 4 in muscle of high-fat diet-fed C57BL/6J mice. J. Agric. Food Chem. 2010, 58, 12916–12923. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Wang, L.; Tinshun, Z.; Nakamura, T.; Ashida, H. Fermented tea improves glucose intolerance in mice by enhancing translocation of glucose transporter 4 in skeletal muscle. J. Agric. Food Chem. 2012, 60, 11366–11371. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Liu, A.; Liu, C.; Tang, Q.; Zhan, L.; Xiao, W.; Huang, J.; Liu, Z.; Zhang, S. Theaflavin Promotes Mitochondrial Abundance and Glucose Absorption in Myotubes by Activating the CaMKK2-AMPK Signal Axis via Calcium-Ion Influx. J. Agric. Food Chem. 2021, 69, 8144–8159. [Google Scholar] [CrossRef] [PubMed]
- Nomura, M.; Takahashi, T.; Nagata, N.; Tsutsumi, K.; Kobayashi, S.; Akiba, T.; Yokogawa, K.; Moritani, S.; Miyamoto, K. Inhibitory mechanisms of flavonoids on insulin-stimulated glucose uptake in MC3T3-G2/PA6 adipose cells. Biol. Pharm. Bull. 2008, 31, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Cameron, A.R.; Anton, S.; Melville, L.; Houston, N.P.; Dayal, S.; McDougall, G.J.; Stewart, D.; Rena, G. Black tea polyphenols mimic insulin/insulin-like growth factor-1 signalling to the longevity factor FOXO1a. Aging Cell 2008, 7, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-Y.; Kunitake, Y.; Hirasaki, N.; Tanaka, M.; Matsui, T. Theaflavins enhance intestinal barrier of Caco-2 Cell monolayers through the expression of AMP-activated protein kinase-mediated Occludin, Claudin-1, and ZO-1. Biosci. Biotechnol. Biochem. 2015, 79, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ohland, C.; Jobin, C.; Sang, S. Black Tea Theaflavin Detoxifies Metabolic Toxins in the Intestinal Tract of Mice. Mol. Nutr. Food Res. 2021, 65, e2000887. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, W.; Lin, H.; Zhang, L.; Wu, F.; Wang, Y.; Yu, S.; Peng, X.; Cheng, W.; Li, M.; et al. Effect of theaflavin-3,3′-digallate on leptin-deficient induced nonalcoholic fatty liver disease might be related to lipid metabolism regulated by the Fads1/PPARδ/Fabp4 axis and gut microbiota. Front. Pharmacol. 2022, 13, 925264. [Google Scholar] [CrossRef]
- Rungratanawanich, W.; Qu, Y.; Wang, X.; Essa, M.M.; Song, B.-J. Advanced glycation end products (AGEs) and other adducts in aging-related diseases and alcohol-mediated tissue injury. Exp. Mol. Med. 2021, 53, 168–188. [Google Scholar] [CrossRef] [PubMed]
- Allaman, I.; Bélanger, M.; Magistretti, P.J. Methylglyoxal, the dark side of glycolysis. Front. Neurosci. 2015, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Uribarri, J.; Cai, W.; Ramdas, M.; Goodman, S.; Pyzik, R.; Chen, X.; Zhu, L.; Striker, G.E.; Vlassara, H. Restriction of advanced glycation end products improves insulin resistance in human type 2 diabetes: Potential role of AGER1 and SIRT1. Diabetes Care 2011, 34, 1610–1616. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Kim, Y.J.; Khang, A.R.; Eckel, R.H. Postprandial dyslipidemia after a standardized high-fat meal in BMI-matched healthy individuals, and in subjects with prediabetes or type 2 diabetes. Clin. Nutr. 2021, 40, 5538–5546. [Google Scholar] [CrossRef] [PubMed]
- Duez, H.; Lamarche, B.; Uffelman, K.D.; Valero, R.; Cohn, J.S.; Lewis, G.F. Hyperinsulinemia is associated with increased production rate of intestinal apolipoprotein B-48-containing lipoproteins in humans. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1357–1363. [Google Scholar] [CrossRef] [PubMed]
- Vergès, B. Intestinal lipid absorption and transport in type 2 diabetes. Diabetologia 2022, 65, 1587–1600. [Google Scholar] [CrossRef]
- Simonen, P.P.; Gylling, H.K.; Miettinen, T.A. Diabetes Contributes to Cholesterol Metabolism Regardless of Obesity. Diabetes Care 2002, 25, 1511–1515. [Google Scholar] [CrossRef] [PubMed]
- Veilleux, A.; Grenier, E.; Marceau, P.; Carpentier, A.C.; Richard, D.; Levy, E. Intestinal lipid handling: Evidence and implication of insulin signaling abnormalities in human obese subjects. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 644–653. [Google Scholar] [CrossRef]
- Roden, M. Mechanisms of Disease: Hepatic steatosis in type 2 diabetes—Pathogenesis and clinical relevance. Nat. Clin. Pract. Endocrinol. Metab. 2006, 2, 335–348. [Google Scholar] [CrossRef]
- Taylor, R.; Al-Mrabeh, A.; Sattar, N. Understanding the mechanisms of reversal of type 2 diabetes. Lancet Diabetes Endocrinol. 2019, 7, 726–736. [Google Scholar] [CrossRef]
- Wagner, R.; Eckstein, S.S.; Yamazaki, H.; Gerst, F.; Machann, J.; Jaghutriz, B.A.; Schürmann, A.; Solimena, M.; Singer, S.; Königsrainer, A.; et al. Metabolic implications of pancreatic fat accumulation. Nat. Rev. Endocrinol. 2022, 18, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Heiskanen, M.A.; Motiani, K.K.; Mari, A.; Saunavaara, V.; Eskelinen, J.J.; Virtanen, K.A.; Koivumäki, M.; Löyttyniemi, E.; Nuutila, P.; Kalliokoski, K.K.; et al. Exercise training decreases pancreatic fat content and improves beta cell function regardless of baseline glucose tolerance: A randomised controlled trial. Diabetologia 2018, 61, 1817–1828. [Google Scholar] [CrossRef] [PubMed]
- Fromenty, B.; Roden, M. Mitochondrial alterations in fatty liver diseases. J. Hepatol. 2023, 78, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Pinti, M.V.; Fink, G.K.; Hathaway, Q.A.; Durr, A.J.; Kunovac, A.; Hollander, J.M. Mitochondrial dysfunction in type 2 diabetes mellitus: An organ-based analysis. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E268–E285. [Google Scholar] [CrossRef] [PubMed]
- Larsen, N.; Vogensen, F.K.; van den Berg, F.W.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Al-Soud, W.A.; Sørensen, S.J.; Hansen, L.H.; Jakobsen, M. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS ONE 2010, 5, e9085. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.l.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R.m. Changes in Gut Microbiota Control Metabolic Endotoxemia-Induced Inflammation in High-Fat Diet–Induced Obesity and Diabetes in Mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef] [PubMed]
- Marrano, N.; Biondi, G.; Borrelli, A.; Rella, M.; Zambetta, T.; Di Gioia, L.; Caporusso, M.; Logroscino, G.; Perrini, S.; Giorgino, F.; et al. Type 2 Diabetes and Alzheimer’s Disease: The Emerging Role of Cellular Lipotoxicity. Biomolecules 2023, 13, 183. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.; Onodera, T.; Scherer, P.E. Lipotoxicity and β Cell Maintenance in Obesity and Type 2 Diabetes. J. Endocr. Soc. 2019, 3, 617–631. [Google Scholar] [CrossRef]
- Maedler, K.; Spinas, G.A.; Dyntar, D.; Moritz, W.; Kaiser, N.; Donath, M.Y. Distinct Effects of Saturated and Monounsaturated Fatty Acids on β-Cell Turnover and Function. Diabetes 2001, 50, 69–76. [Google Scholar] [CrossRef]
- Lee, H.M.; Kim, J.J.; Kim, H.J.; Shong, M.; Ku, B.J.; Jo, E.K. Upregulated NLRP3 inflammasome activation in patients with type 2 diabetes. Diabetes 2013, 62, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Sha, W.; Hu, F.; Xi, Y.; Chu, Y.; Bu, S. Mechanism of Ferroptosis and Its Role in Type 2 Diabetes Mellitus. J. Diabetes Res. 2021, 2021, 9999612. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; An, R.; Li, Q.; Sun, L.; Lai, X.; Chen, R.; Li, D.; Sun, S. Theaflavin TF3 Relieves Hepatocyte Lipid Deposition through Activating an AMPK Signaling Pathway by targeting Plasma Kallikrein. J. Agric. Food Chem. 2020, 68, 2673–2683. [Google Scholar] [CrossRef] [PubMed]
- Hartley, L.; Flowers, N.; Holmes, J.; Clarke, A.; Stranges, S.; Hooper, L.; Rees, K. Green and black tea for the primary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2013, 2013, Cd009934. [Google Scholar] [CrossRef] [PubMed]
- Stensvold, I.; Tverdal, A.; Solvoll, K.; Foss, O.P. Tea consumption. relationship to cholesterol, blood pressure, and coronary and total mortality. Prev. Med. 1992, 21, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Maron, D.J.; Lu, G.P.; Cai, N.S.; Wu, Z.G.; Li, Y.H.; Chen, H.; Zhu, J.Q.; Jin, X.J.; Wouters, B.C.; Zhao, J. Cholesterol-lowering effect of a theaflavin-enriched green tea extract: A randomized controlled trial. Arch. Intern. Med. 2003, 163, 1448–1453. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, T.; Yamamoto, A.; Ueno, T. Effect of oral theaflavin administration on body weight, fat, and muscle in healthy subjects: A randomized pilot study. Biosci. Biotechnol. Biochem. 2017, 81, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Hamdaoui, M.H.; Snoussi, C.; Dhaouadi, K.; Fattouch, S.; Ducroc, R.; Le Gall, M.; Bado, A. Tea decoctions prevent body weight gain in rats fed high-fat diet; black tea being more efficient than green tea. J. Nutr. Intermed. 2016, 6, 33–40. [Google Scholar] [CrossRef]
- Birari, R.B.; Bhutani, K.K. Pancreatic lipase inhibitors from natural sources: Unexplored potential. Drug Discov. Today 2007, 12, 879–889. [Google Scholar] [CrossRef]
- Glisan, S.L.; Grove, K.A.; Yennawar, N.H.; Lambert, J.D. Inhibition of pancreatic lipase by black tea theaflavins: Comparative enzymology and in silico modeling studies. Food Chem. 2017, 01, 296–300. [Google Scholar] [CrossRef]
- Uchiyama, S.; Taniguchi, Y.; Saka, A.; Yoshida, A.; Yajima, H. Prevention of diet-induced obesity by dietary black tea polyphenols extract in vitro and in vivo. Nutrition 2011, 27, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Ichitani, M.; Suzuki, Y.; Unno, T.; Sugawara, T.; Yamahira, T.; Kato, M.; Takihara, T.; Sagesaka, Y.; Kakuda, T.; et al. Black-tea polyphenols suppress postprandial hypertriacylglycerolemia by suppressing lymphatic transport of dietary fat in rats. J. Agric. Food Chem. 2009, 57, 7131–7136. [Google Scholar] [CrossRef] [PubMed]
- Shishikura, Y.; Khokhar, S.; Murray, B.S. Effects of tea polyphenols on emulsification of olive oil in a small intestine model system. J. Agric. Food Chem. 2006, 54, 1906–1913. [Google Scholar] [CrossRef] [PubMed]
- Meier, J.J.; Gethmann, A.; Götze, O.; Gallwitz, B.; Holst, J.J.; Schmidt, W.E.; Nauck, M.A. Glucagon-like peptide 1 abolishes the postprandial rise in triglyceride concentrations and lowers levels of non-esterified fatty acids in humans. Diabetologia 2006, 49, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Shen, H.; Liu, M.; Yang, Q.; Zheng, S.; Sabo, M.; D’Alessio, D.A.; Tso, P. GLP-1 reduces intestinal lymph flow, triglyceride absorption, and apolipoprotein production in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 288, G943–G949. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Müller, T.D. Incretin hormones and type 2 diabetes. Diabetologia 2023, 66, 1780–1795. [Google Scholar] [CrossRef] [PubMed]
- Takashima, Y.; Ishikawa, K.; Miyawaki, R.; Ogawa, M.; Ishii, T.; Misaka, T.; Kobayashi, S. Modulatory Effect of Theaflavins on Apical Sodium-Dependent Bile Acid Transporter (ASBT) Activity. J. Agric. Food Chem. 2021, 69, 9585–9596. [Google Scholar] [CrossRef]
- Vermeer, M.A.; Mulder, T.P.; Molhuizen, H.O. Theaflavins from black tea, especially theaflavin-3-gallate, reduce the incorporation of cholesterol into mixed micelles. J. Agric. Food Chem. 2008, 56, 12031–12036. [Google Scholar] [CrossRef]
- Kudo, N.; Arai, Y.; Suhara, Y.; Ishii, T.; Nakayama, T.; Osakabe, N. A Single Oral Administration of Theaflavins Increases Energy Expenditure and the Expression of Metabolic Genes. PLoS ONE 2015, 10, e0137809. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.T.; Wang, X.; Wu, X.D.; Tian, W.X. Keemun black tea extract contains potent fatty acid synthase inhibitors and reduces food intake and body weight of rats via oral administration. J. Enzyme Inhib. Med. Chem. 2005, 20, 349–356. [Google Scholar] [CrossRef]
- Yeh, C.W.; Chen, W.J.; Chiang, C.T.; Lin-Shiau, S.Y.; Lin, J.K. Suppression of fatty acid synthase in MCF-7 breast cancer cells by tea and tea polyphenols: A possible mechanism for their hypolipidemic effects. Pharmacogenom. J. 2003, 3, 267–276. [Google Scholar] [CrossRef]
- Huang, H.C.; Lin, J.K. Pu-erh tea, green tea, and black tea suppresses hyperlipidemia, hyperleptinemia and fatty acid synthase through activating AMPK in rats fed a high-fructose diet. Food Funct. 2012, 3, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Shackelford, D.B.; Shaw, R.J. The LKB1-AMPK pathway: Metabolism and growth control in tumour suppression. Nat. Rev. Cancer. 2009, 9, 563–575. [Google Scholar] [CrossRef]
- Lin, C.L.; Huang, H.C.; Lin, J.K. Theaflavins attenuate hepatic lipid accumulation through activating AMPK in human HepG2 cells. J. Lipid Res. 2007, 48, 2334–2343. [Google Scholar] [CrossRef]
- Lu, S.; Archer, M.C. Sp1 coordinately regulates de novo lipogenesis and proliferation in cancer cells. Int. J. Cancer 2010, 126, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Luo, T.; Li, X.; Liu, X.; Deng, Z.Y. Comparison of fresh and browning lotus roots (Nelumbo nucifera Gaertn.) on modulating cholesterol metabolism via decreasing hepatic cholesterol deposition and increasing fecal bile acid excretion. Curr. Res. Food Sci. 2023, 7, 100630. [Google Scholar] [CrossRef] [PubMed]
- Boß, M.; Kemmerer, M.; Brüne, B.; Namgaladze, D. FABP4 inhibition suppresses PPARγ activity and VLDL-induced foam cell formation in IL-4-polarized human macrophages. Atherosclerosis 2015, 240, 424–430. [Google Scholar] [CrossRef]
- Feener, E.P.; Zhou, Q.; Fickweiler, W. Role of plasma kallikrein in diabetes and metabolism. Thromb. Haemost. 2013, 110, 434–441. [Google Scholar] [CrossRef]
- Kim, J.E.; Kim, J.S.; Jo, M.J.; Cho, E.; Ahn, S.Y.; Kwon, Y.J.; Ko, G.J. The Roles and Associated Mechanisms of Adipokines in Development of Metabolic Syndrome. Molecules 2022, 27, 334. [Google Scholar] [CrossRef]
- Wang, Z.V.; Scherer, P.E. Adiponectin, the past two decades. J. Mol. Cell Biol. 2016, 8, 93–100. [Google Scholar] [CrossRef]
- Straub, L.G.; Scherer, P.E. Metabolic Messengers: Adiponectin. Nat. Metab. 2019, 1, 334–339. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Adipocytokines: Mediators linking adipose tissue, inflammation and immunity. Nat. Rev. Immunol. 2006, 6, 772–783. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yu, Z.; Zhou, H.H.; Feng, Y.; Bu, Y.; Zhai, D.; Zhang, G.; Ding, S.; Wang, E.; Mi, Y.; et al. Effect of flavonoid intake on circulating levels of adiponectin and leptin: A systematic review and meta-analysis of randomized controlled clinical trials. Phytother. Res. 2022, 36, 4139–4154. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.A.; Park, Y.L.; Yoon, S.H.; Kim, K.Y.; Cho, S.B.; Lee, W.S.; Chung, I.J.; Joo, Y.E. Black tea polyphenol theaflavin suppresses LPS-induced ICAM-1 and VCAM-1 expression via blockage of NF-κB and JNK activation in intestinal epithelial cells. Inflamm. Res. 2011, 60, 493–500. [Google Scholar] [CrossRef]
- Luczaj, W.; Welerowicz, T.; Skrzydlewska, E.; Buszewski, B. Chromatographic Examinations of Tea’s Protection Against Lipid Oxidative Modifications. Toxicol. Mech. Methods. 2008, 18, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Saliminejad, K.; Khorram Khorshid, H.R.; Soleymani Fard, S.; Ghaffari, S.H. An overview of microRNAs: Biology, functions, therapeutics, and analysis methods. J. Cell. Physiol. 2019, 234, 5451–5465. [Google Scholar] [CrossRef] [PubMed]
- Ye, T.; Yang, X.; Liu, H.; Lv, P.; Lu, H.; Jiang, K.; Peng, E.; Ye, Z.; Chen, Z.; Tang, K. Theaflavin protects against oxalate calcium-induced kidney oxidative stress injury via upregulation of SIRT1. Int. J. Biol. Sci. 2021, 17, 1050–1060. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, J.; Wan, Z.; Li, G.; Chen, L.; Guo, Y. Theaflavin ameliorates renal ischemia/reperfusion injury by activating the Nrf2 signalling pathway in vivo and in vitro. Biomed. Pharmacother. 2021, 134, 111097. [Google Scholar] [CrossRef]
- Zeng, J.; Deng, Z.; Zou, Y.; Liu, C.; Fu, H.; Gu, Y.; Chang, H. Theaflavin alleviates oxidative injury and atherosclerosis progress via activating microRNA-24-mediated Nrf2/HO-1 signal. Phytother. Res. 2021, 35, 3418–3427. [Google Scholar] [CrossRef]
- Grelle, G.; Otto, A.; Lorenz, M.; Frank, R.F.; Wanker, E.E.; Bieschke, J. Black tea theaflavins inhibit formation of toxic amyloid-β and α-synuclein fibrils. Biochemistry 2011, 50, 10624–10636. [Google Scholar] [CrossRef]
- Phan, H.T.T.; Samarat, K.; Takamura, Y.; Azo-Oussou, A.F.; Nakazono, Y.; Vestergaard, M.C. Polyphenols Modulate Alzheimer’s Amyloid Beta Aggregation in a Structure-Dependent Manner. Nutrients 2019, 11, 756. [Google Scholar] [CrossRef] [PubMed]
- Jing, Z.; Jianan, H.; Shuxian, C.; Xiaoqin, Y.; Jianjun, L.; Yingzi, W.; Lili1, T.; Zhonghua, L. Theaflavins and EGCG Protect SH-SY5Y Cells from Oxidative Damage Induced by Amyloid-β 1-42 and Inhibit the Level of Aβ42 in vivo and in vitro. Tea Sci. 2016, 36, 655–662. [Google Scholar] [CrossRef]
- Zhu, Q.; Zheng, Z.P.; Cheng, K.W.; Wu, J.J.; Zhang, S.; Tang, Y.S.; Sze, K.H.; Chen, J.; Chen, F.; Wang, M. Natural polyphenols as direct trapping agents of lipid peroxidation-derived acrolein and 4-hydroxy-trans-2-nonenal. Chem. Res. Toxicol. 2009, 22, 1721–1727. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Ni, S.; Xu, N.; Yin, G.; Yu, Y.; Zhou, B.; Zhao, G.; Wang, L.; Zhu, R.; Jiang, S.; et al. Theaflavin-3,3′-Digallate Inhibits Erastin-Induced Chondrocytes Ferroptosis via the Nrf2/GPX4 Signaling Pathway in Osteoarthritis. Oxid. Med. Cell. Longev. 2022, 2022, 3531995. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.-C.; Wu, B.-T.; Tsuei, Y.-W.; Shih, L.-J.; Kuo, Y.-L.; Kao, Y.-H. Green Tea Catechins: Inhibitors of Glycerol-3-Phosphate Dehydrogenase. Planta Med. 2010, 76, 694–696. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-Y.; Li, W.; Xu, Y.; Jin, E.-H.; Tu, Y.-Y. Evaluation of the antioxidant effects of four main theaflavin derivatives through chemiluminescence and DNA damage analyses. J. Zhejiang Univ. Sci. B 2011, 12, 744–751. [Google Scholar] [CrossRef]
- Wenying, Y. Gut hormones in obesity and diabetes. Chin. J. Diabetes 2015, 23, 1142–1148. [Google Scholar] [CrossRef]
- Gribble, F.M.; Reimann, F. Metabolic Messengers: Glucagon-like peptide 1. Nat. Metab. 2021, 3, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Heppner, K.M.; Perez-Tilve, D. GLP-1 based therapeutics: Simultaneously combating T2DM and obesity. Front. Neurosci. 2015, 9, 92. [Google Scholar] [CrossRef]
- Andersen, A.; Lund, A.; Knop, F.K.; Vilsbøll, T. Glucagon-like peptide 1 in health and disease. Nat. Rev. Endocrinol. 2018, 14, 390–403. [Google Scholar] [CrossRef]
- King, A.; Yang, Q.; Huesman, S.; Rider, T.; Lo, C.C. Lipid transport in cholecystokinin knockout mice. Physiol. Behav. 2015, 151, 198–206. [Google Scholar] [CrossRef]
- Hammoud, R.; Drucker, D.J. Beyond the pancreas: Contrasting cardiometabolic actions of GIP and GLP1. Nat. Rev. Endocrinol. 2023, 19, 201–216. [Google Scholar] [CrossRef]
- Nogueiras, R.; Nauck, M.A.; Tschöp, M.H. Gut hormone co-agonists for the treatment of obesity: From bench to bedside. Nat. Metab. 2023, 5, 933–944. [Google Scholar] [CrossRef]
- Dotson, C.D.; Zhang, L.; Xu, H.; Shin, Y.K.; Vigues, S.; Ott, S.H.; Elson, A.E.; Choi, H.J.; Shaw, H.; Egan, J.M.; et al. Bitter taste receptors influence glucose homeostasis. PLoS ONE 2008, 3, e3974. [Google Scholar] [CrossRef]
- Tuzim, K.; Korolczuk, A. An update on extra-oral bitter taste receptors. J. Transl. Med. 2021, 19, 440. [Google Scholar] [CrossRef] [PubMed]
- Le Nevé, B.; Foltz, M.; Daniel, H.; Gouka, R. The steroid glycoside H.g.-12 from Hoodia gordonii activates the human bitter receptor TAS2R14 and induces CCK release from HuTu-80 cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, 1368–1375. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, T.; Sagisaka, M.; Ikeda, R.; Nakamura, T.; Matsuda, N.; Ishii, T.; Nakayama, T.; Watanabe, T. The human bitter taste receptor hTAS2R39 is the primary receptor for the bitterness of theaflavins. Biosci. Biotechnol. Biochem. 2014, 78, 1753–1756. [Google Scholar] [CrossRef] [PubMed]
- Steffensen, K.R.; Gustafsson, J.-A.k. Putative Metabolic Effects of the Liver X Receptor (LXR). Diabetes 2004, 53, S36–S42. [Google Scholar] [CrossRef]
- Ben Aissa, M.; Lewandowski, C.T.; Ratia, K.M.; Lee, S.H.; Layden, B.T.; LaDu, M.J.; Thatcher, G.R.J. Discovery of Nonlipogenic ABCA1 Inducing Compounds with Potential in Alzheimer’s Disease and Type 2 Diabetes. ACS Pharmacol. Transl. Sci. 2021, 4, 143–154. [Google Scholar] [CrossRef]
- Loren, J.; Huang, Z.; Laffitte, B.A.; Molteni, V. Liver X receptor modulators: A review of recently patented compounds (2009–2012). Expert Opin. Ther. Pat. 2013, 23, 1317–1335. [Google Scholar] [CrossRef]
- Griffett, K.; Burris, T.P. Development of LXR inverse agonists to treat MAFLD, NASH, and other metabolic diseases. Front. Med. 2023, 10, 1102469. [Google Scholar] [CrossRef] [PubMed]
- Adigun, T.O.; Danazumi, A.U.; Umar, H.I.; Na’Allah, A.; Alabi, M.A.; Joel, W.O.; Aberuagba, A.; Alejolowo, O.O.; Bamidele, J.O.; Omotayo, O.S.; et al. In silico molecular modeling and simulations of black tea theaflavins revealed theaflavin-3′-gallate as putative liver X receptor-beta agonist. J. Biomol. Struct. Dyn. 2023, 41, 13015–13028. [Google Scholar] [CrossRef]
- Yao, W.; Yinqiao, J.; Shuangmei, W.; Guoping, T.; Chaoke, T. Role of EOXO1 in glucose metabolism and lipid metabolism and hypertriglyceridemia. Life Sci. 2021, 41, 642–647. [Google Scholar] [CrossRef]
- Singh, B.N.; Rawat, A.K.; Bhagat, R.M.; Singh, B.R. Black tea: Phytochemicals, cancer chemoprevention, and clinical studies. Crit. Rev. Food Sci. Nutr. 2017, 57, 1394–1410. [Google Scholar] [CrossRef] [PubMed]
- Filhoulaud, G.; Guilmeau, S.; Dentin, R.; Girard, J.; Postic, C. Novel insights into ChREBP regulation and function. Trends Endocrinol. Metab. 2013, 24, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Li, M.V.; Chen, W.; Harmancey, R.N.; Nuotio-Antar, A.M.; Imamura, M.; Saha, P.; Taegtmeyer, H.; Chan, L. Glucose-6-phosphate mediates activation of the carbohydrate responsive binding protein (ChREBP). Biochem. Biophys. Res. Commun. 2010, 395, 395–400. [Google Scholar] [CrossRef]
- Arden, C.; Tudhope, S.J.; Petrie, J.L.; Al-Oanzi, Z.H.; Cullen, K.S.; Lange, A.J.; Towle, H.C.; Agius, L. Fructose 2,6-bisphosphate is essential for glucose-regulated gene transcription of glucose-6-phosphatase and other ChREBP target genes in hepatocytes. Biochem. J. 2012, 443, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.; Ai, Z.; Liu, S.; Zhang, H.; Chen, Y.; Wang, Y.; Ni, D. Study on mechanism of low bioavailability of black tea theaflavins by using Caco-2 cell monolayer. Drug Deliv. 2021, 28, 1737–1747. [Google Scholar] [CrossRef] [PubMed]
- Mulder, T.P.J.; van Platerink, C.J.; Wijnand Schuyl, P.J.; van Amelsvoort, J.M.M. Analysis of theaflavins in biological fluids using liquid chromatography–electrospray mass spectrometry. J. Chromatogr. B Biomed. Sci. Appl. 2001, 760, 271–279. [Google Scholar] [CrossRef]
- Sun, L.; Su, Y.; Hu, K.; Li, D.; Guo, H.; Xie, Z. Microbial-Transferred Metabolites of Black Tea Theaflavins by Human Gut Microbiota and Their Impact on Antioxidant Capacity. Molecules 2023, 28, 5871. [Google Scholar] [CrossRef]
- Henning, S.M.; Aronson, W.; Niu, Y.; Conde, F.; Lee, N.H.; Seeram, N.P.; Lee, R.P.; Lu, J.; Harris, D.M.; Moro, A.; et al. Tea polyphenols and theaflavins are present in prostate tissue of humans and mice after green and black tea consumption. J. Nutr. 2006, 136, 1839–1843. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zheng, T.; Jin, W.; Shi, Y.; Huang, Q. Enhancing Intestinal Permeability of Theaflavin-3,3′-digallate by Chitosan-Caseinophosphopeptides Nanocomplexes. J. Agric. Food Chem. 2022, 70, 2029–2041. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Inoue, K.; Betsumiya, Y.; Mine, C.; Kouno, I. Two types of oxidative dimerization of the black tea polyphenol theaflavin. J. Agric. Food Chem. 2001, 49, 5785–5789. [Google Scholar] [CrossRef] [PubMed]
- Zi-yin, Y. Isolation, Identification of Polyphenols in Black Tea and Tea (Camellia sinensis) Flower and Studies on Their Antioxidant Function and Mechanism. Ph.D. Thesis, Zhejiang University, Hangzhou, China, 2007. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, S.; Chen, Y.; Gong, Y. Improvement of Theaflavins on Glucose and Lipid Metabolism in Diabetes Mellitus. Foods 2024, 13, 1763. https://doi.org/10.3390/foods13111763
Xu S, Chen Y, Gong Y. Improvement of Theaflavins on Glucose and Lipid Metabolism in Diabetes Mellitus. Foods. 2024; 13(11):1763. https://doi.org/10.3390/foods13111763
Chicago/Turabian StyleXu, Shiyu, Ying Chen, and Yushun Gong. 2024. "Improvement of Theaflavins on Glucose and Lipid Metabolism in Diabetes Mellitus" Foods 13, no. 11: 1763. https://doi.org/10.3390/foods13111763





