Neuroprotective Effects of Curcumin in Neurodegenerative Diseases
Abstract
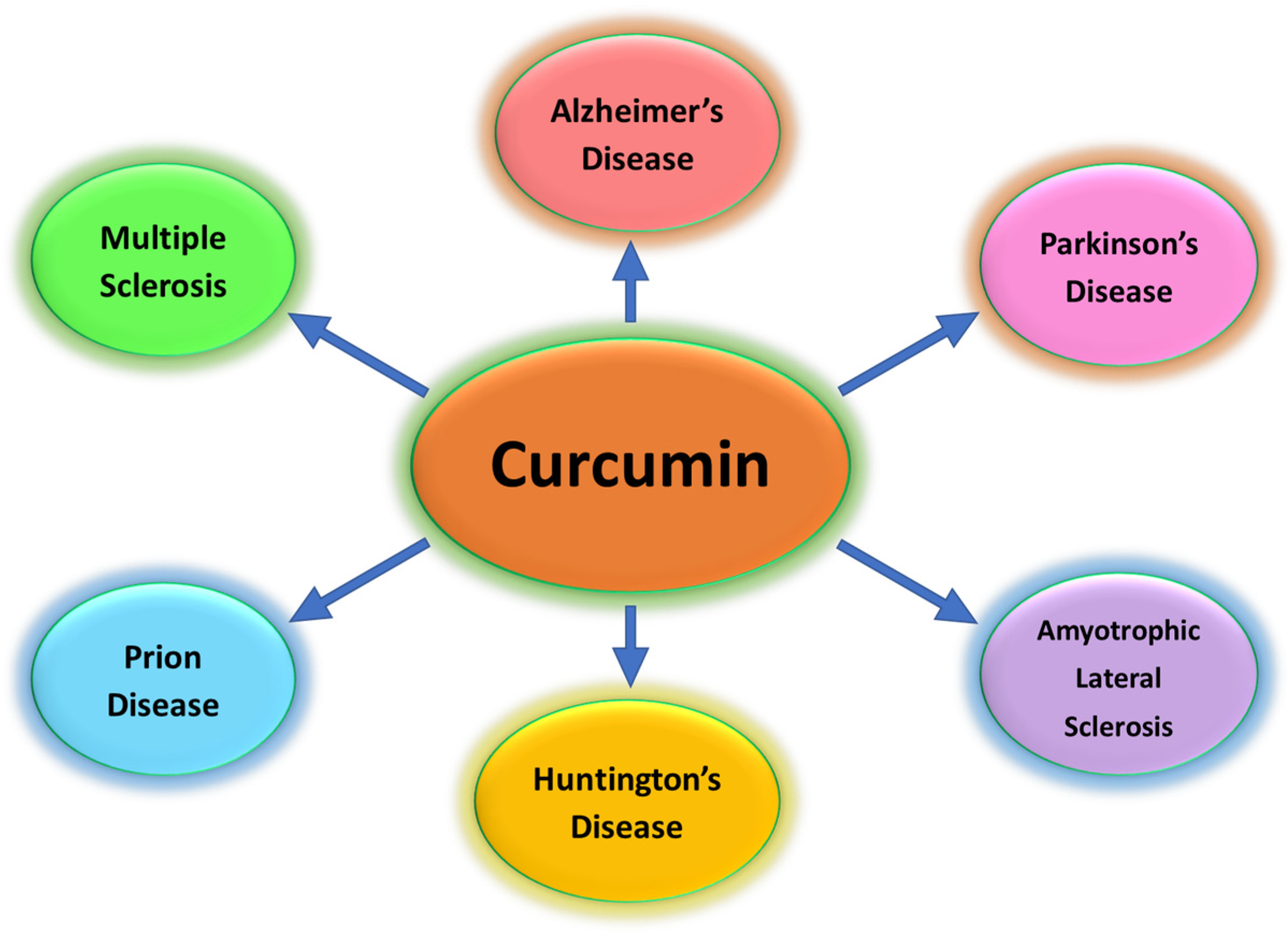
1. Introduction
2. Curcumin: Bioavailability and Metabolism
3. Curcumin Nanoparticles
3.1. Curcumin Nanoparticles in Parkinson’s Disease
3.2. Curcumin Nanoparticles in Alzheimer’s Disease
3.3. Curcumin Nanoparticles in Huntington’s Disease
3.4. Curcumin Nanoparticles in Amyotrophic Lateral Sclerosis
3.5. Curcumin Nanoparticles in Multiple Sclerosis
3.6. Curcumin Nanoparticles in Prion Disease
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Halliwell, B. Role of free radicals in the neurodegenerative diseases: Therapeutic implications for antioxidant treatment. Drugs Aging 2001, 18, 685–716. [Google Scholar] [CrossRef] [PubMed]
- Golpich, M.; Amini, E.; Mohamed, Z.; Azman Ali, R.; Mohamed Ibrahim, N.; Ahmadiani, A. Mitochondrial dysfunction and biogenesis in neurodegenerative diseases: Pathogenesis and treatment. CNS Neurosci. Ther. 2017, 23, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Cannon, J.R.; Greenamyre, J.T. The role of environmental exposures in neurodegeneration and neurodegenerative diseases. Toxicol. Sci. 2011, 124, 225–250. [Google Scholar] [CrossRef] [PubMed]
- Bássoli, R.M.F.; Audi, D.; Ramalho, B.J.; Audi, M.; Quesada, K.R.; Barbalho, S.M. The Effects of Curcumin on Neurodegenerative Diseases: A Systematic Review. J. Herb. Med. 2023, 42, 100771. [Google Scholar] [CrossRef]
- Spanoudaki, M.; Papadopoulou, S.K.; Antasouras, G.; Papadopoulos, K.A.; Psara, E.; Vorvolakos, T.; Solovos, E.; Chrysafi, M.; Psallas, M.; Mentzelou, M.; et al. Curcumin as a Multifunctional Spice Ingredient against Mental Disorders in Humans: Current Clinical Studies and Bioavailability Concerns. Life 2024, 14, 479. [Google Scholar] [CrossRef] [PubMed]
- Gitler, A.D.; Dhillon, P.; Shorter, J. Neurodegenerative disease: Models, mechanisms, and a new hope. Dis. Model. Mech. 2017, 10, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Ceramella, J.; De Maio, A.C.; Basile, G.; Facente, A.; Scali, E.; Andreu, I.; Sinicropi, M.S.; Iacopetta, D.; Catalano, A. Phytochemicals Involved in Mitigating Silent Toxicity Induced by Heavy Metals. Foods 2024, 13, 978. [Google Scholar] [CrossRef] [PubMed]
- Korczowska-Łącka, I.; Słowikowski, B.; Piekut, T.; Hurła, M.; Banaszek, N.; Szymanowicz, O.; Jagodziński, P.P.; Kozubski, W.; Permoda-Pachuta, A.; Dorszewska, J. Disorders of endogenous and exogenous antioxidants in neurological diseases. Antioxidants 2023, 12, 1811. [Google Scholar] [CrossRef] [PubMed]
- Iacopetta, D.; Ceramella, J.; Scumaci, D.; Catalano, A.; Sinicropi, M.S.; Tundis, R.; Alcaro, S.; Borges, F. An Update on Recent Studies Focusing on the Antioxidant Properties of Salvia Species. Antioxidants 2023, 12, 2106. [Google Scholar] [CrossRef]
- Basile, G.; De Maio, A.C.; Catalano, A.; Ceramella, J.; Iacopetta, D.; Bonofiglio, D.; Saturnino, C.; Sinicropi, M.S. Ancient Wheat as Promising Nutraceuticals for the Prevention of Chronic and Degenerative Diseases. Curr. Med. Chem. 2023, 30, 3384–3403. [Google Scholar] [CrossRef]
- Zia, A.; Farkhondeh, T.; Pourbagher-Shahri, A.M.; Samarghandian, S. The role of curcumin in aging and senescence: Molecular mechanisms. Biomed. Pharmacother. 2021, 134, 111119. [Google Scholar] [CrossRef] [PubMed]
- Emami, M.H.; Sereshki, N.; Malakoutikhah, Z.; Dehkordi, S.A.E.; Fahim, A.; Mohammadzadeh, S.; Maghool, F. Nrf2 signaling pathway in trace metal carcinogenesis: A cross-talk between oxidative stress and angiogenesis. Comp. Biochem. Physiol. C Toxicol. Pharm. 2022, 254, 109266. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Alves, H.; Marques, V.; Durazzo, A.; Lucarini, M.; Alves, T.; Morsink, M.; Willemen, N.; Eder, P.; Chaud, M.; et al. Properties, extraction methods, and delivery systems for curcumin as a natural source of beneficial health effects. Medicina 2020, 56, 336. [Google Scholar] [CrossRef] [PubMed]
- Mohseni, M.; Sahebkar, A.; Askari, G.; Johnston, T.P.; Alikiaii, B.; Bagherniya, M. The clinical use of curcumin on neurological disorders: An updated systematic review of clinical trials. Phytother. Res. 2021, 35, 6862–6882. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Harsha, C.; Parama, D.; Girisa, S.; Daimary, U.D.; Mao, X.; Kunnumakkara, A.B. Current clinical developments in curcumin-based therapeutics for cancer and chronic diseases. Phytother. Res. 2021, 35, 6768–6801. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Tang, Z.; Liu, H.; Kang, Y.; Chen, L.; Dong, J.; Chen, W.; Kong, N.; Tao, W.; Xie, T. Nanoheterojunction-Mediated Thermoelectric Strategy for Cancer Surgical Adjuvant Treatment and β-Elemene Combination Therapy. Adv. Mater. 2023, 35, 2207391. [Google Scholar] [CrossRef] [PubMed]
- Svolacchia, F.; Brongo, S.; Catalano, A.; Ceccarini, A.; Svolacchia, L.; Santarsiere, A.; Scieuzo, C.; Salvia, R.; Finelli, F.; Milella, L.; et al. Natural Products for the Prevention, Treatment and Progression of Breast Cancer. Cancers 2023, 15, 2981. [Google Scholar] [CrossRef] [PubMed]
- Catalano, A.; Iacopetta, D.; Ceramella, J.; Mariconda, A.; Rosano, C.; Scumaci, D.; Saturnino, C.; Longo, P.; Sinicropi, M.S. New Achievements for the Treatment of Triple-Negative Breast Cancer. Appl. Sci. 2022, 12, 5554. [Google Scholar] [CrossRef]
- Kali, A.; Charles, M.P. Curcumin as a Promising Therapy for COVID-19: A Review. Glob. J. Med. Pharm. Biomed. Update 2024, 19, 1. [Google Scholar] [CrossRef]
- Catalano, A.; Iacopetta, D.; Ceramella, J.; de Maio, A.C.; Basile, G.; Giuzio, F.; Bonomo, M.G.; Aquaro, S.; Walsh, T.J.; Sinicropi, M.S.; et al. Are nutraceuticals effective in COVID-19 and post-COVID prevention and treatment? Foods 2022, 11, 2884. [Google Scholar] [CrossRef]
- Priyadarsini, K.I. The chemistry of curcumin: From extraction to therapeutic agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, K.I. Chemical and structural features influencing the biological activity of curcumin. Curr. Pharm. Des. 2013, 19, 2093–2100. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Rauf, A.; Akash, S.; Trisha, S.I.; Nasim, A.H.; Akter, M.; Dhar, P.S.; Ogaly, H.A.; Hemeg, H.A.; Wilairatana, P.; et al. Targeted therapies of curcumin focus on its therapeutic benefits in cancers and human health: Molecular signaling pathway-based approaches and future perspectives. Biomed. Pharmacother. 2024, 170, 116034. [Google Scholar] [CrossRef] [PubMed]
- Heger, M.; Van Golen, R.F.; Broekgaarden, M.; Michel, M.C. The molecular basis for the pharmacokinetics and pharmacodynamics of curcumin and its metabolites in relation to cancer. Pharmacol. Rev. 2014, 66, 222–307. [Google Scholar] [CrossRef] [PubMed]
- Jeong, G.S.; Oh, G.S.; Pae, H.O.; Jeong, S.O.; Kim, Y.C.; Shin, M.K.; Seo, B.Y.; Han, S.Y.; Lee, H.S.; Jeong, J.G.; et al. Comparative effects of curcuminoids on endothelial heme oxygenase-1 expression: Ortho-methoxy groups are essential to enhance heme oxygenase activity and protection. Exp. Mol. Med. 2006, 38, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Khayatan, D.; Razavi, S.M.; Arab, Z.N.; Hosseini, Y.; Niknejad, A.; Momtaz, S.; Abdolghaffari, A.H.; Sathyapalan, T.; Jamialahmadi, T.; Kesharwani, P.; et al. Superoxide dismutase: A key target for the neuroprotective effects of curcumin. Mol. Cell. Biochem. 2024, 479, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Xu, L.; Kang, Y.; Cheng, Q.; He, Y.; Ji, X. Platelet-derived drug delivery systems: Pioneering treatment for cancer, cardiovascular diseases, infectious diseases, and beyond. Biomaterials 2024, 306, 122478. [Google Scholar] [CrossRef]
- Jacob, S.; Kather, F.S.; Morsy, M.A.; Boddu, S.H.; Attimarad, M.; Shah, J.; Shinu, P.; Nair, A.B. Advances in Nanocarrier Systems for Overcoming Formulation Challenges of Curcumin: Current Insights. Nanomaterials 2024, 14, 672. [Google Scholar] [CrossRef]
- Bertoncini-Silva, C.; Vlad, A.; Ricciarelli, R.; Giacomo Fassini, P.; Suen, V.M.M.; Zingg, J.M. Enhancing the Bioavailability and Bioactivity of Curcumin for Disease Prevention and Treatment. Antioxidants 2024, 13, 331. [Google Scholar] [CrossRef]
- Ravindranath, V.; Chandrasekhara, N. In vitro studies on the intestinal absorption of curcumin in rats. Toxicology 1981, 20, 251–257. [Google Scholar] [CrossRef]
- Ravindranath, V.; Chandrasekhara, N. Metabolism of curcumin-studies with [3H] curcumin. Toxicology 1982, 22, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Y.; Alam, W.; Ullah, H.; Dacrema, M.; Daglia, M.; Khan, H.; Arciola, C.R. Antimicrobial Potential of Curcumin: Therapeutic Potential and Challenges to Clinical Applications. Antibiotics 2022, 11, 322. [Google Scholar] [CrossRef] [PubMed]
- Vaithiyalingam, M.; Sumathi, D.L.; Sabarathinam, S. Isolation and In Silico Study of Curcumin from Curcuma longa and Its Anti-Diabetic Activity. Appl. Biochem. Biotechnol. 2023, 195, 947–957. [Google Scholar] [CrossRef]
- Dehzad, M.J.; Ghalandari, H.; Nouri, M.; Askarpour, M. Antioxidant and Anti-inflammatory Effects of Curcumin/turmeric Supplementation in Adults: A GRADE-assessed Systematic Review and Dose–response Meta-analysis of Randomized Controlled Trials. Cytokine 2023, 164, 156144. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Ao, M.; Dong, B.; Jiang, Y.; Yu, L.; Chen, Z.; Hu, C.; Xu, R. Anti-Inflammatory Effects of Curcumin in the Inflammatory Diseases: Status, Limitations and Countermeasures. Drug Des. Dev. Ther. 2021, 15, 4503–4525. [Google Scholar] [CrossRef]
- Cox, F.F.; Misiou, A.; Vierkant, A.; Ale-Agha, N.; Grandoch, M.; Haendeler, J.; Altschmied, J. Protective Effects of Curcumin in Cardiovascular Diseases-Impact on Oxidative Stress and Mitochondria. Cells 2022, 11, 342. [Google Scholar] [CrossRef]
- Pourbagher-Shahri, A.M.; Farkhondeh, T.; Ashrafizadeh, M.; Talebi, M.; Samargahndian, S. Curcumin and cardiovascular diseases: Focus on cellular targets and cascades. Biomed. Pharmacother. 2021, 136, 111214. [Google Scholar] [CrossRef]
- Ciuca, M.D.; Racovita, R.C. Curcumin: Overview of Extraction Methods, Health Benefits, and Encapsulation and Delivery Using Microemulsions and Nanoemulsions. Int. J. Mol. Sci. 2023, 24, 8874. [Google Scholar] [CrossRef]
- Xie, X.; Wang, H.; Williams, G.R.; Yang, Y.; Zheng, Y.; Wu, J.; Zhu, L.M. Erythrocyte Membrane Cloaked Curcumin-Loaded Nanoparticles for Enhanced Chemotherapy. Pharmaceutics 2019, 11, 429. [Google Scholar] [CrossRef]
- Jia, G.; Han, Y.; An, Y.; Ding, Y.; He, C.; Wang, X.; Tang, Q. NRP-1 targeted and cargo-loaded exosomes facilitate simultaneous imaging and therapy of glioma in vitro and in vivo. Biomaterials 2018, 178, 302–316. [Google Scholar] [CrossRef]
- Wang, D.; Jiang, S.; Zhang, F.; Ma, S.; Heng, B.C.; Wang, Y.; Zhu, J.; Xu, M.; He, Y.; Wei, Y.; et al. Cell Membrane Vesicles with Enriched CXCR4 Display Enhances Their Targeted Delivery as Drug Carriers to Inflammatory Sites. Adv. Sci. 2021, 8, 2101562. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Xing, S.; Liu, Y. Engineered Cell Membrane-Camouflaged Nanomaterials for Biomedical Applications. Nanomaterials 2024, 14, 413. [Google Scholar] [CrossRef] [PubMed]
- Fontana, F.; Molinaro, G.; Moroni, S.; Pallozzi, G.; Ferreira, M.P.; Tello, R.P.; Elbadri, K.; Torrieri, G.; Correia, A.; Kemell, M.; et al. Biomimetic Platele-Cloaked Nanoparticles for the Delivery of Anti-Inflammatory Curcumin in the Treatment of Atherosclerosis. Adv. Healthc. Mater. 2024; in press. [Google Scholar] [CrossRef] [PubMed]
- Leyva-Gómez, G.; Cortés, H.; Magaña, J.J.; Leyva-García, N.; Quintanar-Guerrero, D.; Florán, B. Nanoparticle technology for treatment of Parkinson’s disease: The role of surface phenomena in reaching the brain. Drug Discov. Today 2015, 20, 824–837. [Google Scholar] [CrossRef]
- Agrawal, M.; Ajazuddin, D.K.; Tripathi, S.; Saraf, S.; Saraf, S.G.; Antimisiaris, S.G.; Mourtas, S.; Hammarlund-Udenaese, M.; Alexander, A. Recent advancements in liposomes targeting strategies to cross blood-brain barrier (BBB) for the treatment of Alzheimer’s disease. J. Control. Release 2017, 260, 61–77. [Google Scholar] [CrossRef] [PubMed]
- Sinicropi, M.S.; Rovito, N.; Carocci, A.; Genchi, G. Acetyl-L-carnitine in Parkinson’s disease. In Mechanisms in Parkinson’s Disease—Models and Treatments; Dushanova, J., Ed.; InTech: London, UK, 2012; Chapter 19; pp. 367–392. ISBN 978-953-307-876-2. [Google Scholar]
- Pringsheim, T.; Jette, N.; Frolkis, A.; Steeves, T.D. The prevalence of Parkinson’s disease: A systematic review and meta-analysis. Mov. Disord. Off. J. Mov. Disord. Soc. 2014, 29, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Ricci, S.; Casalini, S.; Parkula, V.; Selvaraj, M.; Saygin, G.D.; Greco, P.; Biscarini, F.; Mas-Torrent, M. Label-free immunodetection of α-synuclein by using a microfluidics coplanar electrolyte-gated organic field-effect transistor. Biosens. Bioelectron. 2020, 167, 112433. [Google Scholar] [CrossRef]
- Siddique, Y.H.; Khan, W.; Singh, B.R.; Naqvi, A.H. Synthesis of Alginate-Curcumin Nanocomposite and Its Protective Role in Transgenic Drosophila Model of Parkinson’s Disease. ISRN Pharmacol. 2013, 2013, 794582. [Google Scholar] [CrossRef] [PubMed]
- Bollimpelli, V.S.; Kumar, P.; Kumari, S.; Kondapi, A.K. Neuroprotective effect of curcumin-loaded lactoferrin nano particles against rotenone induced neurotoxicity. Neurochem. Int. 2016, 95, 37–45. [Google Scholar] [CrossRef]
- Kundu, P.; Das, M.; Tripathy, K.; Sahoo, S.K. Delivery of dual drug loaded lipid based nanoparticles across the blood–brain barrier impart enhanced neuroprotection in a rotenone induced mouse model of Parkinson’s disease. ACS Chem. Neurosci. 2016, 7, 1658–1670. [Google Scholar] [CrossRef]
- Taebnia, N.; Morshedi, D.; Yaghmaei, S.; Aliakbari, F.; Rahimi, F.; Arpanaei, A. Curcumin-loaded amine-functionalized mesoporous silica nanoparticles inhibit α-synuclein fibrillation and reduce its cytotoxicity-associated effects. Langmuir 2016, 32, 13394–13402. [Google Scholar] [CrossRef]
- Rakotoarisoa, M.; Angelov, B.; Garamus, V.M.; Angelova, A. Curcumin-and Fish Oil-loaded Spongosome and Cubosome Nanoparticles with Neuroprotective Potential against H2O2-induced Oxidative Stress in Differentiated Human SH-SY5Y Cells. ACS Omega 2019, 4, 3061–3073. [Google Scholar] [CrossRef]
- Sookhaklari, R.; Geramizadeh, B.; Abkar, M.; Moosavi, M. The neuroprotective effect of BSA-based nanocurcumin against 6-OHDA-induced cell death in SH-SY5Y cells. Avicenna J. Phytomed. 2019, 9, 92–100. [Google Scholar] [PubMed] [PubMed Central]
- Salehi, B.; Calina, D.; Docea, A.O.; Koirala, N.; Aryal, S.; Lombardo, D.; Pasqua, L.; Taheri, Y.; Salgado Castillo, C.M.; Martorell, M.; et al. Curcumin’s Nanomedicine Formulations for Therapeutic Application in Neurological Diseases. J. Clin. Med. 2020, 9, 430. [Google Scholar] [CrossRef]
- den Haan, J.; Morrema, T.H.; Rozemuller, A.J.; Bouwman, F.H.; Hoozemans, J.J. Different Curcumin Forms Selectively Bind Fibrillar Amyloid Beta in Post Mortem Alzheimer’s Disease Brains: Implications for In-vivo Diagnostics. Acta Neuropathol. Commun. 2018, 6, 75. [Google Scholar] [CrossRef]
- Tang, M.; Taghibiglou, C. The Mechanisms of Action of Curcumin in Alzheimer’s Disease. J. Alzheimers Dis. 2017, 58, 1003–1016. [Google Scholar] [CrossRef]
- Barbara, R.; Belletti, D.; Pederzoli, F.; Masoni, M.; Keller, J.; Ballestrazzi, A.; Vandelli, M.A.; Tosi, G.; Grabrucker, A.M. Novel Curcumin loaded nanoparticles engineered for Blood-Brain Barrier crossing and able to disrupt Abeta aggregates. Int. J. Pharm. 2017, 526, 413–424. [Google Scholar] [CrossRef]
- Fan, S.; Zheng, Y.; Liu, X.; Fang, W.; Chen, X.; Liao, W.; Jing, X.; Lei, M.; Tao, E.; Ma, Q. Curcumin-loaded PLGA-PEG Nanoparticles Conjugated with B6 Peptide for Potential Use in Alzheimer’s Disease. Drug Deliv. 2018, 25, 1091–1102. [Google Scholar] [CrossRef]
- Huo, X.; Zhang, Y.; Jin, X.; Li, Y.; Zhang, L. A Novel Synthesis of Selenium Nanoparticles Encapsulated PLGA Nanospheres with Curcumin Molecules for the Inhibition of Amyloid β Aggregation in Alzheimer’s Disease. J. Photochem. Photobiol. B Biol. 2019, 190, 98–102. [Google Scholar] [CrossRef]
- MacDonald, M.E.; Ambrose, C.M.; Duyao, M.P.; Myers, R.H.; Lin, C.; Srinidhi, L.; Barnes, G.; Taylor, S.A.; James, M.; Groot, N.; et al. The Huntington’s Disease Collaborative Research Group: A Novel Gene Containing a Trinucleotide Repeat That is Expanded and Unstable on Huntington’s Disease Chromosomes. Cell 1993, 72, 971–983. [Google Scholar] [CrossRef]
- Garodia, P.; Hegde, M.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin, Inflammation, and Neurological disorders: How Are They Linked? Integr. Med. Res. 2023, 12, 100968. [Google Scholar] [CrossRef]
- Essa, M.M.; Moghadas, M.; Ba-Omar, T.; Walid Qoronfleh, M.; Guillemin, G.J.; Manivasagam, T.; Justin-Thenmozhi, A.; Ray, B.; Bhat, A.; Chidambaram, S.B.; et al. Protective Effects of Antioxidants in Huntington’s Disease: An Extensive Review. Neurotox. Res. 2019, 35, 739–774. [Google Scholar] [CrossRef]
- Labanca, F.; Ullah, H.; Khan, H.; Milella, L.; Xiao, J.; Dajic-Stevanovic, Z.; Jeandet, P. Therapeutic and Mechanistic effects of Curcumin in Huntington’s disease. Curr. Neuropharmacol. 2021, 19, 1007–1018. [Google Scholar] [CrossRef]
- Sandhir, R.; Yadav, A.; Mehrotra, A.; Sunkaria, A.; Singh, A.; Sharma, S. Curcumin Nanoparticles Attenuate Neurochemical and Neurobehavioral Deficits in Experimental Model of Huntington’s Disease. Neuromol. Med. 2014, 16, 106–118. [Google Scholar] [CrossRef]
- Iacopetta, D.; Ceramella, J.; Catalano, A.; Scali, E.; Scumaci, D.; Pellegrino, M.; Aquaro, S.; Saturnino, C.; Sinicropi, M.S. Impact of Cytochrome P450 Enzymes on the Phase I Metabolism of Drugs. Appl. Sci. 2023, 13, 6045. [Google Scholar] [CrossRef]
- Gharaibeh, A.; Maiti, P.; Culver, R.; Heileman, S.; Srinageshwar, B.; Story, D.; Spelde, K.; Paladugu, L.; Munro, N.; Muhn, N. Solid Lipid Curcumin Particles Protect Medium Spiny Neuronal Morphology, and Reduce Learning and Memory Deficits in the YAC128 Mouse Model of Huntington’s Disease. Int. J. Mol. Sci. 2020, 21, 9542. [Google Scholar] [CrossRef]
- Soler, B.; Fadic, R.; von Bernhardi, R. Stem Cells Therapy in Amyotrophic Lateral Sclerosis Treatment. A Critical View. Rev. Neurol. 2011, 52, 426–434. [Google Scholar] [PubMed]
- Meamar, R.; Nasr-Esfahani, M.H.; Mousavi, S.A.; Basiri, K. Stem Cell Therapy in Amyotrophic Lateral Sclerosis. J. Clin. Neurosci. 2013, 20, 1659–1663. [Google Scholar] [CrossRef]
- Thomsen, G.M.; Gowing, G.; Svendsen, S.; Svendsen, C.N. The Past, Present and Future of Stem Cell Clinical Trials for ALS. Exp. Neurol. 2014, 262, 127–137. [Google Scholar] [CrossRef]
- Markov, A.; Thangavelu, L.; Aravindhan, S.; Zekiy, A.O.; Jarahian, M.; Chartrand, M.S.; Pathak, Y.; Marofi, F.; Shamlou, S.; Hassanzadeh, A. Mesenchymal Stem/Stromal Cells as a Valuable Source for the Treatment of Immune-Mediated Disorders. Stem Cell Res. Ther. 2021, 12, 192. [Google Scholar] [CrossRef]
- Tripodo, G.; Chlapanidas, T.; Perteghella, S.; Vigani, B.; Mandracchia, D.; Trapani, A.; Galuzzi, M.; Tosca, M.C.; Antonioli, B.; Gaetani, P.; et al. Mesenchymal stromal cells loading curcumin-INVITE-micelles: A drug delivery system for neurodegenerative diseases. Colloids Surf. B. Biointerfaces 2015, 125, 300–308. [Google Scholar] [CrossRef]
- Yavarpour-Bali, H.; Ghasemi-Kasman, M.; Pirzadeh, M. Curcumin-loaded nanoparticles: A novel therapeutic strategy in treatment of central nervous system disorders. Int. J. Nanomed. 2019, 14, 4449–4460. [Google Scholar] [CrossRef]
- Najafi, S.; Najafi, P.; Kaffash Farkhad, N.; Hosseini Torshizi, G.; Assaran Darban, R.; Boroumand, A.R.; Sahab-Negah, S.; Khodadoust, M.A.; Tavakol-Afshari, J. Mesenchymal stem cell therapy in amyotrophic lateral sclerosis (ALS) patients: A comprehensive review of disease information and future perspectives. Iran. J. Basic Med. Sci. 2023, 26, 872–881. [Google Scholar] [CrossRef]
- Dolati, S.; Babaloo, Z.; Jadidi-Niaragh, F.; Ayromlou, H.; Sadreddini, S.; Yousefi, M. Multiple sclerosis: Therapeutic applications of advancing drug delivery systems. Biomed. Pharmacother. 2017, 86, 343–353. [Google Scholar] [CrossRef]
- Qureshi, M.; Al-Suhaimi, E.A.; Wahid, F.; Shehzad, O.; Shehzad, A. Therapeutic potential of curcumin for multiple sclerosis. Neurol. Sci. 2018, 39, 207–214. [Google Scholar] [CrossRef]
- Natarajan, C.; Bright, J.J. Curcumin inhibits experimental allergic encephalomyelitis by blocking IL-12 signaling through Janus kinase-STAT pathway in T lymphocytes. J. Immunol. 2002, 168, 6506–6513. [Google Scholar] [CrossRef]
- Mohajeri, M.; Sadeghizadeh, M.; Najafi, F.; Javan, M. Polymerized nano-curcumin attenuates neurological symptoms in EAE model of multiple sclerosis through down regulation of inflammatory and oxidative processes and enhancing neuroprotection and myelin repair. Neuropharmacology 2015, 99, 156–167. [Google Scholar] [CrossRef]
- Motavaf, M.; Sadeghizadeh, M.; Babashah, S.; Zare, L.; Javan, M. Dendrosomal nanocurcumin promotes remyelination through induction of oligodendrogenesis in experimental demyelination animal model. J. Tissue Eng. Regen. Med. 2020, 14, 1449–1464. [Google Scholar] [CrossRef]
- Prusiner, S.B. Novel proteinaceous infectious particles cause scrapie. Science 1982, 216, 136–144. [Google Scholar] [CrossRef]
- Prusiner, S.B. Prions. Proc. Natl. Acad. Sci. USA 1998, 95, 13363–13383. [Google Scholar] [CrossRef]
- Abbott, A. Healthy prions protect nerves. Nat. Int. Wkly. J. Sci. 2010. [Google Scholar] [CrossRef]
- Lathe, R.; Darlix, J.L. Prion Protein PRNP: A New Player in Innate Immunity? The Aβ Connection. J. Alzheimers Dis. Rep. 2017, 1, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Caughey, B.; Raymond, L.D.; Raymond, G.J.; Maxson, L.; Silveira, J.; Baron, G.S. Inhibition of protease-resistant prion protein accumulation in vitro by curcumin. J. Virol. 2003, 77, 5499–5502. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-F.; Yu, K.-H.; Jheng, C.-P.; Chung, R.; Lee, C.-I. Curcumin reduces amyloid fibrillation of prion protein and decreases reactive oxidative stress. Pathogens 2013, 2, 506–519. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Genchi, G.; Lauria, G.; Catalano, A.; Carocci, A.; Sinicropi, M.S. Neuroprotective Effects of Curcumin in Neurodegenerative Diseases. Foods 2024, 13, 1774. https://doi.org/10.3390/foods13111774
Genchi G, Lauria G, Catalano A, Carocci A, Sinicropi MS. Neuroprotective Effects of Curcumin in Neurodegenerative Diseases. Foods. 2024; 13(11):1774. https://doi.org/10.3390/foods13111774
Chicago/Turabian StyleGenchi, Giuseppe, Graziantonio Lauria, Alessia Catalano, Alessia Carocci, and Maria Stefania Sinicropi. 2024. "Neuroprotective Effects of Curcumin in Neurodegenerative Diseases" Foods 13, no. 11: 1774. https://doi.org/10.3390/foods13111774
APA StyleGenchi, G., Lauria, G., Catalano, A., Carocci, A., & Sinicropi, M. S. (2024). Neuroprotective Effects of Curcumin in Neurodegenerative Diseases. Foods, 13(11), 1774. https://doi.org/10.3390/foods13111774








