Phytochemicals Involved in Mitigating Silent Toxicity Induced by Heavy Metals
Abstract
1. Introduction
2. Phytochemicals Used to Ameliorate Heavy Metal-Induced Toxicity
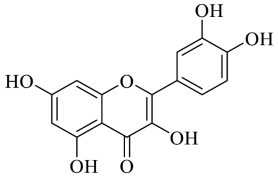
2.1. Flavonoids
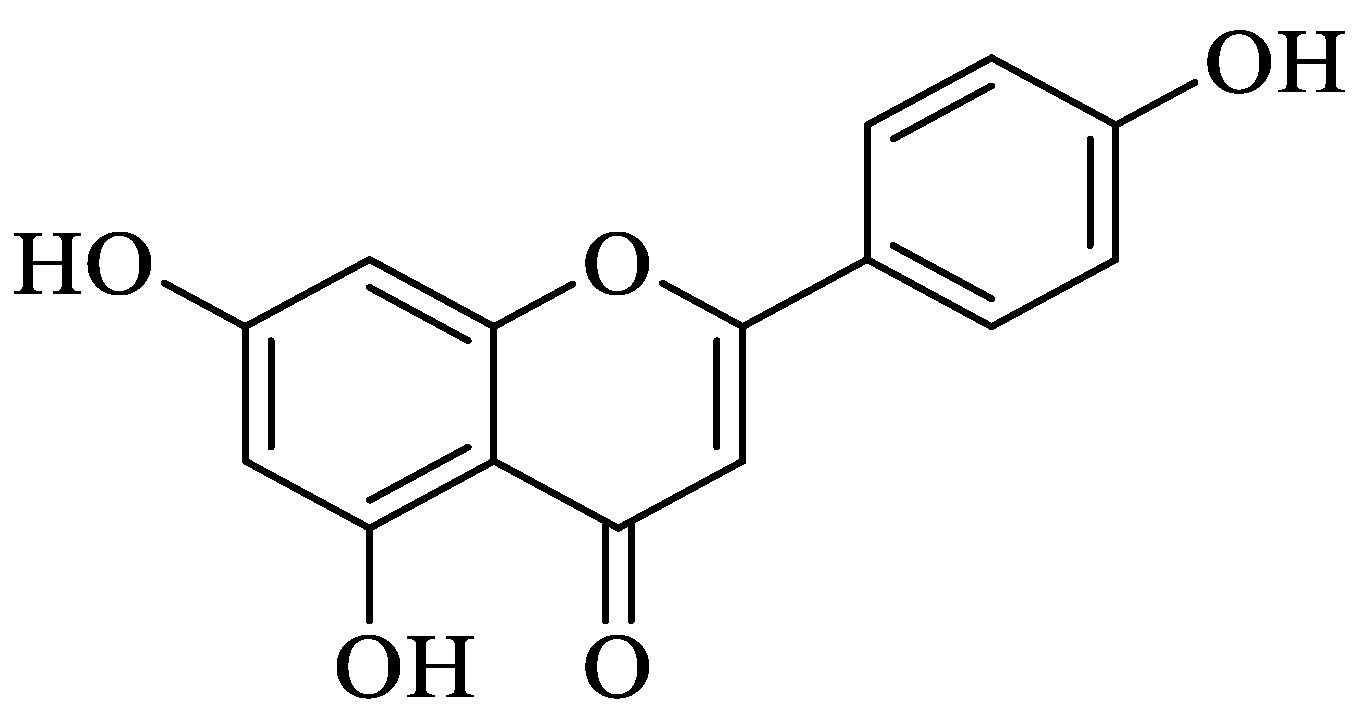
2.1.1. Quercetin
2.1.2. Hesperidin and Hesperetin
2.2. Epigallocatechin Gallate (EGCG)
2.3. Curcumin
2.4. Ferulic Acid
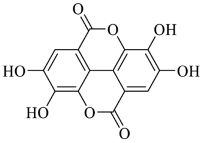
2.5. Ellagic Acid
3. Plant and Herbal Extracts in the Management of Heavy Metal-Induced Toxicity
3.1. Spirulina
3.2. Ginger (Zingiber officinale)
4. Probiotics in the Prevention of Heavy Metal-Induced Toxicity
| Probiotics | Role in Heavy Metal Detoxification | Ref. |
|---|---|---|
| L. rhamnosus GR-1 | Protection against As and Hg absorption in pregnant women and children | [157] |
| P. acidilactici GR-1 | Decrease of Cu (34.45%) and Ni (38.34%) levels | [148] |
| L. delbruekii and L. fermentum | Increased Cd excretion when administered with folic acid | [149] |
| L. plantarum and B. coagulans | Decreased Cd levels in symbiotic diets along with inulin | [150] |
| Ten Lactobacillus strains, including four L. plantarum strains, three L. fermentum strains, L. brevis, L. buchneri, and L. rhamnosus | Reduced Cd toxicity | [151] |
| L. rhamnosus Rosell-11, L. acidophilus Rosell-52, and B. longum Rosell-175 strains | Reduced Cd concentrations in rat blood | [152] |
| L. plantarum CCFM8661 | Pb intestinal sequestration by enhancing bile acid production | [153] |
| Lactobacillus reuteri P16 | Decreased Pb accumulation in tissues of freshwater fish common carp (Cyprinus carpio) | [154] |
| Lactobacillus delbrueckii subsp. bulgaricus KLDS1.0207 | Increased Pb excretion in mice | [155] |
| B. clausii | Decreased Pb and Cd concentrations | [156] |
| L. rhamnosus GR-1 | Pb and Cd sequestration and decreased their absorption across the intestinal epithelium | [157] |
| L. plantarum and B. coagulans | Decreased Hg levels in rat livers and kidneys | [158] |
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siddiqui, R.A.; Moghadasian, M.H. Nutraceuticals and Nutrition Supplements: Challenges and Opportunities. Nutrients 2020, 12, 1593. [Google Scholar] [CrossRef] [PubMed]
- Montano, L.; Maugeri, A.; Volpe, M.G.; Micali, S.; Mirone, V.; Mantovani, A.; Navarra, M.; Piscopo, M. Mediterranean Diet as a Shield against Male Infertility and Cancer Risk Induced by Environmental Pollutants: A Focus on Flavonoids. Int. J. Mol. Sci. 2022, 23, 1568. [Google Scholar] [CrossRef]
- Rahman, Z.; Singh, V.P. The relative impact of toxic heavy metals (THMs) (arsenic (As), cadmium (Cd), chromium (Cr)(VI), mercury (Hg), and Lead (Pb)) on the total environment: An overview. Environ. Monit. Assess. 2019, 191, 419. [Google Scholar] [CrossRef] [PubMed]
- Jannetto, P.J.; Cowl, C.T. Elementary Overview of Heavy Metals. Clin. Chem. 2023, 69, 336–349. [Google Scholar] [CrossRef] [PubMed]
- Sher, S.; Rehman, A. Use of heavy metals resistant bacteria-a strategy for arsenic bioremediation. Appl. Microbiol. Biotechnol. 2019, 103, 6007–6021. [Google Scholar] [CrossRef] [PubMed]
- Carocci, A.; Catalano, A.; Lauria, G.; Sinicropi, M.S.; Genchi, G. Lead Toxicity, Antioxidant Defense and Environment. Rev. Environ. Contam. Toxicol. 2016, 238, 45–67. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.; Lauria, G.; Catalano, A.; Carocci, A.; Sinicropi, M.S. Arsenic: A Review on a Great Health Issue Worldwide. Appl. Sci. 2022, 12, 6184. [Google Scholar] [CrossRef]
- Genchi, G.; Sinicropi, M.S.; Carocci, A.; Lauria, G.; Catalano, A. Mercury Exposure and Heart Diseases. Int. J. Environ. Res. Public Health 2017, 14, 74. [Google Scholar] [CrossRef]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The Effects of Cadmium Toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef]
- Fisher, R.M.; Gupta, V. Heavy Metals; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Rebello, S.; Sivaprasad, M.S.; Anoopkumar, A.N.; Jayakrishnan, L.; Aneesh, E.M.; Narisetty, V.; Sindhu, R.; Binod, P.; Pugazhendhi, A.; Pandey, A. Cleaner technologies to combat heavy metal toxicity. J. Environ. Manag. 2021, 296, 113231. [Google Scholar] [CrossRef]
- Carocci, A.; Catalano, A.; Sinicropi, M.S.; Genchi, G. Oxidative stress and neurodegeneration: The involvement of iron. Biometals 2018, 31, 715–735. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, M.; O’Shea, M.J.; Giere, R.; Krekeler, M.P.S. Road sediment, an underutilized material in environmental science research: A review of perspectives on United States studies with international context. J. Hazard. Mater. 2022, 432, 128604. [Google Scholar] [CrossRef] [PubMed]
- Shahab, A.; Hui, Z.; Rad, S.; Xiao, H.; Siddique, J.; Huang, L.L.; Ullah, H.; Rashid, A.; Taha, M.R.; Zada, N. A comprehensive review on pollution status and associated health risk assessment of human exposure to selected heavy metals in road dust across different cities of the world. Environ. Geochem. Health 2023, 45, 585–606. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Gupta, S.K.; Prakash, J.; Habib, G.; Kumar, P. A global perspective of the current state of heavy metal contamination in road dust. Environ. Sci. Pollut. Res. Int. 2022, 29, 33230–33251. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, Y.; Wang, F.; Luo, Z.; Guo, S.; Strahle, U. Toxicity of mercury: Molecular evidence. Chemosphere 2020, 245, 125586. [Google Scholar] [CrossRef]
- Flora, G.; Gupta, D.; Tiwari, A. Toxicity of lead: A review with recent updates. Interdiscip. Toxicol. 2012, 5, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Rafati Rahimzadeh, M.; Rafati Rahimzadeh, M.; Kazemi, S.; Moghadamnia, A.A. Cadmium toxicity and treatment: An update. Casp. J. Intern. Med. 2017, 8, 135–145. [Google Scholar] [CrossRef]
- Hughes, M.F. Arsenic toxicity and potential mechanisms of action. Toxicol. Lett. 2002, 133, 1–16. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals—Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef]
- Terfi, S.; Djerrad, Z.; Krimat, S.; Sadi, F. Phytochemical composition, cytotoxicity, antioxidant and antimicrobial responses of Lavandula dentata L. grown under different levels of heavy metals stress condition. Drug Chem. Toxicol. 2023, 46, 864–878. [Google Scholar] [CrossRef]
- Musa, J.J.; Musatapha, H.; Bala, J.; Ibrahim, Y.; Akos, M.; Daniel, E.; Oguche, F.; Kuti, A. Heavy Metals in Agricultural Soils in Nigeria: A Review. Arid. Zone J. Eng. Technol. Environ. 2017, 13, 593–603. [Google Scholar]
- Collado-Lopez, S.; Betanzos-Robledo, L.; Tellez-Rojo, M.M.; Lamadrid-Figueroa, H.; Reyes, M.; Rios, C.; Cantoral, A. Heavy Metals in Unprocessed or Minimally Processed Foods Consumed by Humans Worldwide: A Scoping Review. Int. J. Environ. Res. Public Health 2022, 19, 8651. [Google Scholar] [CrossRef] [PubMed]
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Nowicka, B. Heavy metal-induced stress in eukaryotic algae-mechanisms of heavy metal toxicity and tolerance with particular emphasis on oxidative stress in exposed cells and the role of antioxidant response. Environ. Sci. Pollut. Res. Int. 2022, 29, 16860–16911. [Google Scholar] [CrossRef] [PubMed]
- Flora, S.J.; Pachauri, V. Chelation in metal intoxication. Int. J. Environ. Res. Public Health 2010, 7, 2745–2788. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Koyama, H.; Kamogashira, T.; Yamasoba, T. Heavy Metal Exposure: Molecular Pathways, Clinical Implications, and Protective Strategies. Antioxidants 2024, 13, 76. [Google Scholar] [CrossRef]
- Sinicropi, M.S.; Amantea, D.; Caruso, A.; Saturnino, C. Chemical and biological properties of toxic metals and use of chelating agents for the pharmacological treatment of metal poisoning. Arch. Toxicol. 2010, 84, 501–520. [Google Scholar] [CrossRef]
- Rajak, C.; Singh, N.; Parashar, P. Metal toxicity and natural antidotes: Prevention is better than cure. Environ. Sci. Pollut. Res. Int. 2020, 27, 43582–43598. [Google Scholar] [CrossRef]
- Mishra, D.K.; Awasthi, H.; Srivastava, D.; Fatima, Z. Phytochemical: A treatment option for heavy metal induced neurotoxicity. J. Complement. Integr. Med. 2022, 19, 513–530. [Google Scholar] [CrossRef]
- Singh, N.; Sharma, B. Phytochemicals as Therapeutics in Heavy Metal Toxicity. In Advances in Pharmaceutical Biotechnology: Recent Progress and Future Applications; Patra, J.K., Shukla, A.C., Das, G., Eds.; Springer: Singapore, 2020; pp. 91–100. [Google Scholar] [CrossRef]
- Khan, S.S.; Sharma, A.; Flora, S.J.S. Phytochemicals in the Management of Arsenic Toxicity. Chem. Res. Toxicol. 2022, 35, 916–934. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Flora, S.J.S. Nutritional management can assist a significant role in alleviation of arsenicosis. J. Trace Elem. Med. Biol. 2018, 45, 11–20. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.M.; Hu, J.; Chen, C. Potential of natural products in combination with arsenic trioxide: Investigating cardioprotective effects and mechanisms. Biomed. Pharmacother. 2023, 162, 114464. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Q.; Narbad, A.; Chen, W. Dietary strategies for the treatment of cadmium and lead toxicity. Nutrients 2015, 7, 552–571. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Kim, Y.S.; Kumar, V. Heavy metal toxicity: An update of chelating therapeutic strategies. J. Trace Elem. Med. Biol. 2019, 54, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Flora, S.J. Possible role of metal redistribution, hepatotoxicity and oxidative stress in chelating agents induced hepatic and renal metallothionein in rats. Food Chem. Toxicol. 2001, 39, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Amadi, C.N.; Offor, S.J.; Frazzoli, C.; Orisakwe, O.E. Natural antidotes and management of metal toxicity. Environ. Sci. Pollut. Res. Int. 2019, 26, 18032–18052. [Google Scholar] [CrossRef]
- Flora, S.J.; Mittal, M.; Mehta, A. Heavy metal induced oxidative stress & its possible reversal by chelation therapy. Indian J. Med. Res. 2008, 128, 501–523. [Google Scholar]
- Durackova, Z. Some current insights into oxidative stress. Physiol. Res. 2010, 59, 459–469. [Google Scholar] [CrossRef]
- Vezzoli, A.; Mrakic-Sposta, S.; Dellanoce, C.; Montorsi, M.; Vietti, D.; Ferrero, M.E. Chelation Therapy Associated with Antioxidant Supplementation Can Decrease Oxidative Stress and Inflammation in Multiple Sclerosis: Preliminary Results. Antioxidants 2023, 12, 1338. [Google Scholar] [CrossRef]
- Li, A.N.; Li, S.; Zhang, Y.J.; Xu, X.R.; Chen, Y.M.; Li, H.B. Resources and biological activities of natural polyphenols. Nutrients 2014, 6, 6020–6047. [Google Scholar] [CrossRef] [PubMed]
- Islam, F.; Yasmeen, T.; Riaz, M.; Arif, M.S.; Ali, S.; Raza, S.H. Proteus mirabilis alleviates zinc toxicity by preventing oxidative stress in maize (Zea mays) plants. Ecotoxicol. Environ. Saf. 2014, 110, 143–152. [Google Scholar] [CrossRef]
- Brewer, M.S. Natural Antioxidants: Sources, Compounds, Mechanisms of Action, and Potential Applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Li, Y.; Lv, H.; Xue, C.; Dong, N.; Bi, C.; Shan, A. Plant Polyphenols: Potential Antidotes for Lead Exposure. Biol. Trace Elem. Res. 2021, 199, 3960–3976. [Google Scholar] [CrossRef]
- Goncharuk, E.A.; Zagoskina, N.V. Heavy Metals, Their Phytotoxicity, and the Role of Phenolic Antioxidants in Plant Stress Responses with Focus on Cadmium: Review. Molecules 2023, 28, 3921. [Google Scholar] [CrossRef] [PubMed]
- Zagoskina, N.V.; Zubova, M.Y.; Nechaeva, T.L.; Kazantseva, V.V.; Goncharuk, E.A.; Katanskaya, V.M.; Baranova, E.N.; Aksenova, M.A. Polyphenols in Plants: Structure, Biosynthesis, Abiotic Stress Regulation, and Practical Applications (Review). Int. J. Mol. Sci. 2023, 24, 13874. [Google Scholar] [CrossRef] [PubMed]
- Leja, M.; Mareczek, A.; Wyżgolik, G.; Klepacz-Baniak, J.; Czekońska, K. Antioxidative properties of bee pollen in selected plant species. Food Chem. 2007, 100, 237–240. [Google Scholar] [CrossRef]
- Kurek-Górecka, A.; Rzepecka-Stojko, A.; Górecki, M.; Stojko, J.; Sosada, M.; Swierczek-Zieba, G. Structure and antioxidant activity of polyphenols derived from propolis. Molecules 2013, 19, 78–101. [Google Scholar] [CrossRef]
- Borowska, S.; Brzoska, M.M.; Tomczyk, M. Complexation of Bioelements and Toxic Metals by Polyphenolic Compounds—Implications for Health. Curr. Drug Targets 2018, 19, 1612–1638. [Google Scholar] [CrossRef]
- Ademosun, A.O.; Oboh, G.; Bello, F.; Ayeni, P.O. Antioxidative Properties and Effect of Quercetin and Its Glycosylated Form (Rutin) on Acetylcholinesterase and Butyrylcholinesterase Activities. J. Evid. Based Complement. Altern. Med. 2016, 21, NP11–NP17. [Google Scholar] [CrossRef]
- Odbayar, T.O.; Kimura, T.; Tsushida, T.; Ide, T. Isoenzyme-specific up-regulation of glutathione transferase and aldo-keto reductase mRNA expression by dietary quercetin in rat liver. Mol. Cell Biochem. 2009, 325, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.S.; Shukla, R.K.; Sankhwar, M.L.; Patel, D.K.; Ansari, R.W.; Pant, A.B.; Islam, F.; Khanna, V.K. Neuroprotective effect of curcumin in arsenic-induced neurotoxicity in rats. Neurotoxicology 2010, 31, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Stahl, W.; Sies, H. Antioxidant activity of carotenoids. Mol. Asp. Med. 2003, 24, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Singh, S.; Agrawal, A.; Siddiqi, N.J.; Sharma, B. Phytochemicals Mediated Remediation of Neurotoxicity Induced by Heavy Metals. Biochem. Res. Int. 2015, 2015, 534769. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Oliinyk, P.; Lysiuk, R.; Lenchyk, L.; Rathod, S.S.S.; Antonyak, H.; Darmohray, R.; Dub, N.; Antoniv, O.; Tsal, O.; et al. Flavonoids and stilbenoids as a promising arsenal for the management of chronic arsenic toxicity. Environ. Toxicol. Pharmacol. 2022, 95, 103970. [Google Scholar] [CrossRef] [PubMed]
- Visalli, G.; Facciola, A.; Lagana, P.; Di Pietro, A. Food chemoprevention and air pollution: The health comes with eating. Rev. Environ. Health 2020, 35, 471–479. [Google Scholar] [CrossRef]
- Lupea, A.X.; Pop, M.; Cacig, S.I. Structure-Radical Scavenging Activity Relationships of Flavonoids from Ziziphus and Hydrangea Extracts. Rev. Chim. 2008, 59, 309–313. [Google Scholar] [CrossRef]
- Gheldof, N.; Engeseth, N.J. Antioxidant capacity of honeys from various floral sources based on the determination of oxygen radical absorbance capacity and inhibition of in vitro lipoprotein oxidation in human serum samples. J. Agric. Food Chem. 2002, 50, 3050–3055. [Google Scholar] [CrossRef]
- Li, X.; Jiang, X.; Sun, J.; Zhu, C.; Li, X.; Tian, L.; Liu, L.; Bai, W. Cytoprotective effects of dietary flavonoids against cadmium-induced toxicity. Ann. N. Y. Acad. Sci. 2017, 1398, 5–19. [Google Scholar] [CrossRef]
- Singh, P.; Arif, Y.; Bajguz, A.; Hayat, S. The role of quercetin in plants. Plant Physiol. Biochem. 2021, 166, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Wang, T.; Long, M.; Li, P. Quercetin: Its Main Pharmacological Activity and Potential Application in Clinical Medicine. Oxidative Med. Cell Longev. 2020, 2020, 8825387. [Google Scholar] [CrossRef] [PubMed]
- Mishra, D.; Flora, S.J. Quercetin administration during chelation therapy protects arsenic-induced oxidative stress in mice. Biol. Trace Elem. Res. 2008, 122, 137–147. [Google Scholar] [CrossRef]
- Ghosh, A.; Mandal, A.K.; Sarkar, S.; Panda, S.; Das, N. Nanoencapsulation of quercetin enhances its dietary efficacy in combating arsenic-induced oxidative damage in liver and brain of rats. Life Sci. 2009, 84, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Cai, P.; Zhu, Q.; Cao, Q.; Bai, Y.; Zou, H.; Gu, J.; Yuan, Y.; Liu, X.; Liu, Z.; Bian, J. Quercetin and Allicin Can Alleviate the Hepatotoxicity of Lead (Pb) through the PI3K Signaling Pathway. J. Agric. Food Chem. 2021, 69, 9451–9460. [Google Scholar] [CrossRef]
- Srivastava, A.; Kumari, A.; Jagdale, P.; Ayanur, A.; Pant, A.B.; Khanna, V.K. Potential of Quercetin to Protect Cadmium Induced Cognitive Deficits in Rats by Modulating NMDA-R Mediated Downstream Signaling and PI3K/AKT-Nrf2/ARE Signaling Pathways in Hippocampus. Neuromolecular Med. 2023, 25, 426–440. [Google Scholar] [CrossRef]
- Al-Zharani, M.; Mubarak, M.; Rudayni, H.A.; Al-Doaiss, A.A.; Abd-Elwahab, M.M.; Al-Eissa, M.S. Quercetin as a Dietary Supplementary Flavonoid Alleviates the Oxidative Stress Induced by Lead Toxicity in Male Wistar Rats. Nutrients 2023, 15, 1888. [Google Scholar] [CrossRef]
- Famurewa, A.C.; Renu, K.; Eladl, M.A.; Chakraborty, R.; Myakala, H.; El-Sherbiny, M.; Elsherbini, D.M.A.; Vellingiri, B.; Madhyastha, H.; Ramesh Wanjari, U.; et al. Hesperidin and hesperetin against heavy metal toxicity: Insight on the molecular mechanism of mitigation. Biomed. Pharmacother. 2022, 149, 112914. [Google Scholar] [CrossRef]
- Khuntia, G.; Dash, J.R.; Jena, B.; Mishra, U.K.; Parija, S.C. Hesperidin attenuates arsenic trioxide-induced cardiac toxicity in rats. Asian Pac. J. Trop. Biomed. 2023, 13, 156–164. [Google Scholar] [CrossRef]
- Abu-Khudir, R.; Almutairi, H.H.; Abd El-Rahman, S.S.; El-Said, K.S. The Palliative and Antioxidant Effects of Hesperidin against Lead-Acetate-Induced Testicular Injury in Male Wistar Rats. Biomedicines 2023, 11, 2390. [Google Scholar] [CrossRef]
- Bernhoft, R.A. Cadmium toxicity and treatment. Sci. World J. 2013, 2013, 394652. [Google Scholar] [CrossRef] [PubMed]
- Ceramella, J.; Iacopetta, D.; Franchini, A.; De Luca, M.; Saturnino, C.; Andreu, I.; Sinicropi, M.S.; Catalano, A. A Look at the Importance of Chirality in Drug Activity: Some Significative Examples. Appl. Sci. 2022, 12, 10909. [Google Scholar] [CrossRef]
- Pervin, M.; Unno, K.; Takagaki, A.; Isemura, M.; Nakamura, Y. Function of Green Tea Catechins in the Brain: Epigallocatechin Gallate and its Metabolites. Int. J. Mol. Sci. 2019, 20, 3630. [Google Scholar] [CrossRef] [PubMed]
- Zwolak, I. Epigallocatechin Gallate for Management of Heavy Metal-Induced Oxidative Stress: Mechanisms of Action, Efficacy, and Concerns. Int. J. Mol. Sci. 2021, 22, 4027. [Google Scholar] [CrossRef] [PubMed]
- Iheanacho, M.S.; Kandel, R.; Roy, P.; Singh, K.P. Epigallocatechin-3-gallate attenuates arsenic-induced fibrogenic changes in human kidney epithelial cells through reversal of epigenetic aberrations and antioxidant activities. BioFactors 2023. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.L.; Liu, Z.; Qi, Z.J.; Huang, Y.P.; Gao, X.Q.; Zhang, Y.Y. (-)-Epigallocatechin-3-gallate (EGCG) attenuates arsenic-induced cardiotoxicity in rats. Food Chem. Toxicol. 2016, 93, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Parasuraman, S.; Beng, J.Y.K.; Hui, L.C.; Qin, B.N.Y. Effect of Epigallocatechin Gallate on Cadmium Chloride-induced Oxidative Stress in Female Sprague Dawley Rats. Free Radic. Antioxid. 2020, 10, 29–34. [Google Scholar] [CrossRef]
- An, Z.; Qi, Y.; Huang, D.; Gu, X.; Tian, Y.; Li, P.; Li, H.; Zhang, Y. EGCG inhibits Cd2+-induced apoptosis through scavenging ROS rather than chelating Cd2+ in HL-7702 cells. Toxicol. Mech. Methods 2014, 24, 259–267. [Google Scholar] [CrossRef]
- Altenburg, J.D.; Bieberich, A.A.; Terry, C.; Harvey, K.A.; Vanhorn, J.F.; Xu, Z.; Jo Davisson, V.; Siddiqui, R.A. A synergistic antiproliferation effect of curcumin and docosahexaenoic acid in SK-BR-3 breast cancer cells: Unique signaling not explained by the effects of either compound alone. BMC Cancer 2011, 11, 149. [Google Scholar] [CrossRef]
- Baum, L.; Ng, A. Curcumin interaction with copper and iron suggests one possible mechanism of action in Alzheimer’s disease animal models. J. Alzheimers Dis. 2004, 6, 367–377; discussion 443–449. [Google Scholar] [CrossRef]
- Hatamipour, M.; Johnston, T.P.; Sahebkar, A. One Molecule, Many Targets and Numerous Effects: The Pleiotropy of Curcumin Lies in its Chemical Structure. Curr. Pharm. Des. 2018, 24, 2129–2136. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Nino, W.R.; Pedraza-Chaverri, J. Protective effect of curcumin against heavy metals-induced liver damage. Food Chem. Toxicol. 2014, 69, 182–201. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Goel, S.K.; Behari, J.R. Detoxification and antioxidant effects of curcumin in rats experimentally exposed to mercury. J. Appl. Toxicol. 2010, 30, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Moneim, A.M.; El-Toweissy, M.Y.; Ali, A.M.; Awad Allah, A.A.; Darwish, H.S.; Sadek, I.A. Curcumin Ameliorates Lead (Pb2+)-Induced Hemato-Biochemical Alterations and Renal Oxidative Damage in a Rat Model. Biol. Trace Elem. Res. 2015, 168, 206–220. [Google Scholar] [CrossRef]
- Rahaman, M.S.; Banik, S.; Akter, M.; Rahman, M.M.; Sikder, M.T.; Hosokawa, T.; Saito, T.; Kurasaki, M. Curcumin alleviates arsenic-induced toxicity in PC12 cells via modulating autophagy/apoptosis. Ecotoxicol. Environ. Saf. 2020, 200, 110756. [Google Scholar] [CrossRef] [PubMed]
- Mary, C.P.V.; Vijayakumar, S.; Shankar, R. Metal chelating ability and antioxidant properties of Curcumin-metal complexes—A DFT approach. J. Mol. Graph. Model. 2018, 79, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Dędek, K.; Rosicka-Kaczmarek, J.; Nebesny, E.; Kowalska, G. Characteristics and biological properties of ferulic acid. Biotechnol. Food Sci. 2019, 83, 71–85. [Google Scholar] [CrossRef]
- Basile, G.; De Maio, A.C.; Catalano, A.; Ceramella, J.; Iacopetta, D.; Bonofiglio, D.; Saturnino, C.; Sinicropi, M.S. Ancient Wheat as Promising Nutraceuticals for the Prevention of Chronic and Degenerative Diseases. Curr. Med. Chem. 2023, 30, 3384–3403. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, P.; Zhao, J. Ferulic acid mediates prebiotic responses of cereal-derived arabinoxylans on host health. Anim. Nutr. 2022, 9, 31–38. [Google Scholar] [CrossRef]
- Zdunska, K.; Dana, A.; Kolodziejczak, A.; Rotsztejn, H. Antioxidant Properties of Ferulic Acid and Its Possible Application. Skin Pharmacol. Physiol. 2018, 31, 332–336. [Google Scholar] [CrossRef]
- Zhao, Z.; Moghadasian, M.H. Chemistry, natural sources, dietary intake and pharmacokinetic properties of ferulic acid: A review. Food Chem. 2008, 109, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Kassab, R.B.; Lokman, M.S.; Daabo, H.M.A.; Gaber, D.A.; Habotta, O.A.; Hafez, M.M.; Zhery, A.S.; Moneim, A.E.A.; Fouda, M.S. Ferulic acid influences Nrf2 activation to restore testicular tissue from cadmium-induced oxidative challenge, inflammation, and apoptosis in rats. J. Food Biochem. 2020, 44, e13505. [Google Scholar] [CrossRef] [PubMed]
- Sanjeev, S.; Bidanchi, R.M.; Murthy, M.K.; Gurusubramanian, G.; Roy, V.K. Influence of ferulic acid consumption in ameliorating the cadmium-induced liver and renal oxidative damage in rats. Environ. Sci. Pollut. Res. Int. 2019, 26, 20631–20653. [Google Scholar] [CrossRef] [PubMed]
- Kelainy, E.G.; Ibrahim Laila, I.M.; Ibrahim, S.R. The effect of ferulic acid against lead-induced oxidative stress and DNA damage in kidney and testes of rats. Environ. Sci. Pollut. Res. Int. 2019, 26, 31675–31684. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Pan, S.; Zhang, J.; Li, X.; Niu, Y. Ferulic acid exerts Nrf2-dependent protection against prenatal lead exposure-induced cognitive impairment in offspring mice. J. Nutr. Biochem. 2021, 91, 108603. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.M.; Zhao, L.; Li, H.; Xu, H.; Chen, W.W.; Tao, L. Research progress on the anticarcinogenic actions and mechanisms of ellagic acid. Cancer Biol. Med. 2014, 11, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Rios, J.L.; Giner, R.M.; Marin, M.; Recio, M.C. A Pharmacological Update of Ellagic Acid. Planta Med. 2018, 84, 1068–1093. [Google Scholar] [CrossRef]
- Djedjibegovic, J.; Marjanovic, A.; Panieri, E.; Saso, L. Ellagic Acid-Derived Urolithins as Modulators of Oxidative Stress. Oxidative Med. Cell Longev. 2020, 2020, 5194508. [Google Scholar] [CrossRef]
- García-Niño, W.R.; Ibarra-Lara, L.; Cuevas-Magaña, M.Y.; Sánchez-Mendoza, A.; Armada, E. Protective activities of ellagic acid and urolithins against kidney toxicity of environmental pollutants: A review. Environ. Toxicol. Pharmacol. 2022, 95, 103960. [Google Scholar] [CrossRef]
- Bhattacharya, S. The Role of Spirulina (Arthrospira) in the Mitigation of Heavy-Metal Toxicity: An Appraisal. J. Environ. Pathol. Toxicol. Oncol. 2020, 39, 149–157. [Google Scholar] [CrossRef]
- Mumtaz, S.; Ali, S.; Khan, R.; Shakir, H.A.; Tahir, H.M.; Mumtaz, S.; Andleeb, S. Therapeutic role of garlic and vitamins C and E against toxicity induced by lead on various organs. Environ. Sci. Pollut. Res. Int. 2020, 27, 8953–8964. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.; Rao, G.S.; Kumar, M.; Reddy, G. Pharmacokinetic interaction of garlic and atorvastatin in dyslipidemic rats. Indian J. Pharmacol. 2012, 44, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Pourjafar, M.; Aghbolaghi, P.A.; Shakhse-Niaie, M. Effect of Garlic along with Lead Acetate Administration on Lead Burden of Some Tissues in Mice. Pak. J. Biol. Sci. 2007, 10, 2772–2774. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, A.; Ebrahimzadeh Bideskan, A.; Alipour, F.; Fazel, A.; Haghir, H. The Effect of Ascorbic Acid and Garlic Administration on Lead-Induced Neural Damage in Rat Offspring’s Hippocampus. Iran. J. Basic. Med. Sci. 2013, 16, 157–164. [Google Scholar] [PubMed]
- Hernayanti, H.; Lestari, S.R.; Saryono, S.; Lestari, P.E. Anti-inflammatory Test of Centella asiatica Extract on Rat Induced by Cadmium. Molekul 2021, 16, 202–209. [Google Scholar] [CrossRef]
- Flora, S.J.; Gupta, R. Beneficial effects of Centella asiatica aqueous extract against arsenic-induced oxidative stress and essential metal status in rats. Phytother. Res. 2007, 21, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Hussain, N.; Chaudhry, M.N.; Anjum, A.A.; Abbas, N.; Khan, M.N.; Nadeem, S.M. Investigating the Ameliorative Potential of the Aloe barbadensis Aqueous Fraction on Oxidative Stress Markers and Biochemical Parameters in Cadmium-Intoxicated Rabbits. Pol. J. Environ. Stud. 2016, 25, 2423–2433. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Kulkarni, V.H.; Habbu, P.V. Effect of Aloe emodin Against Lead Induced Hepatotoxicity. Int. J. Pharm. Sci. Rev. Res. 2022, 74, 155–160. [Google Scholar] [CrossRef]
- Shokri, F.; Ziarati, P.; Mousavi, Z. Removal of Selected Heavy Metals from Pharmaceutical Effluent by Aloe vera L. Biomed. Pharmacol. J. 2016, 9, 705–713. [Google Scholar] [CrossRef]
- Katubi, K.M.; Amari, A.; Harharah, H.N.; Eldirderi, M.M.; Tahoon, M.A.; Ben Rebah, F. Aloe vera as Promising Material for Water Treatment: A Review. Processes 2021, 9, 782. [Google Scholar] [CrossRef]
- Genchi, G.; Lauria, G.; Catalano, A.; Carocci, A.; Sinicropi, M.S. The Double Face of Metals: The Intriguing Case of Chromium. Appl. Sci. 2021, 11, 638. [Google Scholar] [CrossRef]
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Nickel: Human Health and Environmental Toxicology. Int. J. Environ. Res. Public Health 2020, 17, 679. [Google Scholar] [CrossRef] [PubMed]
- Talha, M.M.H.; Hossain, M.A.; Aktaruzzaman, M.; Islam, M.S.; Khasnobish, A.; Akanda, M.R. Effects of spirulina as a functional ingredient in arsenic-induced broiler diet on growth performance and hematobiochemical parameters. J. Adv. Vet. Anim. Res. 2022, 9, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Musio, B.; Mourad Hussein Ahmed, E.M.F.; Antonicelli, M.; Chiapperini, D.; Dursi, O.; Grieco, F.; Latronico, M.; Mastrorilli, P.; Ragone, R.; Settanni, R.; et al. A spectroscopic study to assess heavy metals absorption by a combined hemp/spirulina system from contaminated soil. Environ. Adv. 2022, 7, 100144. [Google Scholar] [CrossRef]
- Martinez-Galero, E.; Perez-Pasten, R.; Perez-Juarez, A.; Fabila-Castillo, L.; Gutierrez-Salmean, G.; Chamorro, G. Preclinical antitoxic properties of Spirulina (Arthrospira). Pharm. Biol. 2016, 54, 1345–1353. [Google Scholar] [CrossRef] [PubMed]
- Bashandy, S.A.; El Awdan, S.A.; Ebaid, H.; Alhazza, I.M. Antioxidant Potential of Spirulina platensis Mitigates Oxidative Stress and Reprotoxicity Induced by Sodium Arsenite in Male Rats. Oxidative Med. Cell Longev. 2016, 2016, 7174351. [Google Scholar] [CrossRef] [PubMed]
- Misbahuddin, M.; Islam, A.Z.; Khandker, S.; Ifthaker Al, M.; Islam, N.; Anjumanara. Efficacy of spirulina extract plus zinc in patients of chronic arsenic poisoning: A randomized placebo-controlled study. Clin. Toxicol. 2006, 44, 135–141. [Google Scholar] [CrossRef]
- Misbahuddin, M.; Afrin, M. Effect of spirulina on the levels of zinc, vitamin E and linoleic acid in the palm skin extracts of people with prolonged exposure to arsenic. Bangladesh J. Pharmacol. 2013, 8, 84–91. [Google Scholar] [CrossRef]
- Rahman, M.H.; Sikder, M.S.; Islam, A.Z.M.M.; Wahab, M.A. Spirulina as food supplement is effective in arsenicosis. J. Pak. Assoc. Dermatol. 2017, 16, 69–75. [Google Scholar]
- Islam, M.N.; Awal, M.A.; Mostofa, M.; Begum, F.; Khair, A.; Myenuddin, M. Effect of Spirulina on Biochemical Parameters and Reduction of Tissue Arsenic Concentration in Arsenic Induced Toxicities in Ducks. Int. J. Poult. Sci. 2009, 8, 69–74. [Google Scholar] [CrossRef]
- Paniagua-Castro, N.; Escalona-Cardoso, G.; Hernandez-Navarro, D.; Perez-Pasten, R.; Chamorro-Cevallos, G. Spirulina (Arthrospira) protects against cadmium-induced teratogenic damage in mice. J. Med. Food 2011, 14, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Jeyaprakash, K.; Chinnaswamy, P. Effect of spirulina and Liv-52 on cadmium induced toxicity in albino rats. Indian J. Exp. Biol. 2005, 43, 773–781. [Google Scholar]
- Karadeniz, A.; Cemek, M.; Simsek, N. The effects of Panax ginseng and Spirulina platensis on hepatotoxicity induced by cadmium in rats. Ecotoxicol. Environ. Saf. 2009, 72, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Shastri, D.; Kumar, M.; Kumar, A. Modulation of lead toxicity by Spirulina fusiformis. Phytother. Res. 1999, 13, 258–260. [Google Scholar] [CrossRef]
- Upasani, C.D.; Balaraman, R. Protective effect of Spirulina on lead induced deleterious changes in the lipid peroxidation and endogenous antioxidants in rats. Phytother. Res. 2003, 17, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Karaca, T.; Simsek, N. Effects of spirulina on the number of ovary mast cells in lead-induced toxicity in rats. Phytother. Res. 2007, 21, 44–46. [Google Scholar] [CrossRef]
- Ponce-Canchihuaman, J.C.; Perez-Mendez, O.; Hernandez-Munoz, R.; Torres-Duran, P.V.; Juarez-Oropeza, M.A. Protective effects of Spirulina maxima on hyperlipidemia and oxidative-stress induced by lead acetate in the liver and kidney. Lipids Health Dis. 2010, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Gargouri, M.; Ghorbel-Koubaa, F.; Bonenfant-Magne, M.; Magne, C.; Dauvergne, X.; Ksouri, R.; Krichen, Y.; Abdelly, C.; El Feki, A. Spirulina or dandelion-enriched diet of mothers alleviates lead-induced damages in brain and cerebellum of newborn rats. Food Chem. Toxicol. 2012, 50, 2303–2310. [Google Scholar] [CrossRef]
- Saxena, P.S.; Kumar, M. Modulatory potential of Spirulina fusiformis on testicular phosphatases in Swiss albino mice against mercury intoxication. Indian J. Exp. Biol. 2004, 42, 998–1002. [Google Scholar]
- Sharma, M.K.; Patni, R.; Kumar, M.; Kumar, A. Modification of mercury-induced biochemical alterations in blood of Swiss albino mice by Spirulina fusiformis. Environ. Toxicol. Pharmacol. 2005, 20, 289–296. [Google Scholar] [CrossRef]
- Sharma, M.K.; Sharma, A.; Kumar, A.; Kumar, M. Spirulina fusiformis provides protection against mercuric chloride induced oxidative stress in Swiss albino mice. Food Chem. Toxicol. 2007, 45, 2412–2419. [Google Scholar] [CrossRef] [PubMed]
- Bashandy, S.A.; Alhazza, I.M.; El-Desoky, G.E.; Al-Othman, Z.A. Hepatoprotective and hypolipidemic effects of Spirulina platensis in rats administered mercuric chloride. Afr. J. Pharm. Pharmacol. 2011, 5, 175–182. [Google Scholar]
- El-Desoky, G.E.; Bashandy, S.A.; Alhazza, I.M.; Al-Othman, Z.A.; Aboul-Soud, M.A.; Yusuf, K. Improvement of mercuric chloride-induced testis injuries and sperm quality deteriorations by Spirulina platensis in rats. PLoS ONE 2013, 8, e59177. [Google Scholar] [CrossRef] [PubMed]
- Unuofin, J.O.; Masuku, N.P.; Paimo, O.K.; Lebelo, S.L. Ginger from Farmyard to Town: Nutritional and Pharmacological Applications. Front. Pharmacol. 2021, 12, 779352. [Google Scholar] [CrossRef]
- Svolacchia, F.; Brongo, S.; Catalano, A.; Ceccarini, A.; Svolacchia, L.; Santarsiere, A.; Scieuzo, C.; Salvia, R.; Finelli, F.; Milella, L.; et al. Natural Products for the Prevention, Treatment and Progression of Breast Cancer. Cancers 2023, 15, 2981. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Su, J.; Ding, Z.H.; Zheng, Y.T.; Li, Y.; Leng, Y.; Liu, J.K. Chemical constituents and their bioactivities of “Tongling White Ginger” (Zingiber officinale). J. Agric. Food Chem. 2011, 59, 11690–11695. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Tang, Q.; Yang, L. The combination of ginger and zinc supplement could improve lead-induced reproductive dysfunction by inhibiting apoptosis mediated by oxidative damage and inflammation. Andrologia 2022, 54, e14577. [Google Scholar] [CrossRef]
- Motawee, M.E.; Damanhory, A.A.; Sakr, H.; Khalifa, M.M.; Atia, T.; Elfiky, M.M.; Maher, M.; Sakr, H.I. An electron microscopic and biochemical study of the potential protective effect of ginger against Cadmium-induced testicular pathology in rats. Front. Physiol. 2022, 13, 996020. [Google Scholar] [CrossRef]
- Fazal, M.; Veeraraghavan, V.P.; Tahreen, B.; Jayaraman, S.; Gayathri, R. Antioxidant effects of Emblica officinalis and Zingiber officinalis on arsenic and lead induced toxicity on Albino rats. Bioinformation 2021, 17, 295–305. [Google Scholar] [CrossRef]
- Abdel-Megeed, R.M. Probiotics: A Promising Generation of Heavy Metal Detoxification. Biol. Trace Elem. Res. 2021, 199, 2406–2413. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Kumar, P.S.; Jeevanantham, S.; Saravanan, R. A review on bioremediation approach for heavy metal detoxification and accumulation in plants. Environ. Pollut. 2022, 301, 119035. [Google Scholar] [CrossRef] [PubMed]
- Maio, A.C.; Basile, G.; Iacopetta, D.; Catalano, A.; Ceramella, J.; Cafaro, D.; Saturnino, C.; Sinicropi, M.S. The Significant Role of Nutraceutical Compounds in Ulcerative Colitis Treatment. Curr. Med. Chem. 2022, 29, 4216–4234. [Google Scholar] [CrossRef] [PubMed]
- Yoha, K.S.; Nida, S.; Dutta, S.; Moses, J.A.; Anandharamakrishnan, C. Targeted Delivery of Probiotics: Perspectives on Research and Commercialization. Probiotics Antimicrob. Proteins 2022, 14, 15–48. [Google Scholar] [CrossRef] [PubMed]
- Kakade, A.; Salama, E.S.; Usman, M.; Arif, M.; Feng, P.; Li, X. Dietary application of Lactococcus lactis alleviates toxicity and regulates gut microbiota in Cyprinus carpio on exposure to heavy metals mixture. Fish Shellfish Immunol. 2022, 120, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Bisanz, J.E.; Enos, M.K.; Mwanga, J.R.; Changalucha, J.; Burton, J.P.; Gloor, G.B.; Reid, G. Randomized open-label pilot study of the influence of probiotics and the gut microbiome on toxic metal levels in Tanzanian pregnant women and school children. mBio 2014, 5, e01580-14. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.; Yang, J.; Zhao, S.; Ling, Z.; Han, R.; Wu, Y.; Salama, E.S.; Kakade, A.; Khan, A.; Jin, W.; et al. Human supplementation with Pediococcus acidilactici GR-1 decreases heavy metals levels through modifying the gut microbiota and metabolome. NPJ Biofilms Microbiomes 2022, 8, 63. [Google Scholar] [CrossRef] [PubMed]
- Kadry, M.O.; Megeed, R.A. Probiotics as a Complementary Therapy in the Model of Cadmium Chloride Toxicity: Crosstalk of beta-Catenin, BDNF, and StAR Signaling Pathways. Biol. Trace Elem. Res. 2018, 185, 404–413. [Google Scholar] [CrossRef]
- Jafarpour, D.; Shekarforoush, S.S.; Ghaisari, H.R.; Nazifi, S.; Sajedianfard, J.; Eskandari, M.H. Protective effects of synbiotic diets of Bacillus coagulans, Lactobacillus plantarum and inulin against acute cadmium toxicity in rats. BMC Complement. Altern. Med. 2017, 17, 291. [Google Scholar] [CrossRef]
- Kirillova, A.V.; Danilushkina, A.A.; Irisov, D.S.; Bruslik, N.L.; Fakhrullin, R.F.; Zakharov, Y.A.; Bukhmin, V.S.; Yarullina, D.R. Assessment of Resistance and Bioremediation Ability of Lactobacillus Strains to Lead and Cadmium. Int. J. Microbiol. 2017, 2017, 9869145. [Google Scholar] [CrossRef]
- Djurasevic, S.; Jama, A.; Jasnic, N.; Vujovic, P.; Jovanovic, M.; Mitic-Culafic, D.; Knezevic-Vukcevic, J.; Cakic-Milosevic, M.; Ilijevic, K.; Djordjevic, J. The Protective Effects of Probiotic Bacteria on Cadmium Toxicity in Rats. J. Med. Food 2017, 20, 189–196. [Google Scholar] [CrossRef]
- Zhai, Q.; Liu, Y.; Wang, C.; Qu, D.; Zhao, J.; Zhang, H.; Tian, F.; Chen, W. Lactobacillus plantarum CCFM8661 modulates bile acid enterohepatic circulation and increases lead excretion in mice. Food Funct. 2019, 10, 1455–1464. [Google Scholar] [CrossRef] [PubMed]
- Giri, S.S.; Yun, S.; Jun, J.W.; Kim, H.J.; Kim, S.G.; Kang, J.W.; Kim, S.W.; Han, S.J.; Sukumaran, V.; Park, S.C. Therapeutic Effect of Intestinal Autochthonous Lactobacillus reuteri P16 Against Waterborne Lead Toxicity in Cyprinus carpio. Front. Immunol. 2018, 9, 1824. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Jin, D.; Yu, S.; Etareri Evivie, S.; Muhammad, Z.; Huo, G.; Liu, F. In Vitro and In Vivo Evaluation of Lactobacillus delbrueckii subsp. bulgaricus KLDS1.0207 for the Alleviative Effect on Lead Toxicity. Nutrients 2017, 9, 845. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.; Dwivedi, S. In Vitro Evaluation of Bioremediation Capacity of a Commercial Probiotic, Bacillus clausii, for Chromium (VI) and Lead (II) Toxicity. Int. J. Ind. Biotechnol. Biomater. 2018, 4, 1–16. Available online: https://biotech.journalspub.info/?journal=JIBB&page=article&op=view&path%5B%5D=340 (accessed on 23 November 2023).
- Daisley, B.A.; Monachese, M.; Trinder, M.; Bisanz, J.E.; Chmiel, J.A.; Burton, J.P.; Reid, G. Immobilization of cadmium and lead by Lactobacillus rhamnosus GR-1 mitigates apical-to-basolateral heavy metal translocation in a Caco-2 model of the intestinal epithelium. Gut Microbes 2019, 10, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Majlesi, M.; Shekarforoush, S.S.; Ghaisari, H.R.; Nazifi, S.; Sajedianfard, J.; Eskandari, M.H. Effect of Probiotic Bacillus Coagulans and Lactobacillus Plantarum on Alleviation of Mercury Toxicity in Rat. Probiotics Antimicrob. Proteins 2017, 9, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Massoud, R.; Zoghi, A. Potential probiotic strains with heavy metals and mycotoxins bioremoval capacity for application in foodstuffs. J. Appl. Microbiol. 2022, 133, 1288–1307. [Google Scholar] [CrossRef]
- Massoud, R.; Khosravi-Darani, K.; Sharifan, A.; Asadi, G.; Zoghi, A. Lead and cadmium biosorption from milk by Lactobacillus acidophilus ATCC 4356. Food Sci. Nutr. 2020, 8, 5284–5291. [Google Scholar] [CrossRef]
- Massoud, R.; Khosravi-Darani, K.; Sharifan, A.; Asadi, G.H.; Hadiani, M.R. Mercury Biodecontamination from Milk by using L. acidophilus ATCC 4356. J. Pure Appl. Microbiol. 2020, 14, 2313–2321. [Google Scholar] [CrossRef]
- Feng, P.; Ye, Z.; Kakade, A.; Virk, A.K.; Li, X.; Liu, P. A Review on Gut Remediation of Selected Environmental Contaminants: Possible Roles of Probiotics and Gut Microbiota. Nutrients 2018, 11, 22. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, X.; Sheng, H.; Shen, X.; Sun, X.; Yan, Y.; Wang, J.; Yuan, Q. Engineering probiotics as living diagnostics and therapeutics for improving human health. Microb. Cell Fact. 2020, 19, 56. [Google Scholar] [CrossRef]
- Mazhar, S.F.; Afzal, M.; Almatroudi, A.; Munir, S.; Ashfaq, U.A.; Rasool, M.; Raza, H.; Munir, H.M.W.; Rajoka, M.S.R.; Khurshid, M. The Prospects for the Therapeutic Implications of Genetically Engineered Probiotics. J. Food Qual. 2020, 2020, 9676452. [Google Scholar] [CrossRef]
- Chen, R.; Tu, H.; Chen, T. Potential Application of Living Microorganisms in the Detoxification of Heavy Metals. Foods 2022, 11, 1905. [Google Scholar] [CrossRef]


| Pollutant | Test Spice | Spirulina Treatment | Effect | Ref. |
|---|---|---|---|---|
| Arsenic | Human | 250 mg plus 2 mg zinc twice daily in drinking water (16 weeks) | Removal of 47.1% As from scalp hair with melanosis and keratosis improvement | [119] |
| Arsenic | Human | 10 mg daily dissolved in water (6 months) | Reversal of health conditions and restored the patients to normal life | [121] |
| Arsenic | Duck | 30–120 mg/L in drinking water (90 days) | Enhanced body weight and restored haematological parameters | [122] |
| Cadmium | Mice | 62.5–500 mg/kg, p.o. (gestation days 0–17) | Decreased frequency of exencephaly and other foetus malformations | [123] |
| Cadmium | Rat | 500 mg/kg/d p.o. (30 days) | Partially prevented lowering of metal serum concentrations (zinc, iron and selenium). Protective capacity against liver and renal damage due to the antioxidant activity | [124] |
| Cadmium | Rat | 300 mg/kg p.o. (30 days) | Protection against liver damage due to its ability to reduce the vacuolar degeneration, fat infiltration and fibrosis | [125] |
| Lead | Mouse | 800 mg/kg p.o. (15 days before and up to 30 after intoxication) | Decreased affectation on animal and testes weights and tubular diameter, improving the survival time | [126] |
| Lead | Rat | 1500 mg/kg in diet (30 d) | Enhanced SOD, CAT and GSH in liver, lungs, heart and kidneys; in addition, reduced brain metal concentrations and LPO | [127] |
| Lead | Rat | 300 mg/kg in drinking water (30 days) | Reduced increase in the number of mast cells in the ovarian cortex and medulla during the oestrous cycle | [128] |
| Lead | Rat | 20 g diet 5%/d/rat (30 days) | Prevented body weight reduction and liver impairment and protection against oxidative damage in liver and kidneys | [129] |
| Lead | Rat | 5% + dandelion 2% in diet (5th day of gestation to 14th day of lactation) | Minimised lead deposition and oxidative stress in gestation and lactation | [130] |
| Mercury | Mouse | 800 mg/kg p.o. (before and after HgCl2 exposition) | Reduced activity of ACP and ALP in testicles | [131] |
| Mercury | Mouse | 800 mg/kg p.o. (10 days before and 30 days after intoxication) | Modulation of biochemical alterations in blood: calcium and ion levels, acid and alkaline phosphatase activity and lipid peroxidation and GSH level | [132] |
| Mercury | Mouse | 800 mg/kg p.o. (10 days before and 30 days after intoxication) | Protection against renal damage reducing LPO, acid phosphatase activity, tissue degeneration and increased ALP, lactate dehydrogenase and GSH levels | [133] |
| Mercury | Rat | 300 mg/kg p.o. (10 days before and 60 days after) | Reduced hepatotoxicity as well as altered lipid profile through its antioxidant activity | [134] |
| Mercury | Rat | 300 mg/kg p.o. (10 days before and 60 days after) | Protection against testicular damage, re-establishing oxidative stress biomarkers, sperm quality and histopathological alterations | [135] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceramella, J.; De Maio, A.C.; Basile, G.; Facente, A.; Scali, E.; Andreu, I.; Sinicropi, M.S.; Iacopetta, D.; Catalano, A. Phytochemicals Involved in Mitigating Silent Toxicity Induced by Heavy Metals. Foods 2024, 13, 978. https://doi.org/10.3390/foods13070978
Ceramella J, De Maio AC, Basile G, Facente A, Scali E, Andreu I, Sinicropi MS, Iacopetta D, Catalano A. Phytochemicals Involved in Mitigating Silent Toxicity Induced by Heavy Metals. Foods. 2024; 13(7):978. https://doi.org/10.3390/foods13070978
Chicago/Turabian StyleCeramella, Jessica, Azzurra Chiara De Maio, Giovanna Basile, Anastasia Facente, Elisabetta Scali, Inmaculada Andreu, Maria Stefania Sinicropi, Domenico Iacopetta, and Alessia Catalano. 2024. "Phytochemicals Involved in Mitigating Silent Toxicity Induced by Heavy Metals" Foods 13, no. 7: 978. https://doi.org/10.3390/foods13070978
APA StyleCeramella, J., De Maio, A. C., Basile, G., Facente, A., Scali, E., Andreu, I., Sinicropi, M. S., Iacopetta, D., & Catalano, A. (2024). Phytochemicals Involved in Mitigating Silent Toxicity Induced by Heavy Metals. Foods, 13(7), 978. https://doi.org/10.3390/foods13070978